Abstract
Background
Cerebral salt wasting syndrome (CSWS) and the syndrome of inappropriate antidiuretic hormone (SIADH) are both causes of hyponatremia in pediatric neurosurgical patients often with similar presenting symptoms; however, despite similar clinical characteristics the treatment for CSWS and SIADH can be drastically different, which makes the distinction critical for post-operative treatment. Further complicating matters, are the exact mechanism for CSWS which remains unclear, and the incidence and severity of CSWS is not well studied in pediatric neurosurgical patients. We hypothesized that CSWS occurs frequently in post-operative brain tumor patients and is an important cause of post-operative hyponatremia in these patients.
Methods
We designed a single institution retrospective cohort study of all pediatric brain tumor patients undergoing craniotomy for tumor resection at our institution between January 2005 and December 2009.
Results
Of the 282 patients undergoing 291 operations, post-operative CSWS was identified in 15 cases (5%), and was more frequently observed than SIADH (nine cases, 3%). Median onset of CSWS was on post-operative day 3, lasting a median of 2.5 days. Patients with CSWS were more likely to have suffered post-operative stroke (40 vs. 4.6%, P < 0.001), have chiasmatic/hypothalamic tumors (40 vs. 3.8%, P = 0.002), and be younger (mean age 5.9 vs. 9.7 years, P = 0.01) than eunatremic patients. In addition, nearly half of the patients with CSWS (47%) had post-operative hyponatremic seizures.
Conclusion
The diagnosis of CSWS should be strongly considered in hyponatremic pediatric patients with significant natriuresis following brain tumor resection, and a treatment initiated promptly to prevent neurologic sequeleae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyponatremia is a relatively common abnormality in the pediatric neurosurgical patient, with a wide variety of etiologies. Hyponatremia in the post-operative neurosurgical patient can lead to cerebral sodium channel disturbances, which can lower the seizure threshold. Ongoing seizure activity following a primary brain insult, such as surgery, can accelerate secondary brain injury and increase post-operative morbidity and mortality [1]. Post-operative hyponatremia is commonly associated with two conditions: the syndrome of inappropriate anti-diuretic hormone (SIADH) and cerebral salt wasting syndrome (CSWS). Unfortunately, the clinical presentation of the two conditions in the immediate post-operative period can be difficult to distinguish, especially in children where the quantification of volume status is difficult, and invasive central venous pressure monitoring is not routinely used post-operatively. The management for these two conditions (CSWS and SIADH) is radically different, so the distinction is often critical to post-operative care: patients diagnosed with SIADH are treated with fluid restriction, while patients with CSWS are treated with sodium supplementation and plasma volume expansion.
CSWS manifests with a significant natriuresis, subsequent diuresis, and ultimately hypovolemic hyponatremia. Despite recognition as a clinical entity for more than 50 years, CSWS has been often questioned as a unique diagnosis [2, 3]. No definitive pathophysiologic link between central nervous system insult and salt wasting has been established. Atrial and brain natriuretic peptides have been (inconsistently) implicated in the development of, but never definitively proven to cause, CSWS; others have speculated a link between central adrenergic output and CSWS [4]. A salt wasting syndrome has been observed in patients without cerebral insult, leading some authors to rename the syndrome as renal salt wasting [5]. In addition, the classic definition of CSWS requires an intravascular volume depletion, which is difficult to document accurately in many patients without invasive monitoring, and may in fact not present if the patient is quickly resuscitated with plasma volume expansion and salt supplementation [6]. Despite this controversy regarding etiology and diagnostic criteria, we and other authors consider CSWS a useful diagnosis: some hyponatremic patients with a brisk natriuresis do respond to fluid and salt resuscitation, a finding inconsistent with SIADH [6–9].
The incidence of CSWS in pediatric neurosurgical patients is not well established. Furthermore, no study to date has defined either associated or causal patient risk factors for the development of CSWS. Small reports of CSWS in pediatric neurosurgical patients have been published, but the current literature is a mixture of etiologies, including: tumor, closed head injury, and hydrocephalus [7, 9–11]. Here we have undertaken a five-year review of all pediatric patients at our institution undergoing brain tumor resection, to better understand this potentially dangerous, yet treatable condition in a focused population.
Methods
This study was approved by the Children’s Hospital of Philadelphia Institutional Review Board. The Pediatric Neurosurgery Department patient database was queried for the study inclusion criteria: all patients in the age 0–19; without preoperative sodium abnormalities (hyponatremia, hypernatremia, or preoperative DDAVP treatment); undergoing intracranial tumor resection between January 2005 and December 2009. Stereotactic biopsies were excluded from analysis.
Pediatric patients meeting inclusion criteria underwent a meticulous review of their perioperative electronic medical record, including: pre-operative evaluation and laboratory values, intraoperative records (surgical and anesthesia), and all post-operative data. Variables recorded included patient age at surgery, presenting symptoms, co-morbidities, presence of hydrocephalus, tumor location and estimated tumor size, final tumor pathology, intraoperative and post-operative complications, estimated blood loss, and length of admission. Pre-operative tumor volume on MRI was estimated, when imaging was available, using a length x height x width gross approximation. All neurosurgical patients at our institution routinely received normal saline (0.9%) post-operatively for maintenance fluid needs; deviations from this were noted. In patients who developed hyponatremia during their post-operative hospitalization (defined as at least one serum sodium value of <135 mEq/l), the following variables were also recorded: date of onset, date of resolution, minimum serum sodium, clinical symptoms (if any), treatment, average hourly urine output over the 8 h before the first laboratory measurement of hyponatremia, urine and plasma osmolarity, and urine sodium concentration. Patients who were discharged within a week of surgery had their records examined for any emergency room visits and additional inpatient admissions.
All patients with identified hyponatremia had their records assessed in-depth. CSWS was defined (as it has been previously in the literature [9]) as at least one laboratory measurement of hyponatremia (<135 mEq/l) with brisk diuresis (>3 ml/kg/h) and elevated urine sodium (>120 mEq/l), when available, or elevated urine osmolarity (>300 m Osm/kg water). SIADH was defined as at least one laboratory measurement of hyponatremia (<135 mEq/l) with minimal or normal urine output (<2 ml/kg/h) of variable sodium concentration and urine osmolarity without other apparent cause; these patients were identified but not analyzed further. Patients with diabetes insipidus (as identified by the primary team in the medical record) who developed hyponatremia while receiving DDAVP were also identified but not further analyzed. Seizures were defined as any clinical event that the treating team considered ictal; such events were not routinely captured with electroencephalography and were not always witnessed directly by a physician. Statistical analysis was performed using Statis 10.0 software (StataCorp, College Station, TX, USA). Fisher’s exact test was performed to assess unifactorial statistical significance of categorical variables, and Student’s t tests were performed to assess unifactorial statistical significance of continuous variables.
Results
A total of 291 operations for brain tumor resection, comprising 282 unique patients, were identified. From that cohort, 30 patients met the study criteria for post-operative hyponatremia (at least one serum sodium <135 mEq/l as documented by the inpatient laboratory record). Thirteen patients met the study laboratory criteria for CSWS; in addition, all of these patients were diagnosed with CSWS by their providers during the hospitalization. Two additional patients had been diagnosed with CSWS by their inpatient providers, but in retrospect did not meet all study criteria for CSWS defined in our methods. Both had significant diuresis, and responded clinically to hypertonic (3%) saline supplementation, and so were classified as CSWS for the purpose of this study despite not meeting strict laboratory criteria (in one patient, a urine sodium of 100 mmol/l was obtained without a urine osmolarity; in the second patient, no urine labs were sent and successful therapy initiated empirically). These 15 patients are further analyzed below. Nine patients were identified who met the study criteria for SIADH, seven of which had been diagnosed with SIADH by their providers at the time of hospitalization. The other two patients meeting study SIADH criteria had been classified as hyponatremic, but without the explicit diagnosis of SIADH, by their inpatient teams. Six patients had developed hyponatremia in the setting of prior hypernatremia due to diabetes insipidus and subsequent DDAVP treatment. All patients who developed hyponatremia had received normal saline (0.9%) intra- and post-operatively, as is standard for neurosurgical patients at our institution. No patient suffered a demyelinating syndrome from correction of hyponatremia.
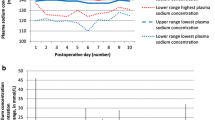
Five percent (15 of 291) of pediatric brain tumor patients developed CSWS in the post-operative period (Table 1). All had onset of CSWS during the initial hospitalization; no patient returned after discharge with new electrolyte disturbances. All received intravenous normal saline for post-operative maintenance fluid requirements before the diagnosis of CSWS. There was a male predominance (11:4), though it was not statistically significant (P > 0.05). A variety of pathologies and tumor locations were observed. Median onset of CSWS, defined as time between surgery and the first low serum sodium value, occurred on post-operative day 3 (range, 0–24 days). CSWS lasted, as defined by normalization of serum sodium and cessation of treatment, a median of 2.5 days (14 patients, range, 2–27 days). One patient continued to require treatment for CSWS in the form of oral salt tabs at the time of discharge to a rehabilitation facility. The average sodium nadir was 124 mEq/l (range, 118–133 mEq/l, see Table 2). The diuresis in the hours before development of hyponatremia averaged 7.8 ml/mg/h (range, 3.08–17.4 ml/kg/h). Urine output was not recorded hourly for all patients, depending on their inpatient unit; therefore, the number of hours over which diuresis was recorded (and thus averaged) varied between one and eight hours. All but one patient had urine sodium labs sent before treatment initiation. Urine sodium values were almost universally high, with an average of 235 mmol/l (range, 100–413 mmol/l).
In a unifactorial analysis, three patient variables were found to be significantly associated with the development of CSWS (Table 3). Patients with CSWS were more likely to be younger (average 5.9 vs. 9.7 years, t = 2.58, df = 274, P = 0.01, difference in means 3.77 years, 95% CI 0.89–6.66 years), and have chiasmatic/hypothalamic tumors (40% of CSWS patients versus 3.8% of eunatremic patients, P = 0.002) than were patients without post-operative sodium abnormalities. Patients who developed post-operative CSWS were also more likely to have suffered post-operative stroke (40% of CSWS patients) than were patients who remained eunatremic (4.6% of eunatremic patients, P < 0.001). All patients with stroke and CSWS were diagnosed with infarct before the development of CSWS. The variables of gender, presenting hydrocephalus, supratentorial location, cortical location other than chiasmatic/hypothalamic, tumor size, and estimated blood loss (either absolute or per-kg body weight) were not significantly different between patients who developed CSWS and patients who remained eunatremic (all P > 0.05). Patients with CSWS had a tenfold increase in the likelihood to develop seizures during the postsurgical admission (47%) than were patients with normal post-operative serum sodium (of whom 4.6% seized, P < 0.001). No patient with seizures developed status epilepticus, and no extubated patient required re-intubation. Long-term rates of epilepsy were not assessed.
Discussion
We have performed a five-year retrospective review to better characterize the landscape of post-operative CSWS in pediatric patients undergoing brain tumor resection. In our pediatric patient population, the overall incidence of CSWS was 5.1%, and was seen slightly more often than was SIADH. Most patients developed CSWS within the first week post-operatively. Two outlying patients developed CSWS several weeks post-operatively (on POD 14 and 24, respectively). The etiology of those patients’ salt abnormalities is not clear, and may not have been related to surgery; however, they were included for completeness as they did develop CSWS during their postsurgical hospitalization. Episodes of CSWS resolved quickly in this patient population, with a median duration of 2.5 days. Only one patient continued to suffer CSWS and required salt supplementation (oral salt tabs) at the time of discharge. Post-operative stroke, chiasmatic/hypothalamic tumors, and younger age at surgery were all associated with the development of CSWS post-operatively.
Strengths of this study include the cohort itself; a large total number of patients, with a wide range of patient ages and diverse tumor pathology. Also, relevant laboratory studies were obtained and available for almost every patient. One weakness of this study is a lack of definitive volume status data; no patient had central venous access in the post-operative hospitalization. At our institution, central venous catheterization is not routinely performed for patients undergoing brain tumor resection. Therefore, distinguishing between CSWS and SIADH was made on laboratory values alone. A second weakness is the retrospective identification of seizure based on the medical record. Certainly, some patients with post-operative twitching and unresponsiveness might not be seizing; however, to avoid introducing reviewer bias into the study all events considered as seizure by the medical team at the time of hospitalization were included. Conversely, seizures might not have been diagnosed in critically ill patients with subtle, sub-clinical ictal events. Furthermore, while seizure is considered a catalyst for secondary brain injury, significant future research is necessary to better define the relationships between the timing, duration, and location of electrical instability in the immature brain and neurological outcomes following brain injury. Another weakness is a consequence of the small number of patients who developed post-operative CSWS: no meaningful multi-factorial analysis was possible to yield independent odds ratios among the three associated patient variables identified: young age; chiasmatic/hypothalamic tumor location; and post-operative stroke. The contribution of young age to development of CSWS is not surprising, considering the relative immaturity of the infantile kidney and the other major electrolyte fluctuations not uncommonly seen in these patients when critically ill. The relative contribution of stroke versus tumor location is less clear. The majority (5/6, 83%) of patients with post-operative stroke and CSWS in this series also had chiasmatic/hypothalamic or sellar tumors (perhaps a trivial spatial distinction, depending on tumor size and surgical approach), and so the independent contributions of each variable are not apparent. The pathophysiology of CSWS is not well defined, so presumably both surgical manipulation of these regions and infarct could each contribute to the condition; the two might even have a synergistic effect. Notably, case reports exist of CSWS after sellar surgery as well as after pediatric ischemic stroke [12, 13].
Regardless of the individual contributions of patient age, tumor location, and stroke, we can make several conclusions based on the present data. First, CSWS appears not infrequently in pediatric patients undergoing brain tumor resection. Secondly, many of these patients experience neurologic sequelae, with 47% of hyponatremic patients having new seizures before hospital discharge. These sequelae could possibly be avoided with vigilant urine output monitoring and aggressive intervention. We consider this vigilance no less important despite the fact that no patient in this series with seizures had a precipitous neurological decline, status epilepticus, or required intubation. All CSWS patients had a significant diuresis (average greater than 7 ml/kg/hr) of relatively concentrated urine for at least two hours before the diagnosis of hyponatremia was made (either from routine lab work noting an asymptomatic low serum sodium, or before the child suffered undue effects such as new seizures and a laboratory sodium was obtained). We recommend a vigilant team approach, in which critical care nursing tracks hourly per-kg urine output, and immediately contacts the physician upon observing a sudden urine output increase in these patients during the first three post-operative days.
Conclusion
CSWS was observed in 5% of pediatric brain tumor patients after undergoing surgical resection in this retrospective cohort study, most frequently in the first three post-operative days. Intervention via plasma volume expansion and salt resuscitation should be considered quickly in any post-operative pediatric brain tumor patient with CSWS, to prevent hyponatremic seizures.
References
Mirski MA, Varelas PN. Seizures and status epilepticus in the critically ill. Crit Care Clin. 2008;24(1):115–47.
Peters JP, Welt LG, Sims EA, Orloff J, Needham J. A salt-wasting syndrome associated with cerebral disease. Trans Assoc Am Physicians. 1950;63:57–64.
Rivkees SA. Differentiating appropriate antidiuretic hormone secretion, inappropriate antidiuretic hormone secretion and cerebral salt wasting: the common, uncommon, and misnamed. Curr Opin Pediatr. 2008;20:448–52.
Cerda-Esteve M, Cuadrado-Godia E, Chillaron JJ, et al. Cerebral salt wasting syndrome: review. Eur J Intern Med. 2008;19:249–54.
Maesaka JK, Imbriano LJ, Ali NM, Ilamathi E. Is it cerebral or renal salt wasting? Kidney Int. 2009;76:934–8.
Singh S, Bohn D, Carlotti AP, Cusimano M, Rutka JT, Halperin ML. Cerebral salt wasting: truths, fallacies, theories, and challenges. Crit Care Med. 2002;30:2575–9.
Berkenbosch JW, Lentz CW, Jimenez DF, Tobias JD. Cerebral salt wasting syndrome following brain injury in three pediatric patients: suggestions for rapid diagnosis and therapy. Pediatr Neurosurg. 2002;36:75–9.
Cole CD, Gottfried ON, Liu JK, Couldwell WT. Hyponatremia in the neurosurgical patient: diagnosis and management. Neurosurg Focus. 2004;16:E9.
Jimenez R, Casado-Flores J, Nieto M, Garcia-Teresa MA. Cerebral salt wasting syndrome in children with acute central nervous system injury. Pediatr Neurol. 2006;35:261–3.
Lin JJ, Lin KL, Hsia SH, Wu CT, Wang HS. Combined central diabetes insipidus and cerebral salt wasting syndrome in children. Pediatr Neurol. 2009;40:84–7.
Bussmann C, Bast T, Rating D. Hyponatraemia in children with acute CNS disease: SIADH or cerebral salt wasting? Childs Nerv Syst. 2001;17:58–62; discussion 63.
Guerrero R, Pumar A, Soto A, et al. Early hyponatraemia after pituitary surgery: cerebral salt-wasting syndrome. Eur J Endocrinol. 2007;156:611–6.
Berger TM, Kistler W, Berendes E, Raufhake C, Walter M. Hyponatremia in a pediatric stroke patient: syndrome of inappropriate antidiuretic hormone secretion or cerebral salt wasting? Crit Care Med. 2002;30:792–5.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hardesty, D.A., Kilbaugh, T.J. & Storm, P.B. Cerebral Salt Wasting Syndrome in Post-Operative Pediatric Brain Tumor Patients. Neurocrit Care 17, 382–387 (2012). https://doi.org/10.1007/s12028-011-9618-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-011-9618-4