Abstract
Background
To observe the effects of the minimally invasive removal of an intracerebral hematoma on the glutamate concentration, blood–brain barrier (BBB) permeability and brain water content in the brain tissue surrounding the hematoma and to provide a theoretical basis for minimally invasive removal of intracerebral hematomas.
Methods
Thirty rabbits (2.8–3.4 kg body weight) were selected to establish a model of intracerebral hemorrhage, and they were randomly divided into a model control group and a minimally invasive group after the model was prepared successfully. The intracerebral hematoma was evacuated by stereotactic procedures in minimally invasive group 6 h after the model was established. The glutamate content, the permeability of the BBB and the brain water content in perihematomal brain tissues were determined and compared between the two groups.
Results
The glutamate content, the permeability of the BBB and the brain water content in the perihematomal brain tissues were significantly decreased compared to the model control group 1, 3, and 7 days after the minimally invasive removal of the intracerebral hematoma.
Conclusions
Minimally invasive surgery for removal of an intracerebral hematoma could significantly reduce the glutamate content, BBB permeability and the brain water content in perihematomal brain tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous intracerebral hemorrhage (ICH) remains a formidable disease. After initial irreversible tissue damage occurs near the hemorrhage nidus, a progressive cascade of elevated local pressures, edema, and excitotoxicity causes additional secondary injury to the surrounding brain tissue [1–3]. This occurs in the days following the initial hemorrhage and commonly results in severe morbidity and high mortality. Much of this secondary process is thought to be attributable to the mass effect of the new hemorrhage, the toxicity associated with hematoma decomposition and the release of inflammatory and free radical mediators. Increased levels of glutamate and aspartate have been detected following subarachnoid hemorrhage (SAH) and intracerebral hemorrhage, and the increased level of glutamate is associated with increased blood–brain barrier (BBB) permeability and brain edema. Cerebral damage as a consequence of glutamate-mediated excitotoxicity is a major consequence of stroke. Recently published data confirmed the correlation between the level of excitatory amino acids (EAAs) and the outcome of ICHs, suggesting that neurochemical monitoring of these substances may have a role in the care of patients [4]. Glutamate-related excitotoxicity may have an important impact on secondary injury [1].
To date, despite promising basic research, the development of effective clinical treatments for this potentially devastating condition has been largely unsuccessful [5]. Clinical studies and case series have demonstrated that clot burden plays a significant role in several forms of intracerebral hemorrhage, suggesting that clot reduction plays an important role in limiting brain edema, additional neuronal injury, and the severity of neurological deficits following an ICH [6–9]. Investigations from the last decade have demonstrated that the extent of ICH-mediated brain injury relates directly to blood clot volume and the duration of blood exposure to brain tissue [10]. As a result, there has been a significant interest in the potential benefits of acute hematoma evacuation. Although ISTICH clearly showed that the majority of patients did not benefit from hematoma removal [11], and traditional medical and surgical approaches have been unable to favorably modify the neurological outcome of patients with intracerebral hemorrhage, investigators did not believe the result of ISTICH directly challenges the usefulness of surgery for spontaneous intracerebral hemorrhage. For deep hematomas, such as those in the basal ganglia or thalamus, benefits will be obtained if a less invasive, safe, and effective method of clot evacuation exists [12]. A trial of CT-guided mechanical aspiration suggested a benefit from stereotactic aspiration of deep intracerebral hematomas under computed tomographic control [7, 13].
Minimally invasive surgical strategies have been devised to minimize injury to the surrounding brain tissue caused by surgery. Recently, the therapeutic effectiveness of minimally invasive evacuation of intracerebral hematomas has been demonstrated by some published clinical studies [10, 14–16]; however, the changes in neurochemicals and BBB permeability in the brain tissue surrounding the hemorrhage following a minimally invasive procedure for intracerebral hematoma evacuation remain poorly understood. There is little information regarding the role of this procedure in terms of the pathophysiological changes in the perihematomal brain tissue. The purpose of the present study was to characterize the changes in the glutamate content in the perihematomal brain tissue after minimally invasive evacuation of spontaneous hematomas and to elucidate the relationship between the neurochemical mechanisms and the perihematomal BBB permeability and brain water content.
Methods and Materials
Materials
Main Reagents
Formamide (molecular formula: HCONH2, Chongqing Chuanjiang Chemical Reagent Factory), urethane (molecular formula: C3H7NO2, Wuxi Yangshan Biochemical), Evan’s Blue (Beijing Hengye Zhongyuan Chemical), 4% paraformaldehyde (Wuhan Boster Biological Technology), urokinase (Guangdong Livzon Pharmaceutical), glutamate (Sigma), derivatization reagent borate buffer (Agilent Technologies, USA), FMOC reagent Agilent PN5061-3337 (Agilent Technologies, USA), OPA reagent Agilent PN5061-3335 (Agilent Technologies, USA, 2,4-DNFB (Japan), and HPLC-grade acetonitrile and methanol (Germany) were used in this study.
Main Instruments
For this study, we used the following instruments: a ZH-Lanxing B-Type rabbit stereotaxic Apparatus (Huaibei Zhenghua Biological Instrument & Equipment), electronic scales (Satourious, Germany), a Rainbow Type-722 grating spectrophotometer (Shandong Gaomi Rainbow Analytical Instrument), a 5415R high-speed centrifuge (Frozen, Heraeus Company), micropipettors (Eppendorf), a 202-2 constant temperature oven (Shanghai Luda Laboratory Apparatus), a digital display thermostat water bath HH-2 (Guohua Electric Appliance), a desktop general centrifuge (TGL-16B; Shanghai Anting Scientific Instrument Factory), a −80°C freezer (Forman Scientific Company), a refrigerator (Qingdao), a CT provided by the Guiyang Medical College, a high performance liquid chromatograph (HP-1100; Agilent Technologies, USA), a G1315 A diode-array detector (DAD, Agilent Technologies, USA), a pH meter (410 A, ORION, USA), an Agilent 1313A Automatic Sampler (Agilent Technologies, USA), a column oven (Agilent Technologies, USA), and scales (Beijing Gangdong Hengye Instrument).
Experimental Groups
The present study was approved by the Animal Care and Use Committee of Guiyang Medical College.
Thirty rabbits (2.8–3.4 kg, either male or female) were provided by the Animal Center of Guiyang Medical College. These rabbits were randomly and equally divided into a minimally invasive group (MI group) and a model control group (MC group), and both groups (15 rabbits each) were equally divided into three subgroups (to be euthanized 1, 3, or 7 days following ICH establishment). An ICH was induced in all animals.
In order to provide the normative values from a normal control group (NC group) so that there is some point of reference with respect to how abnormal values were in the MC group and how much of an improvement to normal the evacuation produced, we performed another experiment. The NC group included 12 normal rabbits, also divided into three subgroups (to be euthanized 1, 3, or 7 days following surgical procedures).
Animal Preparation
Preparation of the ICH Model
The rabbits were fasted for 12 h and water restricted for 4 h before the experiment. The rabbits were then anesthetized by injecting 20% urethane (5 ml/kg) into the ear vein. Slow breathing, a slow corneal reflex and no pain reaction were used as indicators of the animal being fully anesthetized. The head of the rabbit was then sheared to expose the skin for surgery.
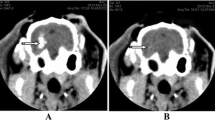
The anesthetized rabbit was fastened to the stereotaxic apparatus, and the skin in the operation field was disinfected using 75% alcohol. A 3-cm incision was made along the mid-line at the line of connection between the two post-orbital margins, and the skull was stripped of subcutaneous fascia to expose the skull. A 3% H2O2 solution was used to open the periosteum and expose the bregma and lambdoid sutures. The head was then adjusted to make the bregma 1.5 mm higher than the lambdoid suture. The position of the internal capsule was located according to the rabbit stereotaxic atlas. The coronal plane crossing the center of bregma was used as the coronal zero plane (AP0), A1 represented the coronal plane 1 mm ahead of AP0 and the internal capsule was estimated to be between A5 and P2. The present experiment used the A1 level and the bregma as base points, using 6 mm left along the coronal suture and 1 mm parallel to the sagittal suture as the puncture point. The skull of the rabbit was drilled, and using a #12 needle and a 1-ml syringe, 0.8 ml autologous arterial blood was taken from the central ear artery. The syringe was then connected to a #7 needle in which the tip was removed. Air was completely removed from the syringe, leaving 0.5 ml of blood. The #7 needle was then inserted vertically and quickly into the skull 12 mm deep, and the blood was slowly injected into the basal ganglia. The injection lasted for approximately 3 min. The needle was left in place for 8 min after the blood was injected to prevent the backflow of the blood, and then the needle was pulled out slowly. Local hemostasis was induced by compression for 2 min. The drill hole was then covered using gutta-percha. A CT scan was performed 3 h later. A high-density shadow in the basal ganglia region with no shadow in the lateral ventricle was considered successful ICH induction (Fig. 1a).
Brain CT showing the area of the intracerebral hematoma before and after evacuation. a Brain CT 3 h after the induction of ICH. The round, high-density shadow in the left basal ganglia region demonstrated the successful induction of the hematoma. b The hematoma in the left basal ganglia region was removed using a minimally invasive procedure, and the high-density shadow disappeared
The rabbits were sent back to the animal room and housed as usual after successful ICH induction confirmed by CT scan. All the animals recovered from anesthesia within 5 h after intravenous injection of 20% urethane. The total anesthesia time was 3–5 h.
The exclusion criteria included visualization of back flow along the needle track, blood in the ventricle, and death of the rabbit.
Minimally Invasive Evacuation of the ICH
In the MI group, surgical procedures for evacuation of the intracerebral hematoma were performed within 6 h of successful ICH induction.
The rabbits were anesthetized again by injecting 20% urethane (5 ml/kg) into the ear vein. They were then placed in the stereotaxic apparatus, and the skin over the operation field was disinfected using 75% alcohol. Using the former drill hole, a #7 needle was inserted into the hematoma, and the liquid part of the hematoma was aspirated. We then injected 5,000 U of urokinase (dissolved in 0.5 ml of 0,9% sodium chloride solution) into the hematoma. The needle was kept in place for 15 min, followed by slow aspiration while withdrawing the needle. A CT scan was performed again, and the high-density shadow was mostly removed (Fig. 1b). The drill hole was then covered using gutta-percha. The skin around the area was disinfected and finally sutured. The rabbits were then placed back in the breeding room for 1, 3, or 7 days.
Treatment of the Normal control Group
Procedures performed in the MC group were also done in the NC group, but without injecting autologous arterial blood into the basal ganglion to induce intracerebral hematoma.
Medical Treatment of the Animals
Animals in each group received only intramuscular injection of penicillin (400,000 U) to prevent infection, and they were housed as usual until they were killed. No other medical treatment was administered.
Brain Tissues Preparation
A 2% Evan’s Blue (2 ml/kg) solution was injected into the ear vein 2 h before the animals were euthanized. The animals were then anesthetized using 20% urethane. The animal’s chest was quickly opened, exposing the heart. A tube was inserted from left ventricle into the aortic root, with a small hole cut off on right auricle to allow the tube to exit. Rabbits were perfused transcardially with 400 ml of 0.9% sodium chloride solution, followed by 100 ml of 4% paraformaldehyde after the fluid flowing out was clear. The brain was then extracted and placed on ice. Using the needle track as the center to prepare a coronal section and a sagittal section, then the brain on the hematoma side was cut and divided into four parts: front-inner, front-outside, back-inner, and back-outside. A total of 5 mm of brain tissue surrounding the hematoma was collected from each part mentioned above. The front-inner part was used for amino acid testing and was stored at −80°C, the front-outside part was used for testing the Evan’s Blue content, the back-inner part was used for testing the water content in the brain and the back-outside part was placed in neutral formaldehyde.
Determination of the Glutamate Level Surrounding the Hematoma using High Performance Liquid Chromatography (HPLC)
Chromatographic Conditions
The chromatographic column used was the ZORBAX clipse-AAA (4.6 × 150 mm, 5 μm). Mobile phase A was 40 mM Na2HPO4, pH 7.8 (5.5 g NaH2PO4·H2O + 1 l water, NaOH was used if needed to make the pH 7.8) and mobile phase B was 45:45:10 (V/V/V) ACN:MeOH:water. The column was run with a flow rate of 2 ml/min. Phase B increased from 0 to 57% between 0 and 18 min and from 57 to 100% between 18.1 and 18.6 min. It remained at 100% between 18.6 and 22.3 min and decreased from 100 to 0% between 22.3 and 23.2 min. Between 23.2 and 26 min, Phase B remained at 0%. The column temperature was 40°C, and the sampling volume was 10 μl. The wavelength of the diode array was 262 nm, and the reference wavelength was 324 nm.
Derivative Solution Preparation
A total of 25 mg OPA was dissolved in 1 ml methanol. Sodium borohydride buffer (4 mol/l) was then added (pH 10.4) and the solution was stirred. The final solution was stored at 4°C.
Standard Solution Preparation
Glutamate and 0.2 mol/l NaHCO3 (pH 9.8) was used to make a standard stock solution at a concentration of 1 g/l.
Biosample Preparation
The brain tissue was defrosted, weighed, and placed into a dry glass homogenizer. Dilute hydrochloric acid (1:5 w/v; 0.1 mmol/l) was added, and the homogenized brains were placed into an ultrasonicator (Temp: 4°C; pulse for 2 s, rest for 2 s; intensity: 20%; 15 times in total). Samples were then centrifuged at 1200 rpm for 20 min at 4°C. Borate saline buffer (2.5 μl) was then added to the supernatant solution and mixed for 20 min, followed by the addition of 0.5 μl OPA. The sample was then mixed for 30 s, FMOC (0.5 μl) was then added and the solution was mixed for another 30 s. Finally, water (32 μl) was added, and the final sample was mixed for 30 s, and 10 μl of the sample was used.
Calculation of Glutamate Concentration
The peak area of glutamate from the HPLC was integrated and used as an external standard for the samples. The glutamate concentration for 1 g of brain tissue was then calculated according to the sample quality.
Measurement of BBB Permeability
Experimental Methods
Evan’s Blue was used as a tracer to measure the BBB permeability. Two hours before each experiment, 2% Evan’s Blue (2 ml/kg) was injected into the ear vein. After 2 h, the brain tissue was quickly removed. The tissue surrounding the hematoma was weighed (with an accuracy of 0.1 mg) and then placed into a test tube with 4 ml of formamide. The tube was then capped and placed in a 54°C water bath for 24 h to allow the Evan’s Blue to spread throughout the brain tissue. The samples were then centrifuged at 2400 rpm for 5 min. A spectrophotometer was used (λ = 632 nm) to measure the absorbance of the supernatant, which was removed using a straw and placed into a quartz cuvette. Absorbency was measured using formamide alone as a blank controller.
Setting up the Standard Curve
Evan’s Blue (4 mg) was placed into a volumetric flask and weighed (within the accuracy of 0.1 mg). A total of 100 ml NS was added, and the solution was stirred. From this solution, 0.3 ml was removed and placed in 5.7 ml of formamide to make the standard buffer solution. A total of 3 ml of this solution was serially diluted in seven tubes containing 3 ml of formamide each. The amount of Evan’s Blue in each of the seven tubes was 8, 4, 2, 1, 0.5, 0.25, and 0.125 μg per ml. The tubes were capped and placed into a 54°C water bath for 24 h. The aforementioned method to measure absorbance was then used. Linear regressions were then calculated for the absorbencies and Evan’s Blue content. The final equation was y = 0.0053x + 0.0608 (R 2 = 0.9833).
Computational Method of Evan’s Blue Content in Brain Tissue
We used the formamide method to measure the Evan’s Blue content in the brain tissue to gauge the severity of the BBB damage. The formula used was as follows: Evan’s Blue content in brain tissue (μg/g wet brain) = B × formamide (ml)/wet weight (g), where B refers to the Evan’s Blue content of the sample (μg/ml) given by the linear regression equation according to standard curve.
Measuring of Water Content of Perihematomal Brain Tissues
The dry and wet weight method was used to measure the water content of the brain tissue. The brains were quickly removed, and brain tissue from the back-outside part of the hematomas was used. First, the weight of the wet tissue was obtained. The samples were then placed in an oven at 100°C for 48 h, and the dried samples were then weighed. The water content of the brain tissue was then calculated as (wet weight – dry weight)/wet weight × 100%.
Statistical Analysis
All data were analyzed using SPSS 11.5. Basic data are expressed as the mean ± standard deviation (X ± SD). A repeated measures ANOVA was used to make comparisons across the whole time series between the MC group and the MI group. A P value less than 0.05 was considered statistically significant. Statistical analysis was performed in consultation with the Department of Biostatistics of Guiyang Medical College.
The normal control data were not included in statistical analysis, but it is provided for normal reference values.
Results
Preparation of the ICH Model
Thirteen rabbits were successfully prepared for ICH model in MC group, however, one rabbit was found to be dead in the animal room without definite cause. In the MI group, ICH model was successfully prepared in all the 15 rabbits, but two animals died of overdose of anesthetic agents during surgery, one animal died on the fifth day because of intracranial infection. Finally, a total of 24 rabbits (12 in MI group, and 12 in MC group, respectively) were included in the present study.
Changes in the Glutamate Concentration in the Brain Tissue Surrounding the Hematoma
The glutamate concentration in the brain tissue surrounding the hematoma on days 1, 3, and 7 following removal of the hematoma was significantly decreased compared to the MC group(F = 30.76, P = 0.0015), suggesting that the minimally invasive procedure for intracerebral hematoma evacuation decreased the glutamate concentration in perihematomal brain tissue (Table 1). A significant difference in perihematomal glutamate concentration was also observed in the MC group and the MI group at different time point (F = 66.2623, P = 0.0000).
Determination of BBB Permeability
The Evan’s Blue content in the brain tissue surrounding the hematoma in the MI group was significantly decreased on days 1, 3, and 7 compared to the MC group, a significant difference was noted (F = 46.34, P = 0.0009,). This suggests that the minimally invasive procedure for intracerebral hematoma evacuation may reduce BBB permeability (Table 1).
Water Content in Brain Tissue Surrounding the Hematoma
The water content in the brain tissue surrounding the hematoma in the MI group was significantly decreased on days 1, 3, and 7 compared to the MC group (F = 14.30, P = 0.0092), suggesting that the minimally invasive procedure for intracerebral hematoma evacuation may reduce the water content of the brain tissue (Table 1).
Discussion
In the present study, the glutamate content in the perihematomal brain tissue of animals that underwent minimally invasive surgery to remove an intracerebral hematoma was decreased at different time points (on days 1, 3, and 7) after hematoma evacuation as compared to the MC group. In addition, the animals that underwent the minimally invasive hematoma evacuation showed a decrease in the Evan’s Blue content, as well as the brain water content in the brain tissue surrounding the hematoma. These findings suggest that minimally invasive removal of intracerebral hematomas may decrease the glutamate content, BBB permeability, and the water content in perihematomal brain tissue.
In recent years, EAAs have become a focus of research examining intracerebral hemorrhages. The glutamate content has been found to be clearly elevated in perihematomal brain tissue, and the prognosis of ICH is closely related with the EAA level [4, 17, 18].
Patients with a spontaneous ICH who presented with more serious brain injuries had a higher concentration of glutamate in the brain and, consequently, a worse prognosis [19]. Glutamate-associated excitotoxicity may have an important impact on secondary injury [1]. Zhu Lingqun et al. [20] examined an animal model of hemorrhagic stroke and found that the glutamate content in the hippocampal area of the pathological group was increased to a large extent, whereas neurons were clearly reduced. In addition to cerebral edema, other phenomena were detected using light microscopy, including the absence of multiple layers of neurons, some physaliphore degeneration and the absence of pyknosis or nuclei. Ultramicrostructural analyses demonstrated edema of colloid cells and neurons, angioedema, organelle swelling in neurons and pyknosis. All of these morphological changes indicate the conformity of the increased EAA concentration in hemorrhagic stroke and the degree of brain injury and show that the neurotoxic effect of EAAs can participate in perihematomal changes during the pathophysiological process.
Increased levels of glutamate have been associated with increased BBB permeability and brain edema, which are the principal pathophysiological changes of secondary brain damage associated with the toxicity involved in hematoma decomposition and the release of inflammatory and free radical mediators. The aforementioned studies have demonstrated that the glutamate level in perihematomal brain tissue increases and results in disruption of the BBB and increased BBB permeability, thereby increasing brain edema. If the effect of glutamate could be antagonized or its content could be reduced, the permeability of the BBB should be reduced, thus decreasing brain edema. Blocking the effect of glutamate with its receptor antagonist MK-801 and felbamate has been shown to reduce the formation of brain edema and help restore BBB permeability in experimental subarachnoid hemorrhages and diffuse brain injuries [21, 22]; however, the development of effective clinical treatments has been largely unsuccessful, and removing intracerebral hematomas by standard open craniotomy commonly causes damage to the uninjured brain tissue overlying the hematoma. This indicates that decreasing the glutamate content in perihematomal brain tissue by minimally invasive evacuation of the intracerebral hematoma should be a better choice. In our experiment, after evacuation of the intracerebral hematoma using a minimally invasive procedure, the ICH-induced neurotoxic glutamate content in the perihematomal brain tissue was reduced, and the mass effect of the hematoma was also decreased, resulting in a decrease in BBB permeability and brain water content. Miller et al. [1] recently obtained similar results. They investigated 12 consecutive patients undergoing frameless stereotactic aspiration and thrombolysis (FAST) of deep ICHs and measured hourly the glucose, lactate, pyruvate, and glutamate levels in the perihematomal tissue of patients undergoing minimally invasive hematoma evacuation. They observed that the glutamate level was elevated in the perihematomal region following ICH, and this level was decreased during hematoma drainage.
Disruption of the blood–brain barrier is a hallmark of ICH-induced brain injury. This disruption contributes to edema formation, the influx of leukocytes and the entry of potentially neuroactive agents into the perihematomal brain, all of which may contribute to brain injury [23]. We found that the increased BBB permeability following ICH was positively correlated with cerebral edema (Table 1). The BBB plays an important role in the adjustment and maintenance of a stable intracerebral microenvironment, and the function of its endothelial cells depends greatly on the changes in the intracerebral microenvironment [24]. Altering the intracerebral microenvironment could, in addition, influence BBB permeability. Neurotoxic substances released as a result of a hematoma following an ICH, such as thrombin and hemoglobin, could be toxic to neurons. The amount of glutamate released from vesicles following an ICH increases, and the energy metabolic disorder around the hematoma influences the glutamate/glutamine dynamics and limits the uptake of glutamate. At the same time, the level of extracellular excitatory neurotransmitters increases due to the inhibition of glutamic acid decarboxylase (GAD) as a result of the energy metabolic disorder and the increased level of neurotoxic substances injures the BBB. Therefore, brain edema has a close relationship with both intracerebral hematoma and BBB permeability [17, 25].
By measuring the degree of BBB damage, measured by testing the Evan’s Blue content in brain tissue at different time points, it has been demonstrated that the BBB begins to break down 6 h after injecting blood. This disruption becomes worse as time increases and peaks after 72 h [26]. The same has been shown with increasing water content in the brain, which is consistent with the results shown here.
Brain edema following intracerebral hemorrhage is the primary reason for the deterioration of the disease condition. Restraining the cerebral edema and relieving the secondary injury to neurons surrounding the hematoma is one of the critical links to reducing the morality rate and lowering the disability rate in the acute stages of ICH. Since cerebral edema is closely related to both hematomas and BBB permeability, removal of an intracerebral hematoma as soon as possible could lower the intracerebral pressure and reduce the cerebral edema and neurotoxic effects caused by thrombin, hemoglobin, and other degradation products. This could relieve the pressure on the surrounding brain tissue from the hematoma, improve microcirculation in the area, facilitate the glutamate/glutamine cycle and expedite the uptake of glutamate. Glutamate can then be converted into γ-aminobutyric acid by glutamic acid decarboxylase, which results in a reduction in the level of extracellular excitatory neurotransmitters, consequently relieving the cerebral edema and making the BBB less permeable. There have been some reports regarding the significant effectiveness of the minimally invasive removal of ICHs during the ultra-early stage on relieving cerebral edema and protecting the BBB [27, 28], which is consistent with the results presented here.
In our previously published data, minimally invasive surgery could decrease the Purdy score of the dog model of ICH, there were significant functional differences in animals treated with minimally invasive procedures compared to control [29], so we did not performed neurological or behavioral assessment in the present study. Although CT scan showed that intracerebral hematoma was evacuated largely or completely after surgical procedures, we performed imaging in all animals to quantify the hemorrhage, the residual hematoma volume was not measured during the histopathological exam, this is a limitation of this manuscript.
In the present study, although significant decreases in glutamate, Evans’s blue, and brain water content were observed in MI group compared with MC group, they were still significantly high as compared with NC group, manifesting that serious pathological changes are very likely even after minimally invasive procedures for hematoma evacuation. These pathological changes may be caused by the residual hematoma after the surgical procedures or/and the procedures itself. Based on the present findings, it might be concluded that the minimally invasive procedures for evacuation of ICHs could significantly reduce the level of excitatory glutamate in perihematomal brain tissues and make the blood–brain barrier less permeable, consequently preventing the formulation and development of secondary cerebral edema. The minimally invasive technique may have benefits from evacuating intracerebral hematoma, but it could only reduce the brain damages caused by the hematoma to some extent, other than completely eliminating them. The significance of the observed changes resulted from hematoma evacuation remains to be determined with respect to neurologic outcome.
References
Miller CM, Vespa PM, McArthur DL, Hirt D, Etchepare M. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduced levels of extracellular cerebral glutamate and unchanged lactate pyruvate ratios. Neurocrit Care. 2007;6:22–9.
Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–9.
Xi G, Keep RF, Hoff JT. Pathophysiology of brain edema formation. Neurosurg Clin N Am. 2002;13:371–83.
Chiang MF, Chiu WT, Lin FJ, Thajeb P, Huang CJ, Tsai SH. Multiparametric analysis of cerebral substrates and nitric oxide delivery in cerebrospinal fluid in patients with intracerebral haemorrhage: correlation with hemodynamics and outcome. Acta Neurochir (Wien). 2006;148:615–21. (dicussion 621).
Hazell AS. Excitotoxic mechanisms in stroke: an update of concepts and treatment strategies. Neurochem Int. 2007;50:941–53.
Wagner KR, Xi G, Hua Y, et al. Ultra-early clot aspiration after lysis with tissue plasminogen activator in a porcine model of intracerebral hemorrhage: edema reduction and blood-brain barrier protection. J Neurosurg. 1999;90:491–8.
Teernstra OP, Evers SM, Lodder J, Leffers P, Franke CL, Blaauw G. Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA). Stroke. 2003;34:968–74.
Vespa P, McArthur D, Miller C, et al. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocrit Care. 2005;2:274–81.
Miller DW, Barnett GH, Kormos DW, Steiner CP. Stereotactically guided thrombolysis of deep cerebral hemorrhage: preliminary results. Cleve Clin J Med. 1993;60:321–4.
Morgan T, Zuccarello M, Narayan R, Keyl P, Lane K, Hanley D. Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial. Acta Neurochir Suppl. 2008;105:147–51.
Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–97.
Nakano T, Ohkuma H. Surgery versus conservative treatment for intracerebral haemorrhage—is there an end to the long controversy? Lancet. 2005;365:361–2.
Hosseini HLC, Hariz M, et al. Stereotactic aspiration of deep intracerebral hematomas under computed tomographic control: a multicentric prospective randomised trial. In: 12th European stroke conference, Section57, Valencia, Spain; 2003.
Gazzeri R, Galarza M, Neroni M, Alfieri A, Esposito S. Minimal craniotomy and matrix hemostatic sealant for the treatment of spontaneous supratentorial intracerebral hemorrhage. J Neurosurg. 2009;110:939–42.
Miller CM, Vespa P, Saver JL, et al. Image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. Surg Neurol. 2008;69:441–6. discussion 446.
Wu G, Wang L, Hong Z, Mao Y, Hu X. Effects of minimally invasive techniques for evacuation of hematoma in basal ganglia on cortical spinal tract from patients with spontaneous hemorrhage: observed by diffusion tensor imaging. Neurol Res. doi:10.1179/016164110X12759951866993.
Hartings JA, Gugliotta M, Gilman C, Strong AJ, Tortella FC, Bullock MR. Repetitive cortical spreading depolarizations in a case of severe brain trauma. Neurol Res. 2008;30:876–82.
Qureshi AI, Ali Z, Suri MF, et al. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit Care Med. 2003;31:1482–9.
Wang E, Ho CL, Lee KK, Ng I, Ang BT. Effects of temperature changes on cerebral biochemistry in spontaneous intracerebral hematoma. Acta Neurochir Suppl. 2008;102:335–8.
Zhu L, Jiang Y, Huang Q. Effect of Xingnaojianshen capsule on hippocampal EAA and neuron of SHRsp with hemorrhagic stroke. Clin Neurol. 1998;11:131–4.
Germano A, Caffo M, Angileri FF, et al. NMDA receptor antagonist felbamate reduces behavioral deficits and blood-brain barrier permeability changes after experimental subarachnoid hemorrhage in the rat. J Neurotrauma. 2007;24:732–44.
Imer M, Omay B, Uzunkol A, et al. Effect of magnesium, MK-801 and combination of magnesium and MK-801 on blood-brain barrier permeability and brain edema after experimental traumatic diffuse brain injury. Neurol Res. 2009;31:977–81.
Keep RF, Xiang J, Ennis SR, et al. Blood-brain barrier function in intracerebral hemorrhage. Acta Neurochir Suppl. 2008;105:73–7.
Nagaraja TN, Keenan KA, Brown SL, Fenstermacher JD, Knight RA. Relative distribution of plasma flow markers and red blood cells across BBB openings in acute cerebral ischemia. Neurol Res. 2007;29:78–80.
MacLellan CL, Davies LM, Fingas MS, Colbourne F. The influence of hypothermia on outcome after intracerebral hemorrhage in rats. Stroke. 2006;37:1266–70.
Dai DW, Wang DS, Li KS, et al. Effect of local mild hypothermia on expression of aquaporin-4 following intracerebral hemorrhage in rats. Zhonghua Yi Xue Za Zhi. 2006;86:906–10.
Jose M, Montes JHW, Fayad PiereB. Stereotactic computed tomographic-guided aspiration and thrombolysis of intracerebral hematoma: protocol and preliminary experience. Stroke. 2000;31:834–40.
Wagner KR. Modeling intracerebal hemorrhage: glutamate, nuclear factor-B signaling and cytokines. Stroke. 2007;38:753–8.
Wu G, Zhong W. Effect of minimally invasive surgery for cerebral hematoma evacuation in different stages on motor evoked potential and thrombin in dog model of intracranial hemorrhage. Neurol Res. 2010;32:127–33.
Acknowledgments
This research was partially supported by Guizhou science and technology fund. We are grateful for the technical help in the high performance liquid chromatography provided by Guizhou physical and chemical institute. We are also indebted to the medical imaging department of Affiliated Hospital, Guiyang Medical College, for valuable discussions about the preparation of ICH model.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, G., Li, C., Wang, L. et al. Minimally Invasive Procedures for Evacuation of Intracerebral Hemorrhage Reduces Perihematomal Glutamate Content, Blood–Brain Barrier Permeability and Brain Edema in Rabbits. Neurocrit Care 14, 118–126 (2011). https://doi.org/10.1007/s12028-010-9473-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-010-9473-8