Abstract
Background
Intra-arterial (IA) nicardipine is often used to treat cerebral vasospasm associated with subarachnoid hemorrhage (SAH). While hypotension has been noted to be a dose-limiting side effect of intravenous infusions, this has seldom been reported for IA administration.
Methods
We reviewed a consecutive series of patients who received IA nicardipine for SAH-associated vasospasm. Nicardipine was titrated to angiographic response, with blood pressure and intracranial pressure monitoring. We analyzed data using Wilcoxon signed rank, Student’s t-test, Spearman’s correlation, and χ2 statistics as appropriate. A P value <0.05 was considered significant.
Results
Thirty patients underwent 50 procedures in which nicardipine was the sole chemical vasodilator (median dose, 15 mg). Median mean arterial pressures (MAP) decreased from 118 to 100 mmHg (P < 0.001), with an intra-operative low of 80 mmHg. Both intra-operative and post-operative decreases in MAP were directly related to nicardipine dose (r s = 0.352, P = 0.022 and r s = 0.308, P = 0.047, respectively). Hypotension (MAP < 70 mmHg) occurred in 22%, and 44% required initiation of or increases in vasopressor therapy. After the first treatment, 11 of 16 patients treated with vasodilator therapy alone, and 5 of 14 patients who underwent additional balloon angioplasty (68.8 vs. 35.7%, P = 0.141), required further endovascular treatments due to recurrent vasospasm on subsequent days.
Conclusions
Intra-arterial nicardipine is associated with significant intra-operative blood pressure lowering, an increased requirement for intra-operative vasopressor therapy, and a tendency toward re-treatment when used as initial monotherapy for vasospasm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) is a significant cause of morbidity and mortality worldwide, with an estimated incidence as high as 23 per 100,000 annually [1]. As many as 19% will develop symptomatic vasospasm [2], which accounts for nearly 13% of death and disability at 6 months [3]. Myriad treatments have been employed to prevent and mitigate the effects of ischemia resulting from vasospasm, including the use of oral nimodipine and “triple-H” therapy. Current endovascular interventions include balloon angioplasty and chemical vasodilation with agents such as papaverine, verapamil, milrinone, and nicardipine.
Nicardipine is a hepatically metabolized dihydropyridine-type calcium channel blocker that causes vasodilation through blockade of L-type calcium channels in vascular smooth muscle cells. Studies on animals and in vitro arterial preparations have shown a relative selectivity for cerebral vasculature [4], and some human studies have suggested that intravenous administration increases cerebral blood flow [5]. When compared to placebo for vasospasm prophylaxis, continuous intravenous (IV) infusions of 0.075 to 0.15 mg/kg/h reduced the risk of symptomatic vasospasm, but caused hypotension in as many as 34% of patients, including 3% with life-threatening hypotension [6].
As an intra-arterial (IA) treatment for vasospasm, nicardipine reduces TCD velocities and improves symptoms, angiographic appearance, and cerebral blood flow [7–11]. In contrast to the frequent hypotension resulting from IV infusions, several small studies have found only short-lived or small decrements in blood pressure, and none has accounted for nicardipine dose [7–12]. Thus, we aimed to assess the relationship between IA nicardipine dose and blood pressure in patients being treated for SAH-associated vasospasm.
Methods
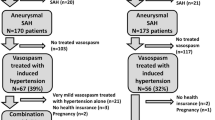
After approval by our institutional review board, we retrospectively identified 30 subjects who received IA nicardipine at least once to treat symptomatic vasospasm from a database of all adult patients admitted with SAH between April 2004 and September 2007. Demographic, clinical, and procedural data were abstracted from the entire patient chart, and for patients who received more than one procedure, each procedure was documented separately.
All subjects were routinely treated with oral nimodipine and received hyperdynamic, hypervolemic (“Triple-H”) therapy for symptomatic vasospasm. Angiography was performed in patients whose deficits did not improve with medical therapy. Patients with angiographic vasospasm involving vessels in the circle of Willis received compliant balloon angioplasty and IA nicardipine (2.5 mg/ml, PDL BioPharma, Inc., Incline Village, NV), while patients with distal vasospasm (i.e., vessels beyond circle of Willis) were treated only with non-diluted IA nicardipine. In some treatment sessions, other vasodilators (e.g., verapamil) were also used, either instead of or in addition to nicardipine. Nicardipine was injected by hand, typically at a rate of 2 ml over 10 min, with the total infusion volume titrated to angiographic improvement of vasospasm. Blood pressure was supported with fluids and vasopressors as needed. The anesthesia team, who determined the choice of anesthetic and sedative drugs, was always informed prior to IA infusion of nicardipine.
Blood pressure was monitored continuously throughout the procedure. We recorded the first, last, and minimum blood pressures as documented on the anesthesia flow sheet, and any intra-operative increases in or initiation of vasopressor agents. We defined hypotension as a mean arterial pressure (MAP) < 70 mmHg. For those subjects whose vasopressor doses were increased following the procedure, we recorded the duration, in hours, for which they remained increased, as documented on intensive care unit nursing flow sheets. For patients with an intracranial pressure (ICP) monitor, the maximal intra-procedural ICP was abstracted from anesthesia records, while pre- and post-procedure ICPs were abstracted from nursing records.
All analyses were performed using SPSS version 14.0 (Chicago, IL). Blood pressures and ICP measurements before, during, and after treatment were compared using paired Student t tests for paired parametric variables and Wilcoxon signed rank tests for paired non-parametric variables. Correlations between nicardipine doses and changes in blood pressure were assessed using Spearman’s ρ coefficient. The requirement for additional endovascular therapy in those initially receiving only chemical vasodilation versus those receiving additional mechanical angioplasty was assessed using the χ2 test. P-values <0.05 were considered significant in all analyses.
Results
Table 1 describes the baseline characteristics of our cohort. Of the 58 total interventions performed in 30 patients, 50 used IA nicardipine as the sole chemical vasodilator. Nicardipine doses in these 50 treatments ranged from 1.8 to 55 mg, with a single patient receiving 82.5 mg (median dose 15 mg). All patients showed angiographic evidence of vasodilation after nicardipine administration.
During these 50 treatments, pre-operative MAP decreased from a median of 118 to 100 mmHg post-operatively (P < 0.001), with an intra-operative low of 80 mmHg (P < 0.001). Both intra-operative and immediate post-operative decreases in MAP were directly related to nicardipine dose (r s = 0.352, P = 0.022, and r s = 0.308, P = 0.047, respectively), as illustrated in Fig. 1. Hypotension (MAP < 70 mmHg) occurred in 11 (22%) procedures, including 3 during which MAP < 60 mmHg (minimum MAP, 53 mmHg) was recorded. In four procedures, hypotension occurred with nicardipine doses ranging from 4 to 7.5 mg. Fifteen (30%) procedures required intra-operative vasopressor initiation and 7 (14%) required intra-operative increases in vasopressor dose. An additional 7 (14%) patients received sustained increases in vasopressor support following the procedure (median 2 h, range 0.5–14).
In the 18 patients with ICP monitors, nicardipine did not cause any overall change in ICP (median pre- and post-procedure ICP 10.0 vs. 8.0 cm H2O, respectively, P = 0.989). Of six patients with intra-operative ICP recordings available, only one developed sustained ICP elevation >20 cm H2O (intra-operative ICP maximum 33 cm H2O, post-operative ICP 10 cm H2O).
Considering all 58 treatments, including those in which non-nicardipine chemical vasodilators were used, 11 of the 16 (68.8%) whose initial intervention consisted of chemical vasodilation alone required further interventions for recurrent vasospasm. In contrast, 5 of 14 (35.7%, P = 0.141) whose initial intervention included balloon angioplasty required further treatments, as illustrated in Fig. 2. This resulted in a median of 2 and 1 interventions per patient, respectively (P = 0.166).
Discussion
In the largest series published to date, we assessed the hemodynamic effects of intra-arterial nicardipine as a therapy for SAH-related vasospasm and found that it was associated with hypotension in a significant proportion of patients. This was manifested as a median reduction in MAP by 18 mmHg, a frequent need for initiation of or increases in vasopressor therapy, and the presence of absolute hypotension (MAP < 70 mmHg) in one-fifth of patients. There was a modest correlation between decreases in MAP and nicardipine dose, although individual responses were variable, and substantial drops occurred even in some patients receiving small doses. Because relative hypotension may antagonize hypertensive or hyperdynamic therapy used to medically treat vasospasm, IA nicardipine for vasospasm treatment should be utilized with caution; treating physicians should be prepared to encounter and manage this potential serious adverse side effect.
In normotensive and awake subjects receiving intravenous boluses of nicardipine, the serum drug concentration declines rapidly such that the half-life is ~60 min [13]. Thus, after 5 h, over 95% of the drug is cleared from the body. When used during general anesthesia for cardiac surgery, intravenous nicardipine boluses decrease MAP in a dose-dependent manner: 2 mg caused a 30 mmHg decrement within 101 s, with 50% recovery of MAP in 3–7 min [14].
In contrast to our findings, most previous studies of IA nicardipine have found smaller degrees and shorter durations of blood pressure lowering over a wide range of doses (Table 2). While most patients in a study by Avitsian et al. [8] required intra-operative vasopressors, their blood pressures tended to increase 10–20 min after nicardipine administration, and only two patients required their use after the procedure. Similarly, Linfante et al. [9] reported that most decreases in blood pressure tended to resolve spontaneously within 10–15 min of nicardipine administration, although three sessions were stopped for systolic blood pressures that were persistently below 90 mmHg. Only the last of these studies specifically assessed for—and did not find—a relationship between nicardipine dose and hypotensive effect.
No single factor clearly accounts for the higher frequency of relative hypotension in our series. Our patients did receive higher concentrations of nicardipine (2.5 mg/ml vs. 0.1–1 mg/ml), but our median dose (15 mg) was similar to that in other studies (5–25 mg). In addition, our rate of infusion was similar to, or less than, that used in other series [8–10]. Oral nimodipine alone has been demonstrated to cause more hypotension after general anesthesia compared to placebo, even given identical baseline blood pressures [15]. Nicardipine may similarly interact with various anesthetic or sedative regimens, potentiating its hypotensive effect with some agents but not others. However, other series have not found the same degree of hypotension, despite having used a variety of sedative and anesthetic techniques [9, 10]. Finally, patient factors such as age, cardiac, and other medical co-morbidities may have contributed to the prolonged hypotensive effect, though our patients were similar to those in other series [7–10].
Consistent with most published accounts, we observed no effect of intra-arterial nicardipine on ICP. A single study reported transient ICP elevations in 6 of 18 patients during nicardipine administration [7] while subsequent studies [8–10] have generally found no ICP changes following IA nicardipine infusion (Table 2).
While this series was not powered to assess clinical outcomes, we noted that those who received only IA chemical vasodilators for vasospasm treatment showed a non-significant trend toward requiring additional endovascular interventions for vasospasm compared to those who underwent angioplasty. This is consistent with a previous study showing that IA nicardipine alone lowered TCD velocities for at least 4 days, with only one patient requiring retreatment, but decreases in TCD velocities were more pronounced when nicardipine was combined with angioplasty [7].
These data suggest that the combined approach may be more effective, although to date, no studies have directly compared the need for re-treatment after mechanical angioplasty versus IA nicardipine alone. Since vasospasm likely reflects subendothelial tissue proliferation and medial collagen deposition and myofibroblast proliferation [16, 17], chemical vasodilation may not be as durable or potent as mechanical intervention. However, angioplasty is typically limited to the proximal intracranial vasculature and is associated with greater procedural risk and complications. Thus, a rational therapeutic approach might incorporate both mechanical angioplasty for proximal vaspospasm and chemical vasodilation for distal vasospasm.
At present, few data guide our choice of intra-arterial chemical vasodilators. While papaverine has become associated with frequent rises in ICP and, more rarely, seizures, nicardipine and verapamil have gained favor more recently [18, 19]. Intra-arterial use of verapamil appears to cause occasional relative hypotension and infrequent increases in ICP [20–23]. Given the lack of controlled studies comparing these agents, the choice of chemical vasodilators requires further prospective study.
This study was performed retrospectively with attendant concerns for selection bias and limited sample size. Consequently, we were unable to control for factors such cardiac status, concurrent medications, or intubation status prior to treatment, which may affect blood pressure responses [8]. Moreover, our study was not powered to fully evaluate whether nicardipine-related hypotension affected clinical outcomes.
Conclusions
We studied the use of IA nicardipine as a vasodilator for use in treating SAH-related vasospasm and found that its use was associated with significant intra-operative blood pressure lowering and an increased requirement for vasopressor therapy. Although it appears to ameliorate angiographic vasospasm, physicians must be prepared to address these hemodynamic effects, particularly given that the medical treatment of vasospasm often calls for hypertension. Ultimately, further controlled studies will be necessary to compare various chemical vasodilators, better define their durability, and elucidate the impact of relative hypotension on clinical outcomes.
References
Bederson JB, Connolly ES Jr, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025.
Longstreth WT Jr, Nelson LM, Koepsell TD, van Belle G. Clinical course of spontaneous subarachnoid hemorrhage: a population-based study in King County, Washington. Neurology. 1993;43:712–8.
Haley EC Jr, Kassell NF, Torner JC. The International Cooperative Study on the Timing of Aneurysm Surgery. The North American experience. Stroke. 1992;23:205–14.
Amenta F, Tomassoni D, Traini E, Mignini F, Veglio F. Nicardipine: a hypotensive dihydropyridine-type calcium antagonist with a peculiar cerebrovascular profile. Clin Exp Hypertens. 2008;30:808–26.
Reddy P, Yeh YC. Use of injectable nicardipine for neurovascular indications. Pharmacotherapy. 2009;29:398–409.
Haley EC Jr, Kassell NF, Torner JC. A randomized controlled trial of high-dose intravenous nicardipine in aneurysmal subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. J Neurosurg. 1993;78:537–47.
Badjatia N, Topcuoglu MA, Pryor JC, et al. Preliminary experience with intra-arterial nicardipine as a treatment for cerebral vasospasm. AJNR Am J Neuroradiol. 2004;25:819–26.
Avitsian R, Fiorella D, Soliman MM, Mascha E. Anesthetic considerations of selective intra-arterial nicardipine injection for intracranial vasospasm: a case series. J Neurosurg Anesthesiol. 2007;19:125–9.
Linfante I, Delgado-Mederos R, Andreone V, Gounis M, Hendricks L, Wakhloo AK. Angiographic and hemodynamic effect of high concentration of intra-arterial nicardipine in cerebral vasospasm. Neurosurgery. 2008;63:1080–6. discussion 1086–1087.
Tejada JG, Taylor RA, Ugurel MS, Hayakawa M, Lee SK, Chaloupka JC. Safety and feasibility of intra-arterial nicardipine for the treatment of subarachnoid hemorrhage-associated vasospasm: initial clinical experience with high-dose infusions. AJNR Am J Neuroradiol. 2007;28:844–8.
Nogueira RG, Lev MH, Roccatagliata L, et al. Intra-arterial nicardipine infusion improves CT perfusion-measured cerebral blood flow in patients with subarachnoid hemorrhage-induced vasospasm. AJNR Am J Neuroradiol. 2009;30:160–4.
Kaku Y, Yonekawa Y, Tsukahara T, Kazekawa K. Superselective intra-arterial infusion of papaverine for the treatment of cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg. 1992;77:842–7.
Campbell BC, Kelman AW, Hillis WS. Noninvasive assessment of the haemodynamic effects of nicardipine in normotensive subjects. Br J Clin Pharmacol. 1985;20(Suppl 1):55S–61S.
Cheung AT, Guvakov DV, Weiss SJ, Savino JS, Salgo IS, Meng QC. Nicardipine intravenous bolus dosing for acutely decreasing arterial blood pressure during general anesthesia for cardiac operations: pharmacokinetics, pharmacodynamics, and associated effects on left ventricular function. Anest Analg. 1999;89:1116–23.
Stullken EH Jr, Balestrieri FJ, Prough DS, McWhorter JM. The hemodynamic effects of nimodipine in patients anesthetized for cerebral aneurysm clipping. Anesthesiology. 1985;62:346–8.
Zubkov AY, Lewis AI, Scalzo D, Bernanke DH, Harkey HL. Morphological changes after percutaneous transluminal angioplasty. Surg Neurol. 1999;51:399–403.
Smith RR, Clower BR, Grotendorst GM, Yabuno N, Cruse JM. Arterial wall changes in early human vasospasm. Neurosurgery. 1985;16:171–6.
Cross DT 3rd, Moran CJ, Angtuaco EE, Milburn JM, Diringer MN, Dacey RG Jr. Intracranial pressure monitoring during intraarterial papaverine infusion for cerebral vasospasm. AJNR Am J Neuroradiol. 1998;19:1319–23.
Carhuapoma JR, Qureshi AI, Tamargo RJ, Mathis JM, Hanley DF. Intra-arterial papaverine-induced seizures: case report and review of the literature. Surg Neurol. 2001;56:159–63.
Sehy JV, Holloway WE, Lin SP, Cross DT III, Derdeyn CP, Moran CJ. Improvement in angiographic cerebral vasospasm after intra-arterial verapamil administration. AJNR Am J Neuroradiol. 2010;31(10):1923–8.
Mazumdar A, Rivet DJ, Derdeyn CP, Cross DT III, Moran CJ. Effect of intraarterial verapamil on the diameter of vasospastic intracranial arteries in patients with cerebral vasospasm. Neurosurg Focus. 2006;21:E15.
Keuskamp J, Murali R, Chao KH. High-dose intraarterial verapamil in the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2008;108:458–63.
Feng L, Fitzsimmons BF, Young WL, et al. Intraarterially administered verapamil as adjunct therapy for cerebral vasospasm: safety and 2-year experience. AJNR Am J Neuroradiol. 2002;23:1284–90.
Acknowledgments
Each author had full access to all of the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors would also like to acknowledge Nicole Rosenberg for her help in preparing the figures and poster presentations of this data.
Disclosure
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosenberg, N., Lazzaro, M.A., Lopes, D.K. et al. High-Dose Intra-Arterial Nicardipine Results in Hypotension Following Vasospasm Treatment in Subarachnoid Hemorrhage. Neurocrit Care 15, 400–404 (2011). https://doi.org/10.1007/s12028-011-9537-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-011-9537-4