Abstract
Advances in oncoimmunology related to the definition of the basic mechanisms of the formation of antitumor immune response, as well as the opening of tumor-associated antigens recognized by immune cells, allowed to start developing ways to influence the effector cells of the immune system to generate effective antitumor cytotoxic response. We investigated the possibility to stimulate an antitumor response in a culture of mononuclear cells of breast cancer patients by dendritic cells transfected with HLA-A*02:01-restricted DNA constructs. We isolated dendritic cells from peripheral blood monocytes and delivered our constructs to these cells by magnetic transfection. Additionally, a series of experiments with loading of dendritic cells with autologous tumor cell lysate antigens was conducted. We have shown that dendritic cells transfected with the HLA-A*02:01-restricted DNA constructs are effective in inducing an antitumor response in a culture of mononuclear cells of breast cancer patients. Dendritic cells transfected with DNA constructor dendritic cells loaded with lysate antigens revealed a comparable stimulated cytotoxic response of mononuclear cells to these two ways of antigen delivery. We conclude that using DNA constructs in conjunction with patient stratification by HLA type allows the application of transfected DCs as an effective method to stimulate antitumor immunity in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue transformation is accompanied by several failings of the antitumor immunity. Dendritic cells (DCs) are responsible for eliciting a cellular immune response, making their role very important. Various aspects of the functional immune system, and in particular the properties of DCs, make it possible to develop immunotherapeutic methods to combat malignant tumors. The use of DCs is one approach for correcting the type and efficiency of the immune response to certain antigens, including those expressed on tumor cells [1]. DCs enhance immunogenicity of tumor antigens, activate different types of immune competent cells, and have the ability to undergo ex vivo generation while preserving their immune properties in vivo.
Strategies for the vaccination of cancer patients involve autologous tumor cells, peptides, native antigens, and DNA constructs [2]. The use of DNA constructs, as a source of antigens, and DCs for their presentation to effector cells enables solving many problems, such as mismatched MHC haplotypes when native antigens are used and an insufficient amount or contamination of a material when a lysate is used, and has certain advantages [3]. DNA constructs give long-term expression of antigens in their natural form with appropriate posttranslational modifications [4]. Since cytotoxic drugs can cause an uncontrolled change in the spectrum of tumor-associated antigen (TAA) expression, DNA constructs have an advantage over tumor cell lysates and native antigens, because their sequences comprise the maximum possible range of immunogenic epitopes of TAAs for a given disease.
DNA constructs can stimulate both CD4+ and CD8+ T cell responses, as well as the humoral immune response specific for target tumor antigens. With this method, a wide range of different epitopes of tumor antigens, without limitation by the MHC molecule type, may be presented to cells of the immune system. A DNA sequence can be easily changed by removing or additionally inserting a special sequence to enhance antigen immunogenicity [5–7].
In our study, allele-specific DNA constructs were generated that contain HLA-A*02:01-digested epitopes of the proteins HER2/neu, mammaglobin-A, NY-BR-1, hMena (ENAH), WT1, telomerase, survivin, and p53, i.e., those specific for breast cancer [DNA (A) construct]. HLA-A*02 is the most common variant of the HLA-A2 family, and the variant HLA-A*02:01 is carried by > 95 % of the HLA-A-positive Caucasian population [8].
We have investigated the stimulation efficacy of the antitumor response in cultures of mononuclear cells from patients with breast cancer using DCs transfected with a mixture of allele-specific constructs containing the HLA-A*02:01-restricted epitopes of TAAs [DNA (A)].
Materials and methods
Study subjects
Peripheral blood of 40 patients aged 35–77 years (mean age, 57.9 years) with breast cancer at stages I–II was used. All patients gave written informed consent, which in simple terms described the basic principles of the research being undertaken. The study was approved by the local ethics committee of the Federal State Budgetary Institution “Research Institute of Clinical Immunology” under the Russian Academy of Medical Sciences Siberian Branch. The presence of the HLA-A*02:01 allele was confirmed by genotyping DNA isolated from peripheral blood cells, using an ALLSET™ GOLD HLA A LOW RES SSP kit (54310D, Invitrogen, USA, Brown Deer, WI).
Preparation of DNA constructs
Cytotoxic T cell epitopes used to produce immunogen were predicted with TEpredict software [2]. We took into account the major steps of mononuclear cell (MNC) class I-restricted antigen presentation: proteasomal cleavage [9] and peptide binding to transporters associated with antigen processing (TAP) [10]. Peptides predicted to have no proteasomal cleavage site at their C terminus, or predicted to be inefficient TAP binders, were excluded from further analysis. The selected candidate epitopes were joined into longer peptides if they overlapped within the original HER2 sequence. Peptides predicted as HLA-A*02:01 binders using either TEpredict or NetMHC [11, 12] were selected to construct the HLA-A*02:01-specific immunogen in the same manner. The HLA-A*02:01 allele was chosen, as it is the most frequently expressed HLA class I allele throughout the world. Individual genes, with modified codon usage, optimized for expression in mammalian cells, were synthesized based on the HLA-A*02:01-specific polyepitope immunogen sequences. DNA inserts were cloned in a eukaryotic pDNAVACCultra5 vector (NTC-DVU5, Nature Technology Corporation, USA) immediately after the CMV promoter. Each DNA plasmid was transformed into E. coli (XL2-blue strain) and confirmed by restriction digestion and DNA sequencing. Plasmid DNA was prepared from suitable clones in bulk, purified using the EndoFree Plasmid Giga Kit (12391, Qiagen, Germany), and resuspended in sterile PBS. DNA concentration was determined by UV spectroscopy. To study the functional properties of our constructs, DNA constructs based on the DNA-vector pDNAVACCultra5 encoding the HLA-A*02:01-specific polyepitopeimmunogens [DNA (A) construct] were obtained. HLA-A*02:01-specific polyepitopeimmunogens [DNA (A) construct] consist of a mixture of pDNA5-BC-A1 (HER2/neu-specific), pDNA5-BC-A2 (TAA-specific), and pDNA5-BC-A3 (TAA-specific) plasmids. pDNA5-BC-A2 contains 73 antigenic determinants from HER2, mammaglobin, NY-BR-1, and hMena; plasmid pDNA5-BC-A3 contains 74 epitopes from WT1, hTERT, survivin, p53, and Muc1. These plasmids were used in equimolar mixtures. The construct based on the DNA-vector pDNAVACCultra5 without inserts of immunogenic peptides was used as the control [DNA (p5) construct].
DC preparation
MNCs from peripheral blood were isolated using the standard Ficoll-Urografin density gradient method. Cells with enhanced ability to adhere to plastic were isolated from the resulting MNC population using a short 2-h incubation in a 75-cm3 culture flask (Corning, USA) in air with 5 % CO2 at 37 °C in RPMI-1640 medium with l-glutamine (Biolot, Russia), supplemented with 10 % fetal bovine serum (FBS; HyClonePerbio, USA), 40 μg/ml gentamicin (KRKA, Slovenia), 200 U/ml benzylpenicillin (“Synthesis”, Russia), 2 mM l-glutamine (Biolot, Russia), 5 × 10−5 M 2-mercaptoethanol (Sigma, USA), and 10 mM HEPES (Biolot, Russia) (complete RPMI-1640). The adherent MNC fraction was cultured in a 48-well plate (Corning, USA) at 1 × 106 cells/ml in 0.5 ml complete RPMI-1640, supplemented with 50 ng/ml recombinant human (rh) GM-CSF (PeproTech, USA) and 100 ng/ml rhIL-4 (PeproTech, USA) for 4 days to produce immature DCs. To obtain DCs loaded with tumor antigens, immature DCs were then exposed to tumor cell lysate at 100 μg/ml for 48 h. For maturation within the next 24 h, the culture was supplemented with rhTNF-α (25 ng/ml; VECTOR, Russia) in fresh culture medium. The non-adherent fraction, containing MNC, was maintained in a 75-cm3 culture flask at 2 × 106 cells/ml in complete RPMI-1640 until decantation.
DC transfection
The magnetic transfection procedure was performed using mature dendritic cells, as per the manufacturer’s instructions, and all reagents were purchased from Promokine, Germany. DCs that had not undergone magnetic transfection were used as controls. DCs at 5 × 105 cells in 0.5 ml of complete RPMI-1640 were cultured in a 48-well plate until maturity. To transfect the cells, a mixture consisting of 0.3 μg DNA and 0.3 μl of MATra-A (nanoparticles) in 25 μl serum-free DMEM medium was prepared. The DNA-MATra-A mixture was incubated at room temperature for 20 min. Before the end of this incubation, the RPMI-1640 was removed from the mature DCs and replaced with 250 μl serum-free medium DMEM containing no additional components. The DNA-MATra-A mixture was then added to cells (25 μl per well). The plate was incubated on a magnetic platform at room temperature for 15 min. After completion of magnetic transfection, the DMEM medium was replaced with 300 μl complete RPMI-1640 to maintain the initial concentration of cells on account of any possible loss of cells during transfection. The plate was retained in the CO2 incubator until required.
DC transfection efficacy
Transfection efficacy was estimated with a Promo-Fluor-500 Nick Translation Labeling Kit (PK-PF500-NTLK-50, Promokine) with further analysis on a FACS Aria flow cytometer (BD, USA), using the Flow-FISH method [13].
Co-culture of DC and MNC
Co-culture of DC and MNC was carried out in several parallel cultures for subsequent functional tests under uniform conditions. The concentration of non-adherent cultured MNC was 1 × 106 cells/ml, the DC:MNC ratio was 1:10, and rhIL-12 (10 ng/ml; PeproTech, USA) and rhIL-18 (100 ng/ml; MBL, USA) were applied to stimulate Th1-polarization. Mononuclear cells used to assess cytotoxicity against autologous tumor cells were cultured for 4 days in complete RPMI-1640 in the presence or absence of recombinant cytokines. Mononuclear cells used for assessing perforin levels were cultured in complete RPMI-1640 in the presence or absence of recombinant cytokines for 2 days; the cultures were subsequently washed of growth factors and cultured for a further 48 h.
Preparation of autologous tumor cells
A tumor sample was washed in RPMI-1640 with a doubled concentration of antibiotics. To obtain autologous tumor cells, a tumor fragment from each patient was crushed and left in 0.25 % trypsin solution at +4 °C overnight. Warm complete RPMI-1640 was used to inactivate the enzyme. The cell suspension was filtered to remove large aggregates, washed twice, and frozen in FBS with 10 % DMSO (Panreacsintesis, Spain). To obtain tumor cell lysates, a tumor fragment was mechanically homogenized and the resulting suspension was successively frozen at −70 °C and thawed at +37 °C through three cycles. Cells were pelleted by centrifugation and sterilized by passing through a 0.45-μm filter. Total protein in the lysates was determined by calculating the 260/280 nm absorbance ratio using a NanoDrop device (Thermo Scientific, USA).
Determination of perforin-positive cell count
The cells to be analyzed were washed once with PBS and fixed with 1 % paraformaldehyde in cold PBS for 20 min. They were centrifuged, and the pellet was resuspended in 0.2 ml PBS containing 0.2 % Tween 20 (Panreacsintesis, Spain) and incubated for 20 min to permeabilize the cell membranes, after which the cells were centrifuged and incubated with fluorochrome-labeled monoclonal antibodies against perforin (perforin-FITC, BD) for 30 min. The cells were washed to remove excess antibody, and the number of positive cells was determined by flow cytometry in the lymphocyte region.
Determination of antitumor cytotoxic effect
Cytotoxicity was assessed by analyzing the lactate dehydrogenase (LDH) content in the conditioned medium obtained by co-culture of MNCs stimulated by transfected or control DCs (effector cells) and tumor cells (target cells), using a CytoTox 96 ® Non-Radioactive Cytotoxicity Assay (G1780, Promega, USA). The effector cell:tumor cell ratio was 10:1. The CytoTox 96 ®assay quantitatively measures lactate dehydrogenase (LDH), a stable cytosolic enzyme that is released upon cell lysis released in radioactive assays. Released LDH in culture supernatants is measured with a 30-min coupled enzymatic assay, which results in the conversion of a tetrazolium salt (INT) into a red formazan product. Visible wavelength absorbance data are collected using a standard 96-well plate reader. The amount of color formed is proportional to the number of lysed cells. To convert the concentration of LDH in the supernatant into percentage cytotoxicity, we applied the formula:
To determine the value OD (maximum target lysis), we lysed the tumor cells by lysis solution.
Statistical analysis
Statistical data were processed using the Statistica 6.0 program. The Friedman test and Newman–Keuls multiple comparison test were used to detect statistically significant differences. The Shapiro–Wilk test was applied to determine sample normality. The data are presented as the mean and standard error for normal distribution, and the median and interquartile range were used for the abnormal distribution.
Results
Generation of antigen constructs
Although immunogenicity of the peptide is crucially determined by its MHC-binding affinity, it is also dependent on the amino acid residues flanking the epitope, which affect the efficiency of its proteasomal release and TAP-dependent transport into the endoplasmic reticulum [14]. Therefore we joined together predicted HLA-A*02:01-specific epitopes into the HLA-A*02:01-specific poly-CTL-epitopes (Table 1). We selected superior spacers for every pair of epitopes, choosing appropriate epitope matchings, and selecting the optimal arrangement of epitopes within the desired construction, thus increasing the efficiency of polyepitope processing and favoring presentation of target epitopes. Thus, in addition to cytotoxic T cell epitopes, efficient antigenic constructs should also contain T helper epitopes. T helper epitopes were predicted using ProPred predictive models [15]. Several fragments of HER2 containing the most promiscuous predicted T helper epitopes were selected. The universal immunogenic peptide, PADRE (Pan DR T Helper Epitope—AKFVAAWTLKAAA), was also incorporated into the poly-Th fragment. Peptides were joined through dibasic K/R-K/R motifs susceptible to cleavage by lysosomal cathepsins B and L participating in the processing of endocytosis antigens [16, 17]. The antigen constructs also possess the N-terminal ER-targeting signal sequence together with the C-terminal lysosomal sorting sequence, redirecting associated immunogens to lysosomes for degradation where peptide fragments may bind to recycling MNC class-II molecules, and are much more efficient in inducing T helper immune responses than similar immunogens lacking such signals [13, 18]. The N-terminal leader peptide (Table 1) was designed from the original HER2 signal peptide using the SignalP web server [19]. The last 11 amino acids of human LAMP-1 protein were selected as the carboxy terminal sorting signal, as suggested in [11], and directly fused to the C terminus of the poly-Th fragment. The poly-CTL epitope with the N-terminal leader peptide and the poly-Th epitope with the C-terminal portion of LAMP1 were then joined together through the spacer, forming a proteasome cleavage site on the C terminus of the poly-CTL portion (Table 1). Our constructs were then transfected into mature DCs by magnetic transfection before co-culture with MNC and investigation of the elicited antitumor response.
The effect of transfected DCs on the generation of MNC cytotoxic response
We generated immature and mature dendritic cells from peripheral blood mononuclear cells in patients with breast cancer. Expression DCs of main surface differential markers CD14, CD83, CD86, HLA-DR before adding TNF-α (stage of immature DCs) was 57.0, 9.38, 76.22, 77.6 %, respectively. Mature DCs (stage after addition of TNF-α and before transfection) expressed significantly higher level CD83, CD86, HLA-DR markers (62.11, 94.31, 95.47 % respectively). Expression CD14 marker was downregulated to 8.14 %. Also, significantly decreased in endocytic activity of mature DCs in dextran uptake compared with immature DCs (398 mean fluorescence intensity (MFI) and 1768 MFI, respectively) that provide the evidence of functional maturation of DCs [20].
The literature provides the data on transfection by genetic constructs both at the stage of immature dendritic cells and at the stage of mature dendritic cells [21, 22]. In this study, magnetic transfection was performed at the stage of mature dendritic cells, 24 h after addition of TNF-α as a maturing stimulus, which yielded the data presented in this article. By using the nick translation and Flow-FISH methods [23], the efficacy of magnetic transfection was confirmed to exceed 40 %. It is supposed that the pronounced effects of DCs transfected at the mature cell stage may be due to the fact that the transfection itself is transient, and the maximum peak of expression of target proteins and their presentation on cells coincides with the moment of interaction between DCs and autologous mononuclear cells in the cellular protocol.
Magnetic transfection with the polyepitope construct did not alter the morphology of mature DCs and the typical markers (CD83, CD86, HLA-DR), expression of which remained at the same level (83.15, 95.07, 95.50 %).
Cytotoxic T cells are the main component of the antitumor immune response, since they can directly lyse tumor cells and secrete immunomodulatory cytokines IL-2, TNF-α, GM-CSF, and IFN-γ, which indirectly affect malignant cells. The protective antitumor immune response involves destruction of malignant target cells, so we have estimated the cytotoxic activity of MNCs activated with transfected DCs against autologous tumor cells by quantitatively determining the content of the cytosolic enzyme, LDH, released from the destroyed cells.
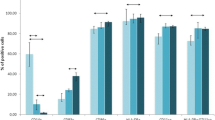
Analysis of the whole patient cohort, without considering the HLA genotype, showed enhanced cytotoxicity following application of DCs transfected with immunogenic DNA constructs compared with all the control groups. The cytotoxicity of the original MNC culture was 9.5 %. However, following co-culture with DCs transfected with a mixture of allele-specific DNA vaccine [DC (A)], this increased to 34.9 %. Upon additional stimulation with IL-12 and IL-18, enhancement of cytotoxicity in group with DC (A) to 43, 91 % compared to cells of all the control groups was found (Fig. 1a). Furthermore, a significant effect of IL-12 and IL-18 on the cytotoxic activity of this culture compared to a similar culture of MNCs with DC (A) without additional stimulants was observed.
Cytotoxicity of MNCs co-cultured with transfected autologous DCs against autologous tumor cells. a Breast cancer patients, n = 38. b HLA-A*02-positive breast cancer patients, n = 19. c HLA-A*02-negative breast cancer patients, n = 19. Data are presented as median (Me) and interquartile range. Arrows indicate statistically significant differences. (P < 0.05). MNC MNC control culture, MNC + DC (0) co-culture of MNCs and DCs without transfection, MNC + DC (p5) co-culture of MNCs and DCs transfected with the DNA-p5 construct, MNC + DC (A) co-culture of MNCs and DCs transfected with HLA-A*02:01-specific DNA constructs
The effect of transfected DCs on the number of perforin-positive cells in MNC culture
Cytotoxicity is a highly organized multifactorial process leading to the death of target cells by means of cytotoxic effector cells. One of the mechanisms causing tumor cell death is granulocyte-dependent endocytosis, the key mediators being perforin, granzyme, and granulysin. Perforin is involved in the formation of pores in the plasma membrane and therefore lysing target cells [24]. To test the assumption that a perforin-dependent mechanism of tumor cell lysis is involved in the antitumor response, we determined the effect of patient MNC co-culture with transfected DCs on perforin expression. Analysis of the whole patient cohort, without considering HLA genotype, indicated significant differences in the numbers of cells expressing perforin in the group treated with DNA (A) constructs compared to the control groups as well as in response to addition of IL-12 and IL-18 (Fig. 2a).
Percentage of perforin-positive cells following co-culture of MNCs and transfected autologous DCs. a Breast cancer patients, n = 38. b HLA-A*02-positive breast cancer patients, n = 19. c HLA-A*02-negative breast cancer patients, n = 19. Data are presented as median (Me) and interquartile range. Arrows indicate statistically significant differences.(P < 0.05). MNC the MNC control culture, MNC + DC (0) co-culture of MNCs and DCs without transfection, MNC + DC (p5) co-culture of MNCs and DCs transfected with the DNA-p5 construct, MNC + DC (A) co-culture of MNCs and DCs transfected with HLA-A*02:01-specific DNA constructs
Determining the MNC cytotoxic response level depending on the presence of the HLA-A*02 allele
The patients were subdivided into groups based on HLA type. In patients expressing the HLA-A*02:01 allele (19 patients), the DNA (A) construct had a pronounced influence on cytotoxicity against autologous tumor cells (Fig. 1b).
Furthermore, significant differences were identified in the DNA (A)-treated groups within the HLA-A*02:01-positive cohort with regards to the percentage of cells expressing intracellular perforin. The HLA-targeted construct induced greater numbers of perforin-positive MNC compared to the control groups, and this effect was further amplified in the presence of IL-12 and IL-18 (Fig. 2b).
In the HLA-A*02:01-negative group, DNA (A) constructs enhanced cytotoxicity to a similar extent compared to the original MNC culture and control constructs. Again, cytotoxicity was slightly elevated in the presence of IL-12 and IL-18 stimulation, and achieved statistical significance of DNA (A) (Fig. 1c).
Investigation of the possible mechanism of MNC cytotoxicity showed a significant increase in the percentage of perforin-positive cells in culture MNCs and DCs transfected with a mixture of allele-specific DNA constructs regardless of adding rhIL-12 and rhIL-18 (Fig. 2c)
Comparison of the MNC-mediated cytotoxic response in the group of HLA-A*02-positive and HLA-A*02-negative breast cancer patients revealed no significant differences.
The influence of DCs loaded with tumor cell lysate antigens on the cytotoxic response of MNC culture
Priming of dendritic cells with autologous tumor cell lysate antigens is a classic method of specific activation of the MNC cytotoxic response. We decided to compare cytotoxic activation of MNCs by transfected DCs and classical lysate-activated DCs to test the efficacy of the developed polyepitopic DNA construct as a source of antigens.
For this purpose, additional experiments involving the use of DCs loaded with tumor lysate antigens were conducted in some patients. A tumor cell lysate is a wide and individual set of tumor antigens that promotes the development of the maximum number of T cell clones with specificity to different immunogenic TAA epitopes characteristic of a given tumor. However, a significant drawback is the lysate immunosuppressive and autoimmune activity due to the heterogeneity of the cellular composition of a tumor material. Also, the immunogenicity of tumor cell lysate antigens enables generating an immune response against a specific tumor tissue and does not span a possible variability of the antigenic composition during the progression and treatment of cancer.
We demonstrated that the use of lysate-activated dendritic cells stimulates the cytotoxic response of the MNC culture that is expressed in an increased cytotoxic activity of MNCs against autologous cells (Fig. 3a) and in the number of perforin-positive cells (Fig. 3b) at levels comparable to those for DCs transfected with DNA constructs.
Cytotoxicity against autologous tumor cells (a) and percentage of perforin-positive cells (b) co-cultured of MNCs and tumor lysate-loaded DCs (n = 20). Data are presented as median (Me) and interquartile range. Arrows indicate statistically significant differences.(P < 0.05). MNC the MNC control culture, MNC + DC (0) co-culture of MNCs and DCs unloaded with tumor lysate antigens, MNC + DC (Lysate) co-culture of MNCs and DCs loaded with tumor lysate antigens
Discussion
The efficient destruction of malignant neoplasms is dependent on the presence of large numbers of functional effector lymphocytes, which recognize and kill tumor cells in vivo [25]. The main challenge of cell-mediated immunotherapy is preparing autologous dendritic cells capable of stimulating antitumor immunity. DCs cultured in vitro are naturally loaded with antigen, thus making it possible to overcome T cell tolerance to tumor antigens and induce a complete immune response [26]. In our study we investigated ability transfected DCs inductions effective cytotoxic T response against autological tumor cells in vitro.
The use of transfection to efficiently deliver antigens for presentation to MNCs by DCs is a modern version of obtaining immune cells to stimulate antitumor immunity in vitro [27, 28]. Designing polyepitope constructs containing only epitopes that stimulate the Th1 and cytotoxic T cell response instead of the whole antigen seems to be the most promising approach, while also limiting the impact of tumor-associated immunosuppression. Polyepitope constructs can contain epitopes from different protein antigens and can at the same time cover a variety of targeted allelic variants of MHC molecules. Certain signal sequences can be incorporated in such constructs to increase immunogenicity and optimize MHC I- or MHC II-dependent presentation of epitopes.
We used polyepitope DNA constructs to activate antitumor cytotoxic T cell responses, comparing these with the conventional loading of DCs with lysates of tumor tissue. The use of dendritic cells to prime an antigen enables efficient activation of the effector functions of immune cells. The effectiveness of a polyepitope construct is affected by various factors, including the presence of T helper epitopes, cleavage in the proteasome, the presence of spacers between epitopes, the role of epitope affinity for dominance, and shifting toward the predominance of a cytotoxic response in vivo. However, the main advantage of DCs transfected with polyepitopic allele-specific DNA constructs comparing with the conventional lysates-loading of DCs is the absence of immunosuppressive and autologous epitopes that can inhibit the development of an effective immune response and even stimulate the autoimmune process. We generated and tested HLA-A*02:01-specific constructs, the design of which was aimed at activation of cytotoxic T lymphocytes through presentation of relevant TAAs on the surface of the DCs. We decided to divide the structure of whole pDNA5-BC-A constructs into three individual DNA construction [pDNA5-BC-A1 (HER2/neu-specific), pDNA5-BC-A2 (TAA-specific), and pDNA5-BC-A3 (TAA-specific)] to facilitate the processing and presentation of antigen in transfected DCs. Each DNA constructs contain the most immunogen cytotoxic epitopes of TAA for breast cancer. DNA constructs containing BC-A1 immunogens were also enhanced with a common T helper epitope. Using DNA construct encoding all researched immunogenic epitopes prevent effective intracellular processing and presentation epitopes by DCs as well as promote low cytotoxic T cells response (data no shown).
An analysis of the cytotoxic activity demonstrated that the use of DCs transfected with the allele-specific DNA construct that encode breast cancer TAA epitopes enhances the antitumor activity of MNCs. We found that, regardless of the presence of the HLA-A*02 genotype in the breast cancer patient, the developed polyepitope DNA construct encoding the most immunogenic TAA epitopes stimulates the cytolytic activity of MNCs of breast cancer patients. Cytokines, such as IL-12 and IL-18, promote a reliable enhancement of the cytotoxic effect of MNC culture. If the obtained effect of the allele-specific DNA construct in the group of HLA-A*02:01-positive patients with breast cancer was the anticipated result, the comparable effect observed in the group of HLA-A*02-negative patients may be related to the cross affinity of allele-specific immunogenic epitopes for other variants of the HLA-A genotype. These data are also consistent with the fact that the use of allele-specific DNA constructs leads to an increased amount of cells containing a cytotoxic response mediator, perforin. In this regard, it may be assumed that genotyping the patient for determining the HLA-A*02 allele is optional if DNA constructs encoding a large number of immunogenic epitopes of various TAAs are used as an antigen source in conjunction with IL-12 and IL-18 cytokines. However, the use of only DCs transfected with polyepitopic allele-specific DNA constructs, without IL-12 and IL-18, in the group of HLA-A*02-positive patients has a stimulating effect on the cytotoxic potential of the MNC culture, which is consistent with our view of affinity of selected immunogenic epitopes comprised by a DNA construct. Therefore, the obtained effect in the group of HLA-A*02-negative breast cancer patients is associated with an additional stimulatory effect of IL-12 and IL-18, which they exert on the effector functions of immune cells.
Conclusions and future perspective
We have shown that the use of different antigen sources, such as tumor cell lysates and DNA constructs to load dendritic cells, gives similar stimulation of cytotoxic activity in vitro. However, lysates can be used only if resection of a tumor or metastases is carried out. The use of DNA constructs is no less effective in these cases than the use of lysates; it allows the use of transfected DCs as an additional means of conservative treatment of patients at the earliest stages of tumor progression when the tumor is not resected. Another prospect for using such DC transfected DNA constructs may be in the preventive vaccination of women predisposed to breast cancer, attested by genetic screening and/or family history.
Usually ex vivo pulsed DCs have shown poor clinical efficiency due to poor migratory capacity of DCs to lymph nodes after injection, weak interaction between transfected DC and naive T cells. In this regard, we assume that the use co-culture of transfected DC and in vitro antigen-activated T cells for modulation anti-tumor response will increase efficiency cytotoxic effect DC-vaccines in vivo.
Abbreviations
- DC:
-
Dendritic cells
- MNC:
-
Mononuclear cells
- TC:
-
Tumor cells
- LDH:
-
Lactate dehydrogenase
- TAAs:
-
Tumor-associated antigens
References
Palucka K, Ueno H, Fay J, Banchereau J. Dendritic cells and immunity against cancer. J Intern Med. 2011;269(1):64–73.
Bei R, Scardino A. TAA polyepitope DNA-based vaccines: a potential tool for cancer therapy. J Biomed Biotechnol. 2010; 102758.
Scardino A, Alimandi M, Correale P, Smith SG, Bei R, Firat H, Cusi MG, Faure O, Graf-Dubois S, Cencioni G, Marrocco J, Chouaib S, Lemonnier FA, Jackson AM, Kosmatopoulos K. A polyepitope DNA vaccine targeted to Her-2/ErbB-2 elicits a broad range of human and murine CTL effectors to protect against tumor challenge. Cancer Res. 2007;67(14):7028–36.
Nakamura M, Iwahashi M, Nakamori M, Ueda K, Ojima T, Naka T, Ishida K, Yamaue H. Dendritic cells transduced with tumor-associated antigen gene elicit potent therapeutic antitumor immunity: comparison with immunodominant peptide-pulsed DCs. Oncology. 2005;68(2–3):163–70.
Boudreau JE, Bonehill A, Thielemans K, Wan Y. Engineering dendritic cells to enhance cancer immunotherapy. Mol Ther. 2011;19(5):841–53.
DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102.
Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140(6):883–99.
Chen KY, Liu J, Ren EC. Structural and functional distinctiveness of HLA-A2 allelic variants. Immunol Res. 2012;53(1–3):182–90.
Toes RE, Nussbaum AK, Degermann S, Schirle M, Emmerich NP, Kraft M, Laplace C, Zwinderman A, Dick TP, Müller J, Schönfisch B, Schmid C, Fehling HJ, Stevanovic S, Rammensee HG, Schild H. Discrete cleavage motifs of constitutive and immunoproteasomes revealed by quantitative analysis of cleavage products. J Exp Med. 2001;194(1):1–12.
Peters B, Bulik S, Tampe R, Van Endert PM, Holzhütter HG. Identifying MHC class I epitopes by predicting the TAP transport efficiency of epitope precursors. J Immunol. 2003;171(4):1741–9.
Antonets DV, Maksiutov AZ. TEpredict: software for T-cell epitope prediction. MolBiol (Mosk). 2010;44(1):130–9.
Lundegaard C, Lamberth K, Harndahl M, Buus S, Lund O, Nielsen M. NetMHC-3.0: accurate web accessible predictions of human, mouse and monkey MHC class I affinities for peptides of length 8-11. Nucleic Acids Res. 2008;36:509–12.
Bonini C, Lee SP, Riddell SR, Greenberg PD. Targeting antigen in mature dendritic cells for simultaneous stimulation of CD4+ and CD8+ T cells. J Immunol. 2001;166(8):5250–7.
Livingston BD, Newman M, Crimi C, McKinney D, Chesnut R, Sette A. Optimization of epitope processing enhances immunogenicity of multiepitope DNA vaccines. Vaccine. 2001;19(32):4652–60.
Singh H, Raghava GP. ProPred: prediction of HLA-DR binding sites. Bioinformatics. 2001;17(12):1236–7.
Zhang T, Maekawa Y, Hanba J, Dainichi T, Nashed BF, Hisaeda H, Sakai T, Asao T, Himeno K, Good RA, Katunuma N. Lysosomal cathepsin B plays an important role in antigen processing, while cathepsin D is involved in degradation of the invariant chain in ovalbumin-immunized mice. Immunology. 2000;100(1):13–20.
Hsieh CS, deRoos P, Honey K, Beers C, Rudensky AY. A role for cathepsin L and cathepsin S in peptide generation for MHC class II presentation. J Immunol. 2002;168(6):2618–25.
Fassnacht M, Lee J, Milazzo C, Boczkowski D, Su Z, Nair S, Gilboa E. Induction of CD4(+) and CD8(+) T-cell responses to the human stromal antigen, fibroblast activation protein: implication for cancer immunotherapy. Clin Cancer Res. 2005;11(15):5566–71.
Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340(4):783–95.
Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182(2):389–400.
Bonehill A, Heirman C, Tuyaerts S, Michiels A, Breckpot K, Brasseur F, Zhang Y, Van Der Bruggen P, Thielemans K. Messenger RNA-electroporated dendritic cells presenting MAGE-A3 simultaneously in HLA class I and class II molecules. J Immunol. 2004;172:6649–57.
O’Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104(8):2235–46.
Rufer N, Brümmendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190(2):157–67.
Voskoboinik I, Dunstone MA, Baran K, Whisstock JC, Trapani JA. Perforin: structure, function, and role in human immunopathology. Immunol Rev. 2010;235(1):35–54.
Pandolfi F, Cianci R, Pagliari D, Casciano F, Bagalà C, Astone A, Landolfi R, Barone C. The immune response to tumors as a tool toward immunotherapy. Clin Dev Immunol. 2011;2011:894704.
Jeras M, Bergant M, Repnik U. In vitro preparation and functional assessment of human monocyte-derived dendritic cells-potential antigen-specific modulators of in vivo immune responses. Transpl Immunol. 2005;14:231–44.
Gattinoni L, Powell DJ Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–93.
Kulikova EV, Kurilin VV, Shevchenko JA, Obleukhova IA, Khrapov EA, et al. Dendritic cells transfected with a DNA construct encoding tumour-associated antigen epitopes induce a cytotoxic immune response against autologous tumour cells in a culture of mononuclear cells from colorectal cancer patients. Scand J Immunol. 2015;82(2):110–7.
Acknowledgments
This work was supported by the Federal target program “Research and development in priority areas of scientific and technological complex development of Russia for 2014-2020” (Agreement No 14.607.21.0043. The unique identifier for Applied Scientific Research RFMEFI60714X0043). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standard
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Rights and permissions
About this article
Cite this article
Sennikov, S.V., Shevchenko, J.A., Kurilin, V.V. et al. Induction of an antitumor response using dendritic cells transfected with DNA constructs encoding the HLA-A*02:01-restricted epitopes of tumor-associated antigens in culture of mononuclear cells of breast cancer patients. Immunol Res 64, 171–180 (2016). https://doi.org/10.1007/s12026-015-8735-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-015-8735-0