Abstract
Background
Recent reports suggest that when thrombolytic agents are administered within the clot, lysis rate accelerates at the expense of increased risk of worsening edema. To test this hypothesis, we report on the volumetric analysis of (1) the intraparenchymal hematoma and, (2) perihematomal edema in a cohort of ICH patients treated with intraclot rtPA.
Methods
A convenience sample of highly selected ICH patients underwent frameless stereotactic aspiration and thrombolysis (FAST) of the clot. Two milligrams of rtPA were administered every 12 h until ICH volume ≤10 cc, or catheter fenestrations were no longer in continuity with the clot. ICH and perihematomal edema volumes were calculated from CT scans. Using random effects linear regression we estimated the rate of hematoma and edema volume resolution as well as their relationship during the first 8 days of lytic therapy.
Results
Fifteen patients were treated, mean age: 60.7 years, median time from ictus to FAST: 1 (range 0–3) day. Using a random effects model that considered volume resolution over the first 8 days following lytic therapy we found that the both percentage hematoma and percentage perihematoma edema resolution per day were quadratic with respect to time. Percentage residual hematoma volume on day K = 97.7% − [24.36%*K] + [1.89%*K 2]; P < 0.001 for both terms. Percentage residual edema on day K = 97.4% − [13.94%*K] + [1.30%*K 2]; P < 0.001 for K and P = 0.01 for K 2. Examination of each patient’s volume data suggests that there exists a strong direct relationship between perihematoma edema volume and same day hematoma volume.
Conclusions
In this cohort of ICH patients treated using FAST, volumetric analysis of ICH and perihematomal edema seems to suggest that local use of rtPA does not exacerbate brain edema formation. Furthermore, there seems to be a strong association between reduction in ICH volume and reduction in edema volume, as would be expected following the concept of “hemotoxicity” postulated by some investigators.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Morbidity and mortality following ICH remain the highest among all forms of cerebrovascular diseases. Although several outcome-modifying clinical variables are well recognized, the impact of perihematomal brain edema on neurological function of patients who survive the acute phase of the disease is less clear.
Plausible mechanisms of neurological deterioration following development of perihematomal edema are: (1) Mass effect introduced by perihematomal edema on an already challenged intracranial compliance, and (2) Secondary neuronal injury triggered by blood/degradation products (“hemotoxicity”) with potential to alter brain function [1, 2]. Unfortunately, directed therapies for ICH-induced brain edema are lacking. It has been only recently (when thrombolytic agents were used to locally lyse intraparenchymal hematomas in animal models of ICH) that efficient reduction in the volume of perihematomal edema has been reported [3]. Okuda and coworkers have also suggested a positive effect of early hematoma removal on perihematomal edema formation in a heterogeneously treated cohort of ICH patients [4]. Similarly, Mayer and coworkers demonstrated an association between hematoma size and volume of perihematoma edema [5]. Although the relative safety of using local thrombolysis in different clinical paradigms of acute brain injury (subarachnoid hemorrhage, ICH, ischemic stroke) is well documented, experimental data suggesting that parenchymal clot lysis using thrombolytic agents potentiate brain edema formation as well as induce direct neurotoxicity have raised concern [6–9]. To better define the impact of recombinant tissue plasminogen activator (rtPA) on clot volume resolution as well as to clarify conflicting results of studies testing the effects of thrombolytic agents on perihematomal edema formation after human ICH, we report on our experience using frameless clot aspiration and thrombolysis (FAST) using rtPA in the treatment of a selected cohort of ICH patients. Specifically, we report on (1) rate of hematoma volume and perihematomal edema volume resolution; and (2) the association between perihematomal edema volume and hematoma volume during the 8 days following the onset of treatment with thrombolysis.
Subjects and Methods
The study cohort consisted of selected ICH patients treated with a FAST protocol using rtPA. This therapy was considered an acceptable alternative to open craniotomy with hematoma evacuation in selected cases at Sinai Grace Hospital rather than a clinical trial designed to test its safety or efficacy. It was applied in a clinically uniform manner to sequentially eligible patients [10, 11]. The decision to perform clot aspiration and thrombolysis was made by a single senior neurosurgeon (RRJ) after patient/family discussions were held. Inclusion criteria when identified within 72 h from the ictus were:
-
1.
Computed tomographic (CT) diagnosis of spontaneous supratentorial ICH of ≥35 cc of volume, following Tuhrim and Broderick estimations that mortality following ICH increases significantly when hematoma volume reaches 30 cc and larger [12–14].
-
2.
Reduced level of consciousness leading to incapacity to follow commands (Score of 2 in the NIH Stroke scale item 1a).
-
3.
Absence of signs of herniation for at least 8 h from ICH onset (To ensure neurological stability prior to the procedure).
-
4.
Patient’s age ≥18 years (as children are not part of the center’s usual patient population).
-
5.
No clinical or radiological evidence of vascular abnormality (e.g. aneurysm, arterio-venous malformation) as cause of ICH.
-
6.
No clinical/laboratory evidence of a bleeding diathesis.
Although no pre-defined time window was specified for the time between patients were first seen in the Emergency Department and the time aspiration/lysis therapy was started, the treating team allocated every effort in expediting this complex process.
The Wayne State University Institutional Review Board approved this project. Data were retrospectively collected onto standardized abstraction forms.
Operative Technique
Following initial resuscitation and radiographic evaluation, potentially eligible patients underwent repeat CT scan with fiducials using a stereotactic protocol that utilized 1- or 2-mm slices that were then loaded onto a Stealth Station (Medtronic, Inc; Minneapolis, MN). Under general anesthesia, the patient’s head was fixed in a Mayfield clamp and a reference arc was attached to the apparatus. Fiducials were identified as landmarks on the CT scan using the Stealth planning software. The goal of placement was to center the catheter along the long axis of the clot with the tip of the catheter near the posterior end of the hematoma to allow maximum exposure of the clot to instilled rtPA. Virtual elongation of the pointer was used to determine burr hole placement and optimal trajectory and depth to the target. Following burr hole placement, the virtual pointer was placed on the dura to reconfirm trajectory and depth. The dura was then opened and a rigid brain cannula (Medtronics Inc, Minneapolis, MN) was placed along the predetermined trajectory to the desired depth. Following placement of the rigid catheter, manual hematoma aspiration was performed. No other methods to mechanically disrupt the clot were attempted. After measuring the aspirate volume, no more than half the pretreatment hematoma volume was removed due to concerns that overly aggressive aspiration could precipitate further hemorrhage. The rigid cannula was then removed and a soft ventriculostomy-type catheter—15-cm long and 1- to 2-mm internal diameter—(Codman, Raynham, MA) was placed along the same trajectory. The intraclot catheter was tunneled under the scalp and sutured in place.
Thrombolysis Protocol
In the operating room, 14/15 patients received 2 mg of rtPA (Alteplase, Genentech, San Francisco, CA) reconstituted in 2 cc of preservative-free 0.9% saline. The catheter then was irrigated with 2 cc of preservative-free 0.9% saline and then clamped from drainage for 1 h. If ICP increased to 20-mm Hg or more for longer than 10 min, the catheter was reopened with an appropriate pressure gradient while adjunctive medical therapies to control ICP were instituted. All patients were managed in a dedicated intensive care unit. Subsequent drug administration was performed using identical and sterile technique at the bedside in the ICU.
Information in regards to the ideal dose of rtPA in this clinical paradigm was extrapolated from available safety information [15–17], such that we administered 2 mg of rtPA into the clot cavity every 12 h in the manner described above. Serial CT scans (Daily or on alternate days) were performed to assess the arbitrarily chosen desired radiographic endpoint for adjunctive thrombolysis, ≤10 cc of residual ICH volume. Treatment was discontinued if catheter fenestrations were no longer in continuity with the clot. Throughout the treatment protocol all patients underwent close neurological and ICP monitoring using standard external ventricular drainage (EVD) placed in the lateral ventricular system. The medical management of these patients followed the current recommendations formulated by the American Heart Association [18]. As is our usual practice, patients received daily coagulation parameter testing (e.g. prothrombin and partial thromboplastin times, platelet count). Following our usual practice, patients did not receive routine antibiotic prophylaxis.
Measurement of Intraparenchymal and Intraventricular Hematoma Volumes
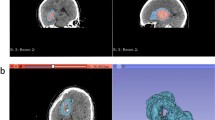
Using the software “ImageJ” for Macintosh (v 1.32e, Wayne Rasband, NIH, Bethesda, MD), volume measurements of blood clots and their perihematomal edema were performed according to their specific signal density on head CT slices (Hyperdensity for hematoma regions and hypodensity within white matter and in perihematomal distribution for brain edema). The distinct boundaries of the hematoma and perihematomal edema areas (Fig. 1) were traced by hand on each slice by one of the investigators (JRC). The cross-sectional area of each slice (square millimeters) was then multiplied by the slice thickness and added up to give the total hematoma and perihematomal edema volumes (cubic millimeters). We and other investigators have previously validated such an approach, with high inter- and intraobserver reliability in the measurements of ICH and perihematomal edema volumes using CT imaging [19, 20]. Single observer variability of less than 1.5% was previously reported by our group [19] when using this methodology in the study of IVH resolution.
All image analyses were performed on a MacBook Pro computer (Apple Computer, Cupertino, CA).
Estimating Rate of Resolution and Association of Perihematomal Edema Volume and Hematoma Volume
Daily percent hematoma and edema resolution rate, and association between perihematomal edema volume and hematoma volume were estimated using random effects generalized least squares regression. The time of each CT from the start of lytic therapy was calculated in days. Rates of resolution for the hematoma and perihematomal edema were carried out taking into account the volume at the start of lytic therapy. Before carrying out regressions to estimate the rate of hemorrhage (or edema) resolution over time, each patient’s data were first standardized into volume expressed as a percent of the patient’s hemorrhage (or edema) volume at the start of lytic therapy (time = 0). As the data are very sparse after the first 8 days (see Fig. 2) we estimated resolution using only data from the first 8 days following the start of therapy. Furthermore we excluded the two patients with hematoma enlargement. The data point for edema for patient 9 on day 5 violated the model’s assumptions about normally distributed residuals and was excluded. All statistical analyses were performed using STATA statistical software (STATA Corporation, College Station, TX) with tests being two-tailed. A P-value of less than 0.05 was considered to indicate statistical significance.
Results
Relevant demographic, clinical, and neuroradiologic characteristics of the study cohort are summarized in Table 1.
Demographic Features
Over 28 consecutive months, approximately 110 patients with diagnosis of ICH were screened. Fifteen patients were treated using the described protocol. Ten patients were male, five female, and their mean age was 60.7 (range 28–86; SD 15.2) years.
Clinical Features
Limited clinical information was available for review in one patient (Patient 7). All the 15 patients had premorbid hypertension. Hematoma location was thalamic (1 patient), lobar (1 patient), and basal ganglia (13 patients). Median GCS on admission was 10.5 (range: 4–15; SD: 3.6), 4 patients had a GCS ≤ 8 and 11 patients had a GCS > 8. Mean admission systolic blood pressure was 229.3 (range: 160–300; SD: 44.7) mmHg; mean diastolic blood pressure was 125.5 (range: 90–170; SD: 24) mmHg. One patient developed bacterial ventriculitis. The ICH drain remained in place 7.9 (range: 4–15; SD: 3.6) days. Patient 3 required repositioning of the ICH drain. There were two deaths (Patients 7 and 13). Care was not withdrawn or withheld in any of the 15 patients. Median GCS at the time of discharge was 11.0 (n = 14; range: 3–15; SD: 3.5). Besides two patients with enlargement of the ICH (Patients 7 and 14), no other clinical or laboratory-based evidence of CNS or systemic bleeding was reported. In patient 7, 20.15% hematoma enlargement was identified at day 3. No clinical information of this patient was available for further analysis. Patient 14 developed 16.97% hematoma enlargement on day 2 of the treatment with an associated GCS decline from 9 to 8. This hematoma enlargement peaked at 105.2% on day 4. Thrombolytic treatment was discontinued after day 2 in this patient.
Treatment
All 15 patients received rtPA for a mean of 4.8 (range: 2–10; SD: 2.3) days. Median time from ictus to clot aspiration and thrombolysis treatment was 1 (range: 0–3; SD: 0.92) day.
Neuroradiologic Features
Mean ICH volume at the start of rtPA lysis was 59.1 (range: 23.5–109; SD: 26.8) cc. Intraventricular hemorrhage was present in five patients, with mean IVH volume of 8.7 (range: 0–53.8; SD: 16.4) cc. No intraventricular thrombolysis was administered in this patient cohort. Radiological evidence of ICH enlargement occurred in patients 7 and 14 (13.3% of the entire cohort). Mean residual hematoma was 69.3% (range: 16–126.1; SD: 30) of the pretreatment hematoma volume at 24 h from the onset of lytic therapy and 17% (range: 1–57.5; SD: 16.2) at the end of the treatment. Two patients demonstrated radiological evidence of non-enlarging, peri-intraventricular catheter tract contusion/hematoma; no such instances were recorded near the intraclot drain. Using a random effects model that considered volume resolution over the first 8 days following lytic therapy we found that the both percentage hematoma and percentage perihematoma edema resolution per day were quadratic with respect to time. Percentage residual hematoma volume on day K = 97.7% − [24.36%*K] + [1.89%*K 2]; P < 0.001 for both terms. Percentage residual edema on day K = 97.4% − [13.94%*K] + [1.30%*K 2]; P < 0.001 for K and P = 0.01 for K 2. The relationship of hematoma and perihematomal edema volumes over the 10 days following the start of treatment is shown in Fig. 2 (by patient). To facilitate a comparison of the relative size of hematoma and perihematoma edema volumes for each patient over time all volumes are represented as percent of the patient’s hematoma volume at the start of lytic therapy. Figure 2 not only depicts the overall downward trend in both hematoma and perihematomal edema volumes that was expressed by the regression equations presented above, but suggests that there exists a strong direct relationship between perihematoma edema volume and same day hematoma volume, occurring even as hematoma volume increased and then decreased in the two patients with hematoma enlargement. Figure 3 shows the estimated hematoma clot resolution over time from the start of lytic therapy and its 95% confidence interval. These are estimated from the regression models described above.
Discussion
We report on the evolution of perihematomal brain edema in the 15 patients treated using FAST. We identified significant rate of hematoma (9.65% per day) and perihematomal edema resolution (4.1% per day) as well as direct association between daily perihematomal edema and hematoma volumes (P < 0.001) within the first 8 days following institution of FAST. The precise mechanism for such association is unclear, although “hemototoxicity” inhibition via removal of blood/blood degradation products remains a plausible hypothesis that needs to be further tested.
Intense investigative effort is currently invested in better understanding mechanisms of secondary neuronal injury following ICH. Perihematomal edema has emerged as an important surrogate of pathological processes within brain tissue surrounding the blood clot that are triggered by the initial insult. It also has the distinct potential to negatively modify the overall outcome of ICH victims, as suggested by Zazulia and coworkers [1]. These investigators reported on a “second wave” of neurological injury that threatens ICH patients within the second and third weeks after the initial ictus. In this report, perihematomal edema was identified as directly or indirectly linked to neurological deterioration in a significant number of ICH patients, likely due to elevated intracranial pressure and tissue shifts. In addition to the mechanical stress that edema inflicts on already challenged cranial vault dynamics, presence of vasogenic edema seems to also represent neuronal stress of different nature. Postulated processes triggered by blood and its degradation products vary from apoptosis to neuronal inflammation [2]. The long-term consequences of such processes on neurological function and recovery are poorly understood.
In view of the apparent lack of additional efficacy of surgical hematoma evacuation over best medical treatment in ICH patients, significant interest has developed in testing alternative therapies such as minimally invasive surgery to remove intracerebral hematomas [21, 22]. Different techniques include simple clot aspiration to more recently the application of fibrinolytic agents into the hematoma following the stereotactic placement of a soft catheter [15, 23–30]. Several small case series in the US have suggested the safety and efficacy of fibrinolytic agents such as urokinase, streptokinase, and recombinant tissue plasminogen activator (rtPA) adjunctively used to enhance hematoma drainage [10, 27, 31]. It is this lack of clinical experience in the use of this modality of treatment and the lack of randomized trial testing this high-risk therapy versus other more conventional/standard forms of therapy in ICH patients that makes FAST largely restricted to centers specialized in the advanced treatment of stroke patients. Although the natural history of intraparenchymal clot resolution is not yet reported, a daily clot resolution rate of 9.12% in our cohort of patients treated using FAST contrasts sharply against the observed spontaneous resolution seen in daily clinical practice. If such accelerated clot lysis modifies favorably survival, then functional neurological outcome and quality of life in these patients become important variables to be prospectively evaluated.
Reports on the effects of rtPA on neuronal tissue have shown conflicting results. In an experimental ischemic stroke paradigm, Wang and coworkers raised the concern that rtPA could promote neurotoxicity [32]. Using an ICH porcine model, Wagner and coworkers reported on the impact of clot removal using rtPA and on the associated reduction in perihematomal edema formation [3]. This positive interaction between clot removal and reduced edema formation seems to conform to the growing evidence assigning thrombin a neurotoxic role in hemorrhagic models of brain injury. Nevertheless, more recently, Thiex and coworkers have reported findings, also in a porcine model of experimental ICH, suggesting the lack of efficacy of clot removal in decreasing inflammation and perihematomal edema [33, 34]. Furthermore, Yepes and coworkers have recently suggested that rtPA opens the blood brain barrier in an experimental model of cerebral ischemia [9]. Although the exact nature of these conflicting results of the effect of parenchymal clot lysis on perihematoma edema is unclear, translational limitations from experimental paradigms of brain injury to the living human model remain a testable hypothesis. Similar to what Wagner and coworkers reported, in our cohort of 15 patients treated with FAST, we identified both, hematoma volume reduction (achieved by the administration of rtPA) and edema volume reduction, as well as a significant association between these volumes each day. If reproduced, these results could be indicative of the beneficial effects of early clot removal, not only on the physical stress that such a mass causes on the cranial compliance of ICH patients, but also on mitigating the secondary neuronal injury mediated by the exposure of viable brain tissue to thrombin and other blood degradation products. Also, prospective confirmation of our results will strengthen the available literature addressing the safety of thrombolytic therapy in paradigms of acute brain injury.
The limitations of the current study are several, however:
-
First, the retrospective and selected nature of the study, as well as small sample size hamper generalizations of these results. Future prospective studies in an open, unbiased population testing the impact of clot aspiration-lysis on neurological outcome of ICH patients should confirm this preliminary association between hematoma resolution and perihematomal edema formation.
-
Second, the methodology used to quantify volumes of interest relies on visual identification and free-hand definition of the boundaries of the intraparenchymal clot (hyperdensity) and perihematomal edema (hypodensity) following standard diagnostic CT criteria. Although the visual identification and discrimination of such structures is less prone to subjectivity in T2 weighted- and FLAIR MR imaging, such an approach using CT imaging has been previously tested and validated by Zimmerman and coworkers [20]. These investigators found good inter- and intraobserver correlation for the measurements of edema volume (intra- and interclass correlation coefficient of 0.73 and 0.72, respectively), when using visually defined, free-hand methodology, as we did. Nevertheless, prospective studies aiming to validate our findings should incorporate MR imaging to their methodology to better define the accuracy of the more globally available CT measures. Furthermore, our patient cohort underwent CT imaging daily or every-other-day, thus minimizing errors in the accurate sequential description of the volumes of interest in this study. Future clinical trial testing thrombolytic therapy in ICH patients should be incorporated in their design daily CT imaging to address this issue.
-
Third, other clinical variables with reported potential to modify edema formation, such as serum glucose and blood pressure elevation, were not prospectively recorded during the treatment phase of these patients. Thus, the analysis of such data and their association to evolution of perihematomal edema was not incorporated in this study. Such variables should carefully be evaluated in prospective studies using clot aspiration and lysis in the treatment of ICH, to properly account for the actual response of perihematomal edema to clot removal.
In summary, our investigation allows us to postulate that parenchymal clot treatment using FAST protocol for ICH patients can lead to clot volume resolution with an associated amelioration of perihematomal edema formation. If confirmed in a prospective manner, these observed effects of rtPA on perihematomal edema formation provide an added benefit to clot lysis acceleration induced by this novel form of therapy in ICH. Furthermore, our observations serve as clinical evidence favoring the concept of blood/blood product induced neurotoxicity suggesting that the evacuation of intraparenchymal blood could become mandatory using minimally invasive procedures, once prospectively reproduced.
References
Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ. Progression of mass effect after intracerebral hemorrhage. Stroke 1999;30:1167–73.
Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63.
Wagner KR, Xi G, Hua Y, Zuccarello M, de Courten-Myers GM, Broderick JP, Brott TG. Ultra-early clot aspiration after lysis with tissue plasminogen activator in a porcine model of intracerebral hemorrhage: edema reduction and blood–brain barrier protection. J Neurosurg. 1999;90:491–8.
Okuda M, Suzuki R, Moriya M, Fujimoto M, Chang CW, Fujimoto T. The effect of hematoma removal for reducing the development of brain edema in cases of putaminal hemorrhage. Acta Neurochir Suppl. 2006;96:74–7.
Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T. Recombinant activated factor vii for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–85.
Lapointe M, Haines S. Fibrinolytic therapy for intraventricular hemorrhage in adults. Cochrane Database Syst Rev. 2002;CD003692.
Figueroa BE, Keep RF, Betz AL, Hoff JT. Plasminogen activators potentiate thrombin-induced brain injury. Stroke 1998;29:1202–1207; discussion 1208.
Rohde V, Rohde I, Thiex R, Ince A, Jung A, Duckers G, Groschel K, Rottger C, Kuker W, Muller HD, Gilsbach JM. Fibrinolysis therapy achieved with tissue plasminogen activator and aspiration of the liquefied clot after experimental intracerebral hemorrhage: rapid reduction in hematoma volume but intensification of delayed edema formation. J Neurosurg. 2002;97:954–62.
Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood–brain barrier via the ldl receptor-related protein. J Clin Invest. 2003;112:1533–40.
Barrett RJ, Hussain R, Coplin WM, Berry S, Keyl PM, Hanley DF, Johnson RR, Carhuapoma JR. Frameless stereotactic aspiration and thrombolysis of spontaneous intracerebral hemorrhage. Neurocrit Care. 2005;3:237–45.
Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, Karimi A, Shaw MD, Barer DH. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (stich): a randomised trial. Lancet 2005;365:387–97.
Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27:617–21.
Tuhrim S, Dambrosia JM, Price TR, Mohr JP, Wolf PA, Hier DB, Kase CS. Intracerebral hemorrhage: external validation and extension of a model for prediction of 30-day survival. Ann Neurol. 1991;29:658–63.
Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 1993;24:987–93.
Schaller C, Rohde V, Meyer B, Hassler W. Stereotactic puncture and lysis of spontaneous intracerebral hemorrhage using recombinant tissue-plasminogen activator. Neurosurgery 1995;36:328–33; discussion 333–325.
Deinsberger W, Lang C, Hornig C, Boeker DK. Stereotactic aspiration and fibrinolysis of spontaneous supratentorial intracerebral hematomas versus conservative treatment: a matched-pair study. Zentralbl Neurochir. 2003;64:145–50.
Naff NJ, Carhuapoma JR, Williams MA, Bhardwaj A, Ulatowski JA, Bederson J, Bullock R, Schmutzhard E, Pfausler B, Keyl PM, Tuhrim S, Hanley DF. Treatment of intraventricular hemorrhage with urokinase: effects on 30-day survival. Stroke 2000;31:841–7.
Broderick JP, Adams HP Jr., Barsan W, Feinberg W, Feldmann E, Grotta J, Kase C, Krieger D, Mayberg M, Tilley B, Zabramski JM, Zuccarello M. Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the stroke council, American heart association. Stroke 1999;30:905–15.
Naff NJ, Williams MA, Rigamonti D, Keyl PM, Hanley DF. Blood clot resolution in human cerebrospinal fluid: evidence of first-order kinetics. Neurosurgery 2001;49:614–9; discussion 619–621.
Zimmerman RD, Maldjian JA, Brun NC, Horvath B, Skolnick BE. Radiologic estimation of hematoma volume in intracerebral hemorrhage trial by ct scan. AJNR Am J Neuroradiol. 2006;27:666–70.
Zuccarello M, Andaluz N, Wagner KR. Minimally invasive therapy for intracerebral hematomas. Neurosurg Clin N Am. 2002;13:349–54.
Zuccarello M, Andrioli GG, Trincia G, Pardatscher K. Spontaneous intracerebral haematomas. Aspects of treatment. Zentralbl Neurochir. 1983;44:209–13.
Lippitz BE, Mayfrank L, Spetzger U, Warnke JP, Bertalanffy H, Gilsbach JM. Lysis of basal ganglia haematoma with recombinant tissue plasminogen activator (rtpa) after stereotactic aspiration: initial results. Acta Neurochir (Wien) 1994;127:157–60.
Matsumoto K, Hondo H. Ct-guided stereotaxic evacuation of hypertensive intracerebral hematomas. J Neurosurg. 1984;61:440–8.
Miller DW, Barnett GH, Kormos DW, Steiner CP. Stereotactically guided thrombolysis of deep cerebral hemorrhage: preliminary results. Cleve Clin J Med. 1993;60:321–4.
Mohadjer M, Braus DF, Myers A, Scheremet R, Krauss JK. Ct-stereotactic fibrinolysis of spontaneous intracerebral hematomas. Neurosurg Rev. 1992;15:105–10.
Montes JM, Wong JH, Fayad PB, Awad IA. Stereotactic computed tomographic-guided aspiration and thrombolysis of intracerebral hematoma: protocol and preliminary experience. Stroke 2000;31:834–40.
Niizuma H, Otsuki T, Johkura H, Nakazato N, Suzuki J. Ct-guided stereotactic aspiration of intracerebral hematoma—result of a hematoma-lysis method using urokinase. Appl Neurophysiol. 1985;48:427–30.
Rohde V, Rohde I, Reinges MH, Mayfrank L, Gilsbach JM. Frameless stereotactically guided catheter placement and fibrinolytic therapy for spontaneous intracerebral hematomas: technical aspects and initial clinical results. Minim Invasive Neurosurg. 2000;43:9–17.
Tzaan WC, Lee ST, Lui TN. Combined use of stereotactic aspiration and intracerebral streptokinase infusion in the surgical treatment of hypertensive intracerebral hemorrhage. J Formos Med Assoc. 1997;96:962–7.
Vespa P, McArthur D, Miller C, O’Phelan K, Frazee J, Kidwell C, Saver J, Starkman S, Martin N. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocrit Care 2005;2:274–81.
Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tpa) increases neuronal damage after focal cerebral ischemia in wild-type and tpa-deficient mice. Nat Med. 1998;4:228–31.
Thiex R, Kuker W, Jungbluth P, Kayser C, Muller HD, Rohde I, Gilsbach JM, Rohde V. Minor inflammation after surgical evacuation compared with fibrinolytic therapy of experimental intracerebral hemorrhages. Neurol Res. 2005;27:493–8.
Thiex R, Kuker W, Muller HD, Rohde I, Schroder JM, Gilsbach JM, Rohde V. The long-term effect of recombinant tissue-plasminogen-activator (rt-pa) on edema formation in a large-animal model of intracerebral hemorrhage. Neurol Res. 2003;25:254–62.
Acknowledgment
Dr. Daniel F. Hanley is partially supported by NIH NINDS 1R01 NS046309-01.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carhuapoma, J.R., Barrett, R.J., Keyl, P.M. et al. Stereotactic Aspiration-Thrombolysis of Intracerebral Hemorrhage and its Impact on Perihematoma Brain Edema. Neurocrit Care 8, 322–329 (2008). https://doi.org/10.1007/s12028-008-9074-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-008-9074-y