Abstract
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with multi-organ inflammation, linked to loss of immune tolerance to self-antigens and the production of a diversity of autoantibodies, with a negative impact on the patients’ quality of life. Regulatory T cells have been reported as deficient in number and function in SLE patients. However, some authors also described an enrichment of this cell type. The hypothesis that certain forms of autoimmunity may result from a conversion of Treg cells into a Th17 cell phenotype has been suggested by some studies. In fact, in SLE patients’ sera, the IL-17 levels were observed as abnormally high when compared with healthy individuals. Environmental factors, such as vitamin D, that is considered a potential anti-inflammatory agent, combined with genetic and hormonal characteristics have been associated with SLE phenotype and with disease progression. The aim of this study was to evaluate the effect of vitamin D supplementation on FoxP3 expression and IL-17A-producing T cells, through FoxP3+/IL-17A ratio. Additionally, disease evolution, serum vitamin D levels, serum autoantibodies levels and calcium metabolism (to assure safety) were also studied. We assessed 24 phenotypically well-characterized SLE patients. All patients were screened before vitamin D supplementation and 3 and 6 months after the beginning of this treatment. Peripheral blood lymphocyte’s subsets were analysed by flow cytometry. Serum 25(OH)D levels significantly increased under vitamin D supplementation (p = 0.001). The FoxP3+/IL-17A ratio in SLE patients after 6 months of vitamin D supplementation was higher than that in the baseline (p < 0.001). In conclusion, this study demonstrated that vitamin D supplementation provided favourable, immunological and clinical impact on SLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE or lupus) is a systemic autoimmune disease with multi-organ inflammation [1, 2], linked to loss of immune tolerance to self-antigens and the production of a diversity of autoantibodies. It mainly affects women of childbearing age. This disease has a female-to-male ratio in adults of approximately 9:1, a peak age of diagnosis between 15 and 44 years and a known negative impact on the quality of life, including reduced levels of employment and income. Despite phenotypic heterogeneity and an unpredictable disease evolution, a strong genetic and environmental contribution to the development of SLE is supported by broad evidence. The autoantibodies are primarily directed against chromatin and ribonuclear particle constituents [nucleosomes, single- and double-stranded DNA (dsDNA), RNPs] [2–5] and play a pathogenic role [3]. The hyperreactivity to these self-antigens leads to the formation of immune complexes that cause local inflammation and tissue damage [6].
With the production of autoantibodies and prolonged cell life, B cell regulation is important in the maintenance of immune balance. B cells from patients with SLE have been shown to present autoantigens, induce CD4+ T helper cells (Th1/Th2), inhibit regulatory T cells (Tregs) and secrete pro-inflammatory cytokines [7].
Regulatory T cells are specialized suppressor cells [5, 8], with the phenotype CD4+FoxP3+CD25highCD127low [9], that have the capacity to regulate the intensity and quality of the immune response [10]. They actively suppress effector cells, including those associated with autoimmune diseases [4], thereby establishing and maintaining immunological self-tolerance [11].
Some studies suggest that regulatory T cells are deficient in number and function in several autoimmune diseases including SLE [12]. However, an enrichment of this cell type has also been reported [13, 14]. These contradictory observations may be either due to the lack of a well-defined specific Treg marker in humans or due to the heterogeneity of SLE phenotypes [12]. Concerning Tregs markers, this population was initially characterized by CD25 alone, which could explain the reported contradictory results. Dysfunction of these cells could also be explained by the fact that FoxP3-expressing cells lose CD25 expression and consequently their suppressive functionality [15, 16].
Besides Th1 and Th2, a third subset of CD4+ effector Th cells was identified, named Th17 because of its unique ability to produce IL-17 (IL-17A and IL-17F). These cells play a critical role in the recruitment, activation and migration of neutrophils [4]. Beyond their protective role in the clearance of extracellular pathogens, a major role of Th17 lymphocytes seems to be their involvement in the induction and maintenance of chronic inflammatory processes [4, 17].
The hypothesis that certain forms of autoimmunity may result from a conversion of Treg cells into a Th17 cell phenotype has been suggested by some studies [18].
In SLE patients’ sera, the IL-17 levels are abnormally high when compared with healthy individuals. IL-17 is also produced by neutrophils, innate lymphoid cells (ILCs) and other T cell types including CD4+, CD8+, double-negative (CD4−CD8−) and TCR-γδ [4]. This phenomenon could promote the autoimmune process by increasing the activation of immune cells itself. Release of IL-17 by infiltrating T cells in specific organs may also contribute to local tissue injury by instigating the inflammatory response [4] (Table 1).
Environmental factors combined with genetic and hormonal characteristics have been associated with SLE phenotype and with disease progression [19, 20].
Vitamin D is one of the environmental factors likely related to SLE pathogenesis, and its deficiency appears to be associated with immunomodulatory abnormalities in this disease [21].
The identification of vitamin D receptors in immune system cells and the discovery that dendritic cells can produce the metabolically active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3; calcitriol) have led to the suggestion that vitamin D is an immune modulator [1]. The importance of vitamin D in several autoimmune disorders has been reported, and vitamin D deficiency has been associated with the pathogenesis and severity of multiple sclerosis (MS), rheumatoid arthritis (RA), systemic sclerosis (SSc) and SLE, among others [22]. In SLE patients, serum vitamin D3 levels seem to correlate inversely with SLEDAI scores [6].
It has been found that one of the consequences of 1,25(OH)2D3 on the immune response is the stimulation of innate immunity and suppression of adaptive immunity [23]. Studies on the immunomodulatory properties of 1,25(OH)2D3 confirmed the inhibition of Th1 cell development via an inhibition of IL-12 production by antigen-presenting cells. Further work documented its ability to drive CD4 T lymphocytes to a Th2 phenotype with a reduction in Th1-type activity [17], by increasing the production of IL-5 and IL-10. Vitamin D indirectly reduces the production of IFN-γ [24]. It also affects B cells causing induction of B cell apoptosis, inhibition of B cell proliferation, and generation of memory B cells, plasma cell differentiation and immunoglobulin production/secretion [24–27]. In human epidermal and dermal cells, it was demonstrated that 1,25(OH)2D3 modulates regulatory T cell numbers and their suppressive abilities through dendritic cells [23]. The effect of vitamin D on dendritic cells includes the differentiation of monocytes into immature dendritic cells, the maturation of dendritic cells and dendritic cell survival [26].
Local vitamin D metabolism allows immune cells to modulate immune responses in an independent way when regulation is required, but the optimization of this autocrine and/or paracrine circuit is strictly dependent on the circulating 25(OH)D (the calcitriol precursor) availability. The levels of circulating 25(OH)D needed to meet the requirements of vitamin D sufficiency are still a matter of debate, especially in the light of the non-classical effects of vitamin D [27]. These evidences justify the motivation to consider vitamin D supplementation as an immunomodulatory intervention in SLE.
The FoxP3 gene has a vitamin D receptor element (VDRE) in its promoter region, being important for its cellular expression [28]. The immunomodulatory effect assessed by the imbalance of FoxP3+/IL-17A CD4+ T lymphocytes is widely recognized [29]. However, it is not known if this immune imbalance can be represented by a ratio between these two cell populations. This ratio can be calculated by dividing the percentage of total lymphocytes that are CD4+ and express FoxP3 by the percentage of total lymphocytes that are CD4+ and synthesize IL-17A.
In this study, using a well phenotypically characterized patients’ cohort and a well-defined therapeutic intervention, the authors investigate whether the FoxP3+/IL-17A ratio and disease activity is modified after vitamin D supplementation (cholecalciferol). For this purpose, we evaluated the effect of vitamin D supplementation on CD4+ FoxP3 expression and CD4+ IL-17A-producing T cells. Additionally, disease evolution, serum vitamin D levels, serum autoantibodies levels and calcium metabolism (to assure safety) were studied.
Therefore, whether the FoxP3+/IL-17A ratio has a positive effect on SLE disease activity is the question addressed in this study.
Materials and methods
Subjects
The study population consisted of SLE patients recruited between 1 November 2012 and 31 January 2013. SLE patients (diagnosed according to the 1997 [30] and 2012 [31] revised ACR criteria for SLE) were selected from the outpatient clinic database of the Clinical Immunology Unit from Centro Hospitalar do Porto (North of Portugal). By protocol all these patients should have more than 5 years of disease and should be in a stable phase of disease and without major flares for at least 1 year. Stability was defined as no new major organ involvement in the last year independently of the SLEDAI-2K score and no changes in steroid dose or immunosuppressive therapy in the last 12 months. Baseline 25(OH)D serum level was measured. Hypovitaminosis D was defined as serum 25(OH)D <75 nmol/L, and patients were selected irrespective of their baseline 25(OH)D levels.
Ethical approval was obtained from the research ethics committee of Centro Hospitalar do Porto, and written informed consent for all analysis was obtained from all subjects.
Study design
A prospective cross-sectional study with 6-month follow-up evaluations of patients with a dose-escalating protocol of vitamin D supplementation was done. Safety of high-dose vitamin D supplementation was also monitored (increase of serum phosphorus or calcium).
We assessed 24 SLE patients for eligibility (1 man and 23 women). The clinical characterization of SLE patients comprised: (1) evaluation of the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) [32]; (2) evaluation of the Systemic Lupus International Collaborating Clinics/American College Rheumatology (ACR) Damage Index (SLICC-SDI) [33]; (3) flare evaluation according to modified SLE Flare Index (SFI) [34–36]; (4) cumulative organ involvement (specific data and treatment); (5) previous and present immunosuppressive therapy and hydroxychloroquine.
All patients were screened before vitamin D supplementation (day 0 or D0) and 3 and 6 months (M3 and M6) after the beginning of this treatment.
At D0, before vitamin D supplementation, relevant data were compiled, namely: baseline SLEDAI-2K, baseline SLICC-SDI, disease duration, concomitant therapy (including steroid doses), previous SLE manifestations (including cumulative organ involvement and number of disease flares), baseline 25(OH)D serum levels, baseline %TCD4+FoxP3+/%TCD4+IL-17A+ ratio (FoxP3+/IL-17A ratio), baseline autoantibodies levels, complement (C3 and C4) as required for SLEDAI-2K, serum calcium, serum phosphorus and serum PTH (parathyroid hormone) levels.
At M3: SLEDAI-2K, concomitant therapy (including variations on steroid doses), flare evaluation, 25(OH)D serum levels, autoantibodies levels and complement levels (C3 and C4) as required for SLEDAI-2K, serum calcium, serum phosphorus and serum PTH levels.
At M6: SLEDAI-2K, SLICC, concomitant therapy (including variations on steroid doses), flare evaluation, 25(OH)D serum levels, %TCD4+FoxP3+/%TCD4+IL-17A+ ratio (FoxP3+/IL-17A ratio), serum autoantibodies levels, complement (C3 and C4) as required for SLEDAI-2K, serum calcium, serum phosphorus and serum PTH levels.
Supplementation protocol
Baseline (day 0)
-
Vitamin D < 50 nmol/l—50,000 UI cholecalciferol/week/8 weeks, then 2000 U/day.
-
Vitamin D > 75 nmol/L—2000 UI/day.
-
Vitamin D > 50 nmol/L and <75 nmol/L—4000 UI/day/8 weeks, then 2000 UI/day.
3-month follow-up (M3)
-
<50 nmol/L—as the baseline.
-
>50 nmol/L and <75 nmol/L—duplicates the baseline dose per 8 weeks, then 4000/day.
-
>75 nmol/L and <125 nmol/L—same dose.
-
>125 nmol/L—50 % reduction.
Vitamin D assessment
25(OH)D protocol: vitamin D total assay for the Elecsys analysers and Cobas modular platforms—Roche®.
Flow cytometry
Peripheral blood lymphocyte’s subsets including T, B, NK, TCD4+FoxP3+, TCD4+IL-17A+ were analysed by flow cytometry, in a Coulter Epics XL-MCL® cytometer. Cells counts (cells/μL) and proportions (%) were established from fresh blood samples using different protocols and monoclonal antibodies (mAbs), conjugated to fluorescein (FITC), phycoerythrin (PE), phycoerythrin-Texas Red (ECD) and phycoerythrin-cyanin 5.1 (PC5).
T (CD3+/CD4+ and CD3+/CD8+), B (CD19+) and NK (CD56+) lymphocytes were analysed using a Beckman Coulter standard protocol with the following mAbs: anti-CD45 FITC (clone B3821F4A), anti-CD3 PC5 (clone UCHT1), anti-CD4 RD1 (clone SFCI12T4D11), anti-CD8 ECD (clone SFCI21Thy2D3), anti-CD19 ECD (clone J4.119) and anti-CD56 FITC (clone N901/NKH-1), all from Beckman Coulter, Fullerton, California, USA.
Effector CD4+ T cells producing IL-17 were quantified after 4-h stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomicin in the presence of brefeldin A. Cells were fixed and permeabilized using the IntraPrep Permeabilization Reagent (Beckman Coulter) buffer system and stained with anti-CD4 PC5 (clone 13B8.2; Immunotech, Marseille, France) and anti-IL-17 PE (clone eBio64DEC17; eBioscience Inc, San Diego, CA).
CD4+FoxP3+ T cells were quantified after cell fixation and permeabilization using the mAbs anti-CD4 PC5 (clone 13B8.2; Beckman Coulter IOTest; Marseille, France) and anti-FoxP3 PE (clone PCH10; eBioscience Inc, San Diego, CA), according to the manufacturer’s staining protocol.
Statistical analysis
We compared measures taken at baseline (before vitamin D supplementation, D0) with those taken at M6, using the nonparametric paired Wilcoxon signed-rank test, because the data did not follow a Gaussian distribution. Significance level was set at α = 0.05. Statistical analysis was performed using the SPSS v.22.
Results
Demographic, clinical and laboratorial features of SLE patients at baseline are described in serum 25(OH)D levels significantly increased under vitamin D supplementation from 59.32 ± 29.59 nmol/L at day 0 to 80.39 ± 24.57 nmol/L at month 3 (p = 0.030) and to 85.25 ± 30.92 nmol/L at month 6 (p = 0.001) (Fig. 1).
Treatment was safe, with no significant increase of serum phosphorus or calcium. Serum calcium significantly decreased from 2.34 ± 0.10 mmol/L at day 0 to 2.27 ± 0.10 mmol/L at month 6 (p = 0.026) but all in the normal range (Fig. 2).
Disease activity, assessed by SLEDAI-2K, significantly decreased from 2.75 ± 4.76 at day 0 to 1.67 ± 2.79 at month 6 (p = 0.026) (Fig. 3).
Serum anti-dsDNA levels (evaluated by immunofluorescence) remained stable during follow-up, while C3 complement fraction significantly decreased from 101.5 ± 23.57 mg/dL at day 0 to 95.46 ± 21.71 mg/dL at month 6 (p = 0.013) (Fig. 4), but no new hypocomplementemia occurred (reference values 88–201 mg/dL).
None of the patients required modification of the prednisone and immunosuppressive dosage or initiation of new immunosuppressive agents. We did not observe SLE flares during the 6-month follow-up period.
The impact of vitamin D supplementation on the proportions of CD3+ T cells, CD4+ and CD8+ T cells is shown in Fig. 5.
The mean proportions at baseline were 80.05 ± 5.64 % for CD3+ T cells (Fig. 5a), 47.8 ± 7.58 % for CD4+ T cells (Fig. 5b) and 30.56 ± 7.73 % for CD8+ T cells (Fig. 5c). At month 6, the proportion of CD3+ T cells, CD4+ and CD8+ T cells remained stable.
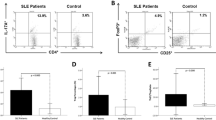
The impact of vitamin D supplementation on CD3+CD4+FoxP3+ T cells was evaluated. The percentage of CD4+FoxP3+ cells at baseline was 7.27 ± 5.92 %. The percentage of CD4+FoxP3+ cells was increased by 11.42 ± 6.54 % at month 6 (p < 0.001) (Fig. 6a). A decrease was observed in CD4+IL-17A from 3.89 ± 1.76 % at day 0 to 2.82 ± 1.03 % at month 6 (p = 0.001) after vitamin D supplementation (Fig. 6b).
Accordingly, the FoxP3+/IL-17A ratio in patients with SLE after 6 months of vitamin D supplementation was higher than that in the baseline (p < 0.001; Fig. 7).
Considering patients with 25(OH)D levels at baseline ≥75 nmol/L, the increase of FoxP3+/IL-17A ratio observed for the entire group after 6 months of vitamin D supplementation was also valid (p = 0.043; Fig. 8).
The relationship between CD4+FoxP3+ and CD4+IL-17A cells and 25(OH)D levels, serum calcium, serum phosphorus and serum PTH levels was also analysed. No associations were found between these features and CD4+FoxP3+ and CD4+IL-17A proportions.
No significant differences were observed for the other laboratory and clinical parameters, such as SLICC score.
Discussion
In this study, we assessed for the first time the safety, the clinical and immunological effects of vitamin D supplementation in patients with SLE in a Portuguese population.
Vitamin D deficiency is now recognized as a pandemic [37], and strong evidence exists that vitamin D deficiency can contribute to a raised risk for autoimmune diseases [38–41]. The definition of vitamin D insufficiency (25–50 nmol/L) and deficiency (<25 nmol/L) varies between studies, and the degree of hypovitaminosis D varies from region to region. While 25(OH)D levels below 75 nmol/L are common worldwide, levels below 25 nmol/L that constitute frank vitamin D deficiency are most commonly seen in groups at risk, in particular, with autoimmune diseases [42] such as MS [43], RA, type 1 diabetes mellitus and SLE [6, 44].
In SLE, some studies have demonstrated an association between higher disease activity and low serum levels of vitamin D, but the results are controversial. Nevertheless, vitamin D supplementation is currently recommended as a treatment for some of these patients. However, data about the effects of its administration in immune regulation in SLE are still missing [45].
To date, only one study has assessed the in vivo benefit of vitamin D supplementation in SLE, but only in vitamin D deficient patients [46]. In our study, we included patients with 25(OH) vitamin D > 75 nmol/L.
We confirmed the high frequency of hypovitaminosis D in SLE patients (70.8 %) described in some studies, and we also demonstrated that vitamin D supplementation significantly increases serum 25(OH)D levels, at 3 and 6 months after the beginning of the study. Furthermore, we could show that this therapy was safe, since no alterations on phosphorus or calcium levels and no side effects were reported.
It is well known that T cells initiate and sustain the secretion of antibodies by B cells. The high degree of hypermutation in SLE-associated autoantibodies demonstrates the T cell dependency of autoantibodies development in these patients. SLE is also associated with pathologically altered immune responses, with hyperactive B cells playing an important role in its pathogenesis. In this context, SLE is a T and B cell-dependent disease, associated with rather functional deficiency of regulatory T cells [47], an increase in T helper lymphocytes producing IL-17 (Th17 cells) [48, 49] and an increased expression of IFN-inducible genes [50]. The immunomodulatory properties of vitamin D have been under increased scrutiny in the last years. 1,25(OH)2D3 was shown to inhibit Th17 responses, probably owing to its capacity to inhibit IL-23 production [46], and to induce the differentiation and/or expansion of FoxP3+ Tregs and an increased expression of CTLA-4 [46].
In our study, after 6 months of vitamin D supplementation, we observed an increase of FoxP3 expression in CD4+ T cells and a decrease in CD4+IL-17A. The FoxP3+/IL-17A ratio was found to be significantly higher at the end of the treatment when compared with D0. This effect was also observed in patients with elevated vitamin D levels at baseline, showing that immunological effects were unrelated to the standard cut-off used for metabolic bone disease. It is well known that the imbalance between Th17 and Treg cells results in inflammation and/or autoimmunity. The shared requirement of cytokines in iTreg and Th17 cell differentiation naturally leads to the hypothesis that an imbalance between these two cell types may lead to tissue inflammation. While Th17 cells promote inflammation and autoimmunity, Treg cells modulate the function of effector T cells, preventing this phenomenon. A reestablishment of this balance may be achieved by decreasing Th17 function and differentiation and by the increase of Treg cells number and function. This hypothesis offers numerous potential pharmacologic targets for immunomodulation. The inflammatory imbalance might be modified to restore immune homoeostasis, resulting in therapeutic benefit. An intriguing alternative approach involves pharmacologically altering iTreg and Th17 cell differentiation or expansion, using cytokines, cytokine inhibitors and small molecule inhibitors of key signalling pathways [18].
Our results suggested that vitamin D supplementation improves the Treg/Th17 ratio, an effect described for the first time in SLE patients, of real benefit, as shown by the effective decrease of the SLEDAI scores. Vitamin D supplementation was shown to be a safe and an efficient treatment for improving stable SLE patients’ condition and flare prevention. Since none of the patients exhibit complications resulting from vitamin D supplementation, perhaps the administered doses could be increased, maintaining the safety and leading to even better results.
In spite of displaying optimal circulating vitamin D levels, the ability to metabolize vitamin D may vary between individuals’ genetics and may therefore contribute to the risk of developing immune abnormalities. These situations are illustrated by the presence of certain gene polymorphisms in the vitamin D metabolizing enzymes [27], such as CYP2R1 (hydroxylates vitamin D3 to 25(OH)D in the liver) or CYP27B1 (activated by PTH and hydroxylates 25(OH)D to 1,25(OH)2D3 in the kidney) [51]. Even if all these steps are properly functioning, the vitamin D active metabolite (1,25(OH)2D3) must be recognized, bound and activated by its receptor (VDR). Polymorphisms in the VDR gene may also play a role in this mechanism [1].
The main limitation of this study is the lack of an adequate Portuguese control population with known vitamin D levels.
In conclusion, this study demonstrated that vitamin D supplementation seems to provide favourable immunological effects in patients with SLE, independently of the 25(OH)D patients’ status. We observed a decrease of IL-17A-producing T cells and an increase in FoxP3 expression, confirming the relationship between vitamin D status and immunological balance. Nevertheless, these results should be interpreted with caution, since previous studies evaluating the supplementation of 25(OH)D in SLE disease have had inconsistent results.
It is important to consider all the existing variables and their specific outcomes in each patient. Therapy should be individualized to take into account all the existing factors and differences between patients. Using the FoxP3+/IL-17A ratio, it may be possible to tailor vitamin D therapy for each patient. An individualized therapy should be undertaken, since some patients will need very high doses of supplementation whereas others will need only modest doses to achieve the same outcome.
Finally, concerning vitamin D therapy safety, screening to monitor PTH levels will be only required if hypocalcaemia occurs.
References
Carvalho C, Marinho A, Leal B, Bettencourt A, Boleixa D, Almeida I et al. Association between vitamin D receptor (VDR) gene polymorphisms and systemic lupus erythematosus in Portuguese patients. Lupus. 2015;24:846–53.
Lauwerys BR, Hachulla E, Spertini F, Lazaro E, Jorgensen C, Mariette X, et al. Down-regulation of interferon signature in systemic lupus erythematosus patients by active immunization with interferon alpha-kinoid. Arthritis Rheum. 2013;65:447–56.
Lauwerys BR, Ducreux J, Houssiau FA. Type I interferon blockade in systemic lupus erythematosus: where do we stand? Rheumatology. 2014;53:1369–76.
Jiang SE. TH17 cells in health and disease. 1st ed. New York: Springer; 2011.
Lahita RG, Tsokos GC, Buyon JP, Koike T. Systemic lupus erythematosus. 5th ed. Cambridge: Academic Press, Elsevier Inc; 2011.
Mandal M, Tripathy R, Panda AK, Pattanaik SS, Dakua S, Pradhan AK, et al. Vitamin D levels in Indian systemic lupus erythematosus patients: association with disease activity index and interferon alpha. Arthritis Res Ther. 2014;16:R49.
Guerra SG, Vyse TJ, Graham DSC. The genetics of lupus: a functional perspective. Arthritis Res Ther. 2012;14:211.
Mocanu V, Oboroceanu T, Zugun-Eloae F. Current status in vitamin D and regulatory T cells—immunological implications. Rev Med Chir Soc Med Nat Iasi. 2013;117:965–73.
Del Pozo-Balado MM, Leal M, Mendez-Lagares G, Pacheco YM. CD4(+)CD25(+/hi)CD127(lo) phenotype does not accurately identify regulatory T cells in all populations of HIV-infected persons. J Infect Dis. 2010;201:331–5.
Smolders J, Thewissen M, Peelen E, Menheere P, Tervaert JW, Damoiseaux J, et al. Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS One. 2009;4:e6635.
Daniel C, von Boehmer H. Extrathymic generation of regulatory T cells—chances and challenges for prevention of autoimmune disease. Adv Immunol. 2011;112:177–213.
Sawla P, Hossain A, Hahn BH, Singh RP. Regulatory T cells in systemic lupus erythematosus (SLE); role of peptide tolerance. Autoimmun Rev. 2012;11:611–4.
Lin SC, Chen KH, Lin CH, Kuo CC, Ling QD, Chan CH. The quantitative analysis of peripheral blood FOXP3-expressing T cells in systemic lupus erythematosus and rheumatoid arthritis patients. Eur J Clin Invest. 2007;37:987–96.
Suarez A, Lopez P, Gomez J, Gutierrez C. Enrichment of CD4+ CD25 high T cell population in patients with systemic lupus erythematosus treated with glucocorticoids. Ann Rheum Dis. 2006;65:1512–7.
Bonelli M, Savitskaya A, Steiner CW, Rath E, Smolen JS, Scheinecker C. Phenotypic and functional analysis of CD4+ CD25− Foxp3+ T cells in patients with systemic lupus erythematosus. J Immunol. 2009;182:1689–95.
Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+ CD25 high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–88.
Bansal AS, Henriquez F, Sumar N, Patel S. T helper cell subsets in arthritis and the benefits of immunomodulation by 1,25(OH)(2) vitamin D. Rheumatol Int. 2012;32:845–52.
Eisenstein EM, Williams CB. The T(reg)/Th17 cell balance: a new paradigm for autoimmunity. Pediatr Res. 2009;65:26R–31R.
Choi J, Kim ST, Craft J. The pathogenesis of systemic lupus erythematosus—an update. Curr Opin Immunol. 2012;24:651–7.
Kunz M. Lupus erythematosus. Part I: epidemiology, genetics and immunology. JDDG. 2013;11:709–19.
Schneider L, Dos SA, Santos M, da Silva Chakr RM, Monticielo OA. Vitamin D and systemic lupus erythematosus: state of the art. Clin Rheumatol. 2014;33:1033–8.
Cutolo M. Vitamin D and autoimmune rheumatic diseases. Rheumatology. 2009;48:210–2.
Hart PH, Gorman S. Exposure to UV wavelengths in sunlight suppresses immunity. To what extent is UV-induced Vitamin D3 the mediator responsible? Clin Biochem Rev. 2013;34:3–13.
Guillot X, Semerano L, Saidenberg-Kermanac’h N, Falgarone G, Boissier MC. Vitamin D and inflammation. Joint Bone Spine Rev Rhum. 2010;77:552–7.
Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev. 2012;12:127–36.
Kamen D, Aranow C. Vitamin D in systemic lupus erythematosus. Curr Opin Rheumatol. 2008;20:532–7.
Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–96.
Kang SW, Kim SH, Lee N, Lee WW, Hwang KA, Shin MS, et al. 1,25-Dihyroxyvitamin D3 promotes FOXP3 expression via binding to vitamin D response elements in its conserved noncoding sequence region. J Immunol. 2012;188:5276–82.
Astry B, Venkatesha SH, Moudgil KD. Involvement of the IL-23/IL-17 axis and the Th17/Treg balance in the pathogenesis and control of autoimmune arthritis. Cytokine. 2015;74:54–61.
Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–86.
Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–91.
Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9.
Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:953–62.
Petri M, Buyon J, Kim M. Classification and definition of major flares in SLE clinical trials. Lupus. 1999;8:685–91.
Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353:2550–8.
Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S.
Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66:1137–42.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81.
Kriegel MA, Manson JE, Costenbader KH. Does vitamin D affect risk of developing autoimmune disease? a systematic review. Semin Arthritis Rheum. 2011;40(512–31):e8.
Shoenfeld N, Amital H, Shoenfeld Y. The effect of melanism and vitamin D synthesis on the incidence of autoimmune disease. Nat Clin Pract Rheumatol. 2009;5:99–105.
Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807–20.
Royal W 3rd, Mia Y, Li H, Naunton K. Peripheral blood regulatory T cell measurements correlate with serum vitamin D levels in patients with multiple sclerosis. J Neuroimmunol. 2009;213:135–41.
Ben-Zvi I, Aranow C, Mackay M, Stanevsky A, Kamen DL, Marinescu LM, et al. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS One. 2010;5:e9193.
Wahono CS, Rusmini H, Soelistyoningsih D, Hakim R, Handono K, Endharti AT, et al. Effects of 1,25(OH)2D3 in immune response regulation of systemic lupus erithematosus (SLE) patient with hypovitamin D. Int J Clin Exp Med. 2014;7:22–31.
Terrier B, Derian N, Schoindre Y, Chaara W, Geri G, Zahr N, et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res Ther. 2012;14:R221.
Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175:8392–400.
Crispin JC, Tsokos GC. Interleukin-17-producing T cells in lupus. Curr Opin Rheumatol. 2010;22:499–503.
Dolff S, Quandt D, Wilde B, Feldkamp T, Hua F, Cai X, et al. Increased expression of costimulatory markers CD134 and CD80 on interleukin-17 producing T cells in patients with systemic lupus erythematosus. Arthritis Res Ther. 2010;12:R150.
Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–23.
Reimers LL, Crew KD, Bradshaw PT, Santella RM, Steck SE, Sirosh I, et al. Vitamin D-related gene polymorphisms, plasma 25-hydroxyvitamin D, and breast cancer risk. CCC. 2015;26:187–203.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marinho, A., Carvalho, C., Boleixa, D. et al. Vitamin D supplementation effects on FoxP3 expression in T cells and FoxP3+/IL-17A ratio and clinical course in systemic lupus erythematosus patients: a study in a Portuguese cohort. Immunol Res 65, 197–206 (2017). https://doi.org/10.1007/s12026-016-8829-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-016-8829-3