Abstract
Background
Although intracranial hemorrhage and infarction have been reported in patients with H1N1 influenza infection treated with extracorporeal membrane oxygenation (ECMO), the clinical outcomes of these patients are not well described.
Methods
The authors present two patients with H1N1 influenza infection and diffuse cerebrovascular injury in the setting of ECMO.
Results
Diffuse cerebrovascular injury including intraparenchymal hemorrhage was found on head CT and brain MRI in both cases and confirmed by autopsy in one patient who died. Punctate foci of susceptibility effect were seen in both patients on T2* susceptibility-weighted or susceptibility-sensitive gradient echo sequences. These foci of susceptibility effect were consistent with infarction on histopathologic evaluation in the patient who died. The other patient made an excellent clinical recovery.
Conclusions
Frequent and early surveillance imaging should be obtained in patients with H1N1 influenza infection undergoing ECMO, although the presence of diffuse cerebral injury, including intraparenchymal hemorrhage and multifocal punctate susceptibility effect, does not necessarily portend a poor prognosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the first case of 2009 H1N1 influenza A virus infection was confirmed in the United States in April 2009, more than 10,000 H1N1-related deaths, many because of refractory hypoxemia, have occurred [1, 2]. For critically ill, often young patients with H1N1 influenza infection, extracorporeal membrane oxygenation (ECMO) has been used worldwide for hypoxemia refractory to conventional mechanical ventilation, despite the lack of robust evidence in its favor [3, 4]. Intracranial hemorrhage and infarction have been reported in patients with H1N1 influenza infection treated with ECMO, but the range of clinical outcomes in these patients is not well described. The authors present two cases of critically ill patients with 2009 H1N1 influenza infection complicated by diffuse cerebrovascular injury in the setting of rescue ECMO for severe respiratory distress syndrome.
Case Summaries
Case 1
A 41-year-old man presented to a local hospital with 5 days of dyspnea, fever, and cough. Oxygen saturation (SpO2) was 70% on room air at the time of presentation. He was started on broad-spectrum antibiotics and oseltamivir and was intubated for hypoxic respiratory failure. H1N1 influenza virus was detected by reverse transcriptase-polymerase chain reaction (RT-PCR) from a nasal swab. He was then transferred to this hospital after 11 days for management of refractory respiratory failure. While on increasing ventilatory support, including fraction of inspired oxygen (FiO2) up to 1.0, positive end-volume expiratory pressure (PEEP) of 18 and respiratory rate of 30, SpO2 ranged from 72 to 96%. He became hypotensive and developed acute renal failure requiring continuous veno-venous hemodialysis (CVVH) and vasopressor therapy.
Veno-venous ECMO was initiated for refractory hypoxia on hospital day (HD) 3 and continued for 18 days. On ECMO, SpO2 measured by serial arterial blood gases ranged from 80 to 100% (mean % ± standard deviation = 89.2 ± 5.9), with FiO2 ranging from 0.4 to 1.0. While receiving heparin for ECMO, his platelet nadir was 55,000/mm3, peak international normalized ratio (INR) was 1.3, and peak partial thromboplastin time (PTT) was 50.7 s.
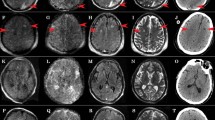
Head computed tomography (CT) imaging performed on HD 25 demonstrated two right temporal lobe hyperdensities consistent with intraparenchymal hemorrhage, measuring 2 and 1 cm, respectively, in largest diameter (Fig. 1). The susceptibility-weighted T2* magnetic resonance imaging sequence acquired on HD 27 demonstrated innumerable punctate foci of susceptibility effect throughout the white matter, more widespread in the cerebrum than in the cerebellum, with marked involvement of the corpus callosum and relative sparing of the brainstem (Fig. 1). Restricted diffusion and T2 hyperintensity were also present in the corpus callosum. Severe, persistent hypoxia in the lateral decubitus position prevented lumbar puncture from being performed safely, and paralytics and sedation could not be lightened for a more detailed neurologic examination. His clinical condition continued to decline and he died on HD 38.
Case 1: a Coronal noncontrast CT image from HD 25, showing a 2 cm hyperdense parenchymal hematoma in the right temporal lobe. b and c Axial susceptibility-weighted T2* magnetic resonance images from HD 27 showing the right temporal hematoma, as well as innumerable foci of susceptibility effect throughout the white matter, more widespread in the cerebrum than the cerebellum, with relative sparing of the brainstem and marked involvement of the corpus callosum. d Coronal section of the fixed brain demonstrating a discrete intraparenchymal hematoma in the right posterior temporal lobe, as well as punctate lesions in the white matter. e Numerous yellow, slightly sunken lesions are seen in the corpus callosum. f A lesion from the corpus callosum consists of a focus of infarction filled with macrophages, small amounts of hemosiderin and surrounding reactive changes in the form of reactive astrocytes and axonal spheroids (LH&E stain)
Post-mortem examination of the brain performed 1 day after death confirmed the presence of two right temporal lobe hematomas (Fig. 1) without associated vascular malformations or evidence of vasculitis. In the corpus callosum and hemispheric white matter, there were numerous small slightly sunken yellowish lesions (Fig. 1), consistent with infarcts. These were not associated with vasculitis or ring hemorrhages, and occasionally contained small amounts of hemosiderin and perilesional reactive gliosis (Fig. 1). There were no viral cytopathic changes in neurons, glia, arachnoid cells, or vascular endothelium.
Case 2
A 24-year-old man with sickle cell trait presented to a local hospital with 5 days of fever, vomiting, and cough. SpO2 was 85% on room air at the time of presentation. He was started on broad-spectrum antibiotics and oseltamivir and was intubated for hypoxic respiratory failure on the day of presentation. While intubated, SpO2 was as low as 75% despite an increase in FiO2 to 1.0, PEEP of 27 and respiratory rate of 50. He developed rhabdomyolysis with peak serum creatine kinase of 53,000 units/liter and thrombocytopenia with a platelet count of 123,000/mm3. H1N1 influenza virus was confirmed by RT-PCR from a nasal swab. He was transferred to our hospital after 5 days when his hypoxia worsened despite aggressive ventilatory support.
Veno-venous ECMO was initiated on the night of transfer and continued for 13 days. On ECMO, SpO2 measured by serial arterial blood gases ranged from 76 to 100% (mean% ± standard deviation = 91.4 ± 4.9), with FiO2 ranging from 0.4 to 1.0. He also developed acute renal failure requiring CVVH. His thrombocytopenia worsened with a platelet nadir of 57,000/mm3. Heparin was used while on ECMO, with PTT measurements ranging mostly from 50 to 70 s, although several were supratherapeutic (>150 s). Peak INR was 1.7. On HD 15, a head CT scan demonstrated a hyperdense lesion involving the splenium of the corpus callosum, measuring 4.8 cm in largest diameter, thought to reflect subacute hemorrhage, with surrounding edema in the splenium and body of the corpus callosum, centrum semiovale, right frontoparietal region, forceps major, and right occipital cortex. There were also multiple punctate foci of subcortical hemorrhage in the right parietal lobe. Subsequent CT imaging demonstrated stability of the hemorrhage in the splenium of the corpus callosum. On HD 22, the patient began to spontaneously open his eyes as sedation was weaned, but he was unable to follow commands and did not exhibit any spontaneous movement of his extremities.
Brain MRI on HD 33 demonstrated expected evolution of the hemorrhage in the splenium of the corpus callosum and subacute infarcts in the right parietal and occipital lobes with petechial hemorrhagic conversion. The susceptibility-sensitive T2*-weighted gradient echo sequence showed innumerable punctate susceptibility foci within the corpus callosum, bilateral internal capsules and bilateral cerebral and cerebellar hemispheres (Fig. 2). Punctate foci of diffusion restriction were also seen in the right corona radiata. On HD 34, the patient was able to follow simple commands and mouthed the words “I can’t breathe.” He began to purposefully move his upper and lower extremities against gravity. He was discharged to a rehabilitation facility on HD 48.
Case 2: a and b Axial susceptibility-sensitive T2*-weighted gradient echo magnetic resonance images acquired on HD 33 demonstrating evolving hemorrhage in the splenium of the corpus callosum and numerous punctate susceptibility foci in the genu (a arrowhead), body of the corpus callosum (b arrow), and bilateral internal capsules (a arrow). c Axial susceptibility-sensitive T2*-weighted gradient echo magnetic resonance images acquired 10 months after initial MRI again demonstrating numerous susceptibility foci in the genu of the corpus callosum (c arrowhead), body of the corpus callosum (d arrow), and bilateral internal capsules (c arrow)
He was seen in neurology clinic 2 months after discharge, at which point his examination was notable for a left homonymous hemianopsia, impaired recall, mild inattention, decreased verbal fluency, and difficulty with abstraction. At 9 months, he had only a partial left visual field deficit, and there had been significant improvement in attention, language, memory, and abstract thinking. A repeat brain MRI demonstrated persistence of the punctate foci of susceptibility effect on the susceptibility-sensitive T2*-weighted gradient echo sequence (Fig. 2).
Discussion
Intracranial hemorrhage is a common cause of morbidity and mortality in patients undergoing ECMO [5]. Although the use of ECMO in patients with 2009 H1N1 influenza infection has been less extensively studied, uniformly fatal intracranial hemorrhage has been described in one case series [3]. Intracranial hemorrhage can also occur in the setting of influenza-associated encephalopathy, often accompanied by multi-organ failure, disseminated intravascular coagulation, and diffuse cerebral edema [6, 7]. This well-described neurological complication of influenza infection makes it difficult to determine with certainty whether the pathogenesis of intracranial hemorrhage in critically ill patients with H1N1 influenza infection undergoing ECMO is a result of influenza-related vascular injury, an underlying hemorrhagic diathesis secondary to hypercytokinemia-associated thrombocytopenia and elevated prothrombin time, an adverse effect of ECMO, anticoagulation required for ECMO, or some combination thereof.
At autopsy, the histopathologic appearance of the multifocal areas of susceptibility effect noted in case 1’s MRI was consistent with infarction, rather than hemorrhage, raising the possibility that the susceptibility effect was due to air emboli. Gaseous microemboli detected by transcranial Doppler ultrasound have been described as a complication of ECMO [8, 9]. Air, like blood, causes hypointensity in MRI pulse sequences that are sensitive to magnetic susceptibility effects. Once a cerebral air embolus is visible on CT scan, the time required for it to become undetectable in subsequent scans has been determined to be on the order of hours to days [10]. In CT, air emboli are visible as hypodense foci because of a reduction in X-ray attenuation that is confined to the emboli, whereas, in susceptibility-sensitive MRI, air emboli are visible because of perturbations of the magnetic field that extend far outside of the embolus, resulting in characteristic “blooming” that can make the embolus appear far larger than it really is. Therefore, it is reasonable to expect that, as air emboli gradually decrease in size because of absorption, they would remain visible for a longer time on MRI than on CT. A delay in absorption of air emboli might make it possible for trapped air emboli to cause end-arteriole damage leading to ischemic injury [11].
Another potential etiology for the multifocal areas of susceptibility effect observed in case 1’s MRI is microvascular thrombi occluding the end arterioles or capillaries. Although a repeat MRI could not be obtained in case 1, the serial MRI findings in case 2 suggest the presence of intravascular thrombi causing susceptibility effect [12–14]. Indeed, the unchanged appearance of susceptibility-related signal loss on repeat brain MRI for case 2 almost 1 year after being on ECMO is not consistent with air emboli, which would have been removed from the microvasculature in hours to days. By contrast, microvascular thrombi could persist for a longer period of time, producing susceptibility effect up to 1 year later. While multifocal microvascular hemorrhage is another etiologic consideration for the susceptibility effect observed in case 2, this etiology is inconsistent with the histopathological findings in case 1. Thus, if the mechanism of injury were similar for the two patients described, microvascular thrombi best explain the radiological and histopathological findings in both cases.
The two cases presented here support the recommendation that brain imaging should be considered in critically ill patients with H1N1 influenza infection on ECMO, particularly those in whom sedation and pharmacologic paralysis render neurologic examination extremely challenging, in order to minimize further neurologic complications and guide management [5]. Given that neurologic complications are often identified in patients within the first few days of initiating ECMO, frequent and early surveillance imaging should be pursued even in the absence of overt neurologic symptoms. Select radiographic findings on ECMO, including significant hemorrhage or generalized edema, may alter management. However, although diffuse cerebral injury during ECMO is often associated with a poor prognosis, the excellent clinical outcome of case 2 speaks to the wide range of potential outcomes in individuals with H1N1 influenza infection who have undergone ECMO, provided that the systemic illness can be treated. The possibility of neurological recovery in an H1N1 influenza patient with diffuse cerebrovascular injury in the setting of ECMO should be considered during discussions regarding goals of care.
References
CDC Estimates of 2009 H1N1 Influenza Cases: Hospitalizations and deaths in the United States. April 2009–February 13, 2010. 2010. www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm.
Nin N, Soto L, Hurtado J, et al. Clinical characteristics and outcomes of patients with 2009 influenza A (H1N1) virus infection with respiratory failure requiring mechanical ventilation. J Crit Care. 2010; doi:10.1016/j.crc.2010.05.031.
Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–95.
Miller RR 3rd, Markewitz BA, Rolfs RT, et al. Clinical findings and demographic factors associated with ICU admission in Utah due to novel 2009 influenza A(H1N1) infection. Chest. 2010;137:752–8.
Lidegran MK, Mosskin M, Ringertz HG, et al. Cranial CT for diagnosis of intracranial complications in adult and pediatric patients during ECMO: clinical benefits in diagnosis and treatment. Acad Radiol. 2007;14:62–71.
Weitkamp JH, Spring MD, Brogan T, et al. Influenza A virus-associated acute necrotizing encephalopathy in the United States. Pediatr Infect Dis J. 2004;23:259–63.
Lyon JB, Remigio C, Milligan T, Deline C. Acute necrotizing encephalopathy in a child with H1N1 influenza infection. Pediatr Radiol. 2010;40:200–5.
Zanatta P, Forti A, Bosco E, et al. Microembolic signals, strategy to prevent gas embolism during extracorporeal membrane oxygenation. J Cardiothorac Surg. 2010;5:5.
Jonas RA. The effect of extracorporeal life support on the brain: cardiopulmonary bypass. Semin Perinatol. 2005;29:51–7.
Dexter F, Hindman BJ. Recommendations for hyperbaric oxygen therapy of cerebral air embolism based on a mathematical model of bubble absorption. Anesth Analg. 1997;84:1203–7.
Jeon S-B, Kang D-W. Neurological picture. Cerebral air emboli on T2-weighted gradient-echo magnetic resonance imaging. J Neurol Neurosurg Psychiatr. 2007;78:871.
Idbaih A, Boukobza M, Crassard I, et al. MRI of clot in cerebral venous thrombosis: high diagnostic value of susceptibility-weighted images. Stroke. 2006;37:991–5.
Boukobza M, Crassard I, Bousser MG, Chabriat H. MR imaging features of isolated cortical vein thrombosis: diagnosis and follow-up. AJNR Am J Neuroradiol. 2009;30:344–8.
Kim HS, Lee DH, Choi CG, et al. Progression of middle cerebral artery susceptibility sign on T2*-weighted images: its effect on recanalization and clinical outcome after thrombolysis. AJR Am J Roentgenol. 2006;187:W650–7.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chow, F.C., Edlow, B.L., Frosch, M.P. et al. Outcome in Patients with H1N1 Influenza and Cerebrovascular Injury Treated with Extracorporeal Membrane Oxygenation. Neurocrit Care 15, 156–160 (2011). https://doi.org/10.1007/s12028-011-9534-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-011-9534-7