Abstract
Background
Prognostication for survivors of cardiac arrest is a frequent challenge to neurologists. Our aim was to determine whether the FOUR (Full Outline of UnResponsiveness) score is an accurate predictor of outcome in patients after cardiac arrest and to compare its performance to the Glasgow Coma Scale (GCS).
Methods
We prospectively identified patients surviving cardiac arrest from June 2006 to October 2009. Neurologic exams were grouped into two time intervals following cardiac arrest: 1–2 days and 3–5 days. The FOUR score and the Glasgow coma scale (GCS) were determined for each examination. Primary outcome was in-hospital mortality.
Results
Of 136 patients, 112 (82%) were examined on days 1–2 after cardiac arrest and 87 (64%) on days 3–5. Forty-seven patients (35%) survived to hospital discharge and 89 (65%) died during hospitalization. No patients with a sum FOUR score ≤4 at exam days 3–5 survived (false positive rate [FPR] 0% C.I. 0.000–0.0345), whereas one patient (2%) with sum GCS score of 3 survived to discharge (FPR 2.2%, C.I. <0.0001–0.1758). At days 3–5 after arrest, 41 of 45 (91%) patients with a sum FOUR score >8 survived (P < 0.0001), while 39 of 45 (87%) with a sum GCS > 6 survived (P < 0.0001). A 2-point improvement in FOUR score, but not GCS, in serial exams was associated with survival. Sensitivities, specificities, positive, and negative predictive values were comparable between both scales.
Conclusion
The FOUR score, a simple clinical tool, is an accurate predictor of outcome in patients surviving cardiac arrest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Following resuscitation after cardiac arrest, an accurate prognostic opinion regarding survival and functional outcome is needed. This can present a major challenge because of the broad range of possible outcomes, from death to independent functional recovery. Although advances in brain imaging, biochemical markers, and electrophysiologic studies have aided in accurate prognostication [1–5], the clinical neurologic examination remains the foundation of the assessment. Certain signs, such as absent pupillary or corneal reflexes at day 3 after cardiac arrest, motor response no better than extensor at day 3, as well as the presence of myoclonus status epilepticus, are known predictors of poor outcome [6–11].
The Glasgow Coma Scale (GCS) has been used to predict outcome following cardiac arrest [12, 13]. While the GCS remains widely used in clinical evaluation, a more informative scale, the Full Outline of UnResponsiveness (FOUR) score, was recently developed to provide a more comprehensive assessment. The FOUR score includes additional information not assessed by the GCS including brainstem reflexes, visual tracking, breathing patterns, and respiratory drive [14] (Fig. 1). It is also more practical for evaluating critically ill intubated patients, as it does not depend on an evaluation of the verbal response. It has already been validated in various populations of comatose patients [15–17].
Full Outline of UnResponsiveness (FOUR) score. Eye response: E4 eyelids open or opened, tracking, or blinking to command; E3 eyelids open but not tracking; E2 eyelids closed but open to loud voice; E1 eyelids closed but open to pain; and E0 eyelids remain closed with pain. Motor response: M4 thumbs-up, fist, or peace sign; M3 localizing to pain; M2 flexion response to pain; M1 extension response to pain; and M0 no response to pain or generalized myoclonus status. Brainstem reflexes: B4 pupil and corneal reflexes present; B3 one pupil wide and fixed; B2 pupil or corneal reflexes absent; B1 pupil and corneal reflexes absent; and B0 absent pupil, corneal, and cough reflex. Respiration pattern: R4 not intubated, regular breathing pattern; R3 not intubated, Cheyne-Stokes breathing pattern; R2 not intubated, irregular breathing; R1 breathes above ventilatory rate; and R0 breathes at ventilator rate or apnea
The aim of our study was to determine whether the FOUR score accurately predicts outcome in comatose patients after cardiac arrest and to compare its performance to the GCS in this setting.
Methods
We prospectively identified patients who survived cardiac arrest for whom a neurologic consultation was requested at our institution from June 2006 to October 2009. Detailed neurologic examinations were performed and recorded exclusively by consultant neurointensivists. Exams were performed at two intervals, 1–2 days and 3–5 days after cardiac arrest. When information was available from several examinations during these two periods, the earlier exam was used for analysis. When feasible, patients were examined in the absence of effects of sedative medications. At our institution, patients with out-of-hospital ventricular fibrillation arrests are candidates for therapeutic hypothermia. Patients who were treated with therapeutic hypothermia were examined after rewarming. The Full Outline of UnResponsiveness (FOUR) score and the Glasgow coma scale (GCS) were determined upon each examination. Survival and neurologic outcome according to the Cerebral Performance Categories (CPC) scale were assessed at hospital discharge [18]. The CPC scale is a measure of outcome defined by the following: 1—good cerebral performance (conscious, alert, able to work, may have mild deficit), 2—moderate cerebral disability (disabled but independent), 3—severe cerebral disability (conscious but disabled and dependent), 4—coma or vegetative state, and 5—death. The study protocol was approved by the Mayo Clinic Institutional Review Board. A more detailed analysis including extensive neurologic examination findings, neuroimaging, serum neuron-specific enolase measurements, and results of electrophysiologic studies will be presented in a larger analysis.
Statistical Analysis
Data are presented using calculations for sensitivity, specificity, positive and negative predictive values, and false-positive rate (FPR, i.e., 1—specificity). Confidence intervals (95%) were determined using the modified Wald method, except when the frequency of the event was zero. In that circumstance, the upper limit of the confidence interval was determined using Hanley’s formula (3/n). Statistical comparison was performed using two-tailed Fisher’s exact test or Chi-square test. A P value < 0.05 was considered statistically significant.
Results
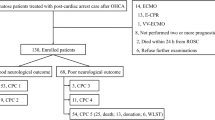
A total of 136 patients were identified. Mean age was 62 years (range 21–87, standard deviation 15). Baseline characteristics are summarized in Table 1. Fifty-one patients (38%) were treated with therapeutic hypothermia. Forty-seven patients (35%) survived to hospital discharge (26 of whom were treated with hypothermia) and 89 (65%) died during hospitalization. All survivors were conscious at hospital discharge and 75% of survivors achieved at least moderate functional outcome, as measured by discharge CPC ≤2. Seventeen patients (36% of survivors and 13% of total population) had a discharge CPC of 2, and 12 (26% of survivors, 9% of total) had discharge CPC of 3. No patients had a CPC of 4 at discharge. Median duration of hospital stay for survivors was 15 days. Of those who did not survive to discharge, median day of death was 4.
One-hundred and twelve patients (82%) were examined within 1–2 days after cardiac arrest. Of these, 36 (32%) had a sum FOUR score ≤4 and only one (0.9%), with a sum score of 4, survived to discharge. With the exception of three patients who had second cardiac arrests, all of the remaining 30 patients with a sum FOUR score >8 survived to discharge. Similarly, all but three patients (two with repeat cardiac arrest) with a sum GCS score >6 (24 of 27) survived to discharge. However, four of the 55 (7%) patients with a sum GCS score of 3, the lowest score possible, also survived to discharge.
Eighty-seven patients (64%) were examined in the interval of 3–5 days following cardiac arrest. The great majority of these (n = 70, 80%) were examined on day 3, while 14 were examined on day 4, and 3 were examined on day 5. The FOUR score and GCS distributions at exam day 3 are provided in Fig. 2. Of 20 patients with a sum FOUR score ≤4, none (0%) survived to discharge, yielding a specificity of 100% and FPR of 0% (C.I. 0.000–0.0345). In contrast, there was one patient (2%) with a sum GCS score of 3 who survived to discharge (FPR 2.2%, C.I. <0.0001–0.1758). This patient had a sum FOUR score of 5 (E0, M0, B4, and R1) and by discharge the only deficit present was reduced visual acuity. She was otherwise awake, alert, ambulating independently and was without significant cognitive deficit. Patients with a sum GCS score of 3 exhibited a wide range of FOUR scores (0–7).
Of 42 patients who died during hospitalization after being examined in days 3–5, 38 (91%) had a sum FOUR score ≤8 and a sum GCS score ≤6. Forty-one of 45 patients (91%) with a sum FOUR score >8 survived (P < 0.0001). The majority of these patients (n = 29, 71%) had scores ≥13. Of the 45 who survived to discharge, 39 (87%) had a sum GCS >6 (P < 0.0001). Using these cutoffs of eight for FOUR score and six for GCS, specificities are similar (91% vs. 87%, respectively) while the sensitivities are equivalent at 91%. The FOUR score and GCS performed identically in our population when using higher cutoffs of 10 and 8, with both producing 92% sensitivity and 80% specificity. Table 2 presents additional data on sensitivity, specificity, and predictive values.
Sixty-two patients were examined at both time intervals. Of these, 22 (35%) had improvement in their coma scale ratings. An increase in 2 points from the first examination to the second was significantly associated with survival when graded by the FOUR score, but not the GCS. There were 29 patients with a FOUR score delta ≥2, 19 of whom survived (P = 0.041). There were 23 patients with a GCS delta ≥2, 15 of whom survived (P = 0.114). The coma scales were divided into three categories based on the results of our sensitivities and specificities for cutoffs of three and eight for the FOUR score and three and six for the GCS. The shift in patient distribution in coma scale grade from first to second examination for these patients is shown in Fig. 3.
Discussion
The results of this study show that in patients surviving cardiac arrest, the FOUR score is an accurate predictor of outcome. The vast majority of patients with a FOUR score >8 at days 3–5 after cardiac arrest survive to hospital discharge. We also found that no patients with a FOUR score ≤4 at days 3–5 after cardiac arrest survived, while in contrast, a GCS of 3 was not invariably associated with death. Improvement in coma scale by 2 points in serial examinations was associated with survival with the FOUR score but not the GCS. The overall performance of the two coma scales was similar.
Previous studies have evaluated the GCS as a predictor for neurologic outcome after cardiac arrest [12, 19]. Though various cutoffs and time periods were used, results of these studies indicated that the GCS was a reasonable predictor of outcome but lacked specificity. Results of a recent study showed that at day 3 after cardiac arrest, a GCS >6 had a specificity of 87% and sensitivity of 73% for predicting good outcome [13]. Though our end point was different and we included patients not treated with hypothermia, we found a comparable specificity (86%) but a higher sensitivity (90%) for a GCS cut off of six at examination day 3 after cardiac arrest.
On serial examination, about 35% of patients improved in coma scale rating, with the majority of these patients moving from moderate into higher ranges of the two scales, while almost all patients with low scores remained low. However, there was remarkable improvement seen in some patients with low scores (for example, one patient with a FOUR score of 4 and GCS of 3 moving to scores of 13 and 15, respectively). Furthermore, an improvement by 2 points in the sum FOUR score on serial examinations was significantly associated with good outcome. This illustrates the value of serial neurologic examinations when estimating prognosis.
This study is limited by non uniformity of examination times, potential confounder effects of residual effects of sedative medications, and relatively small study population. In addition, only two-thirds of our population had documented examinations during the 3–5 day interval which was used for analysis. This is in part because some patients who recovered substantially within 1-2 days did not require repeat examination for prognostication purposes, but also reflects patients in whom support was withdrawn prior to day 3 after cardiac arrest. As it is probably the case in the majority, if not all, previous studies on prognostication in cardiac arrest, mortality in this series was most often preceded by withdrawal of life support measures. Therefore, it is conceivable that the high predictive value of the coma scores could have been favored by an increased rate of withdrawal of life support in deeply comatose patients with estimated poor prognosis leading to a self-fulfilling prophecy. It is important to note that prognostication in this setting should not be done on the basis of a single clinical assessment or a single test. In this study population, several ancillary tests were done during the course of evaluation including serum NSE (69%), head computed tomography scan (72%), somatosensory evoked potentials (36%), and electroencephalogram (34%). We are currently performing a more detailed analysis of this population including more detailed neurologic examination findings, neuroimaging data, serum neuron-specific enolase (NSE) measurements, results of electrophysiologic studies, and main reasons for withdrawal of life support. These will be presented in separate studies and are beyond the scope of this manuscript.
In summary, we found that the FOUR score is an accurate predictor of outcome in survivors of cardiac arrest. It performs at least as well as the GCS in this population. This simple clinical tool should be incorporated along with biomarker and electrophysiologic data to prognosticate in cardiac arrest survivors.
References
Chen R, Bolton CF, Young B. Prediction of outcome in patients with anoxic coma: a clinical and electrophysiologic study. Crit Care Med. 1996;24(4):672–8.
Bassetti C, Bomio F, Mathis J, Hess CW. Early prognosis in coma after cardiac arrest: a prospective clinical, electrophysiological, and biochemical study of 60 patients. J Neurol Neurosurg Psychiatry. 1996;61(6):610–5.
Logi F, Fischer C, Murri L, Mauguiere F. The prognostic value of evoked responses from primary somatosensory and auditory cortex in comatose patients. Clin Neurophysiol. 2003;114(9):1615–27.
Fogel W, Krieger D, Veith M, et al. Serum neuron-specific enolase as early predictor of outcome after cardiac arrest. Crit Care Med. 1997;25(7):1133–8.
Wijman CA, Mlynash M, Caulfield AF, et al. Prognostic value of brain diffusion-weighted imaging after cardiac arrest. Ann Neurol. 2009;65(4):394–402.
Levy DE, Caronna JJ, Singer BH, Lapinski RH, Frydman H, Plum F. Predicting outcome from hypoxic-ischemic coma. Jama. 1985;253(10):1420–6.
Zandbergen EG, Hijdra A, Koelman JH, et al. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology. 2006;66(1):62–8.
Edgren E, Hedstrand U, Kelsey S, Sutton-Tyrrell K, Safar P. Assessment of neurological prognosis in comatose survivors of cardiac arrest. BRCT I study group. Lancet. 1994;343(8905):1055–9.
Wijdicks EF, Parisi JE, Sharbrough FW. Prognostic value of myoclonus status in comatose survivors of cardiac arrest. Ann Neurol. 1994;35(2):239–43.
Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67(2):203–10.
Young GB, Doig G, Ragazzoni A. Anoxic-ischemic encephalopathy: clinical and electrophysiological associations with outcome. Neurocrit Care. 2005;2(2):159–64.
Mullie A, Verstringe P, Buylaert W, et al. Predictive value of glasgow coma score for awakening after out-of-hospital cardiac arrest. Cerebral resuscitation study group of the Belgian society for intensive care. Lancet. 1988;1(8578):137–40.
Schefold JC, Storm C, Kruger A, Ploner CJ, Hasper D. The Glasgow Coma Score is a predictor of good outcome in cardiac arrest patients treated with therapeutic hypothermia. Resuscitation. 2009;80(6):658–61.
Wijdicks EF, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: the FOUR score. Ann Neurol. 2005;58(4):585–93.
Iyer VN, Mandrekar JN, Danielson RD, Zubkov AY, Elmer JL, Wijdicks EF. Validity of the FOUR score coma scale in the medical intensive care unit. Mayo Clin Proc. 2009;84(8):694–701.
Stead LG, Wijdicks EF, Bhagra A, et al. Validation of a new coma scale, the FOUR score, in the emergency department. Neurocrit Care. 2009;10(1):50–4.
Wolf CA, Wijdicks EF, Bamlet WR, McClelland RL. Further validation of the FOUR score coma scale by intensive care nurses. Mayo Clin Proc. 2007;82(4):435–8.
Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–4.
Sandroni C, Barelli A, Piazza O, Proietti R, Mastria D, Boninsegna R. What is the best test to predict outcome after prolonged cardiac arrest? Eur J Emerg Med. 1995;2(1):33–7.
Sponsorship and Disclosures
This study was not sponsored. Dr. Jennifer Fugate reports no disclosures. Dr. Alejandro Rabinstein reports no disclosures. Dr. Daniel Claassen reports no disclosures. Dr. Roger White reports no disclosures. Dr. Eelco Wijdicks reports no disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fugate, J.E., Rabinstein, A.A., Claassen, D.O. et al. The FOUR Score Predicts Outcome in Patients after Cardiac Arrest. Neurocrit Care 13, 205–210 (2010). https://doi.org/10.1007/s12028-010-9407-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-010-9407-5