Abstract
Lung cancer has become the leading cause of cancer-related death worldwide. However, treatment failures still represent enormous challenges, and it is doubtful whether standard treatment modalities could continuously achieve substantial improvements. As one of the novel therapy strategies, PD-L1 has been shown the function of down-regulating T cell activation through receptor PD-1. Moreover, prognosis of cancer patients is based not only on tumor-related factors but also on host-related factors, particularly systemic inflammatory response. Significantly, squamous non-small cell lung cancer (NSCLC) revealed to be divergent clinical and molecular phenotypes compared with non-squamous NSCLC. Monocyte ratio, neutrophils to lymphocytes ratio, PD-L1 immunostaining score and PD-1-positive stained tumor-infiltrating lymphocyte counts were assessed by Fisher’s linear discriminant analysis to discriminate whether overall survival (OS) would exceeding 5 years. Finally, a prediction model was established for OS based on these immunological markers. Furthermore, this prediction model was validated in a second set of squamous NSCLC patients. The model offers a novel tool for survival prediction and could have important clinical implications for patients with squamous NSCLC, thus providing a framework for future individualized therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer, whose incidence is increasing worldwide, has become the leading cause of cancer-related death worldwide [1]. Approximately, non-small cell lung cancer (NSCLC) accounts for 80 % of lung cancers [2]. Under multimodality treatment, modest improvements in the survival rates of NSCLC have been reported [3]. Nevertheless, treatment failures still represent enormous challenges, and it is doubtful whether these standard treatment modalities could continuously achieve substantial improvements [4].
Immune checkpoint pathways, keeping nascent T cell responses and reducing immune attack against normal tissues, significantly down-regulate T cell activation. However, cancer cells could resist detection or avoid elimination from adaptive immune system by exploiting these co-inhibitory pathways [5]. PD-L1 (B7-H1) and PD-L2 (B7-DC), members of the B7 superfamily, have been shown the function of down-regulating T cell activation through receptor PD-1 [6]. Based on these results, PD-1, which has been identified as a receptor for PD-L1 and PD-L2, has been investigated as a target for cancer immunotherapy [7]. Previous clinical trials of NSCLC have suggested PD-L1 expression as biomarker for positive therapeutic response to anti-PD-1 or anti-PD-L1 antibodies [8, 9]. However, data from several small series regarding the prognostic role of PD-L1 and PD-1 in NSCLC remain limited and inconsistent [10–13].
Nowadays, increasing data indicated that prognosis of cancer patients is based not only on tumor-related factors but also on host-related factors, particularly systemic inflammatory response [14, 15]. As significant indicators of patients’ inflammation status, circulating monocyte count, neutrophil ratio and lymphocyte ratio were proved as predictors of prognosis in various cancers [16–18]. Increased counts of neutrophils and/or decreased counts of lymphocytes might serve as suppressor of lymphokine-activated killer cells, which could increase the propensity to metastasis [19, 20].
Squamous NSCLC, revealing to be divergent clinical and molecular phenotypes compared with non-squamous NSCLC, displays a more consistent immune profile in more frequently specific tumor antigens expressing and more extensive CD8+ effector cells infiltrating [21]. Therefore, novel characters in immunological are hoped to be explored and confirmed to develop relative approach in managing squamous NSCLC [22]. Consequently, we analyzed the immunological marker profiles of a set of patients with squamous NSCLC. A prediction model for overall survival (OS) was derived based on immunological markers and further validated in a second set of squamous NSCLC patients.
Methods
Study protocol was approved by the Ethics Committee of Human Experimentation in China. Written informed consent was obtained from each patient: including signed consent for tissue analysis as well as consent to be recorded for potential medical research at the time of sample acquisition. All experiments were performed in accordance with relevant guidelines and regulations.
Chart review was performed on 1286 consecutive patients who suffered from squamous NSCLC with between November 2004 and March 2008. One hundred and fifty-six of the 1286 patients were enrolled in the final analysis, while other patients with squamous NSCLC were excluded from analysis because of incomplete clinical or pathological data, such as unavailable formalin-fixed paraffin-embedded blocks. These 156 patients were randomly assigned (2:1) centrally by computer into training group (n = 104) and validation group (n = 52).
The blood tests were obtained within 24 h after admission for all patients as routine clinical practice in SYSUCC. Monocyte ratio was calculated as absolute monocyte count after initial treatment divided by absolute monocyte count before initial treatment. In a similar way, NLR was calculated as neutrophil count divided by lymphocyte count. Moreover, NLR ratio was calculated as NLR after initial treatment divided by NLR before initial treatment. Then, characteristics of patients and tumors were collected. Surgically resected or biopsied specimens were fixed in formalin and embedded in paraffin for routine histopathological diagnosis and immunohistochemical analysis. All data were reviewed and confirmed by two independent pathologists based on WHO classification of lung cancer.
Immunohistochemistry
Isolated tumors were fixed in 10 % neutral buffered formalin for 48 h and embedded in paraffin according to standard protocols. Sections (thickness, 4 μm) were deparaffinized and rehydrated in a graded series of alcohol solutions. For antigen retrieval, slides were immersed in ethylenediamine tetra-acetic acid (1 mmol/L, pH 8.0) and boiled for 15 min in a microwave oven. Endogenous peroxidase activity was blocked in 3 % H2O2 at room temperature for 15 min, and nonspecific binding was abolished by 5 % bovine serum albumin for 30 min. Sections were then stained with anti-PD-1 (rabbit anti-PD-1 polyclonal antibody; 1:100 dilution; Protein Tech, Shanghai, China) antibody and anti-PD-L1 (rabbit anti-PD-L1 polyclonal antibody; 1:50 dilution; Protein Tech, Shanghai, China) antibody at 4 °C overnight. After washing with phosphate-buffered saline (PBS), sections were incubated with horseradish peroxidase-conjugated secondary antibody (Envision Detection kit, GK500705, Gene Tech, Shanghai, China) at room temperature for 30 min. After washing thrice with PBS, antibody complexes were colored with 3,3′-diaminobenzidine and then counterstained with hematoxylin. Slides were dehydrated and evaluated.
Semi-quantitative method
The total PD-L1 immunostaining score was calculated as the sum of the positively stained tumor cells and staining intensity. Briefly, the percentage of positive staining was scored as “0” (<5 %, negative), “1” (5–25 %, sporadic), “2” (25–50 %, focal) or “3” (>50 %, diffuse). Staining intensity was scored as “0” (no staining), “1” (weak staining), “2” (moderate staining) or “3” (strong staining). Both the percentage of positive cells and the staining intensity were evaluated under double-blind conditions. The total immunostaining score was calculated as the value of percent positivity score × staining intensity score and ranged from 0 to 9. We defined PD-L1 expression levels as: “0” (score 0–1), “1” (2–3), “2” (4–6) and “3” (>6). The score assessment was performed independently by two independent pathologists blinded to the clinical parameters.
Cell counting
The status of PD-1 staining was recorded by counting PD-1-positive stained TILs. After scanning the whole section at low magnifications (100 ×), ten tumor areas were selected. The value of PD-1 was evaluated by the average of ten 200 × field PD-1-positive stained TILs counts. The counts assessment was also performed independently by two pathologists blinded to the clinical parameters with Image J software (National Institute of Health).
Statistical analysis
The data are presented as the number (%) or median (range) unless otherwise stated. The Pearson χ 2 test and Fisher’s exact test were used for categorical data, and an independent sample t test or the Mann–Whitney U test was used for numerical data.
The potential risk factors: PD-L1 immunostaining score, PD-1-positive TILs counts, monocyte ratio, NLR ratio, sex, age, smoking habit, tumor size, tumor location, differentiation and pathological stage, to discriminate whether OS would exceeding 5 years, were assessed by FLDA. A significant difference was declared if the P value from a two-tailed test was <0.05. The results were similar when using a more liberal p value of 0.10. All clinically possible interactions were tested, but none were statistically significant, so they were excluded in the final model.
First, the final model was used to calculate the discriminant score in each study participant. Second, the comparison between the discriminant score with the OS was used to construct a receiver operating characteristic curve. In the meantime, the AUC and its 95 % CI were also reported to describe the accuracy of the model for identifying metastases in our study participants. And the eigenvalue and canonical correlation were used to evaluate model fit (P < 0.05 was considered statistically significant). We internally validated the model using a cross-validation procedure, which enabled us to use the full data set for model development. P values <0.05 were considered statistically significant. Data analysis was performed using Predictive Analytics Software (PASW) Statistics 18.0 for Windows (SPSS Inc, Chicago, IL).
Result
Clinical outcomes
One hundred and fifty-six patients with squamous NSCLC were eligible for the final analysis. The mean age was 60.14 years (range 32–80 years, median 60 years); 147 patients were male (94.2 %) and 9 female (5.8 %). One hundred and thirty-six (87.2 %) patients were smokers. Additionally, stage IA disease occurred in 15 (9.6 %) patients, IB in 44 (28.2 %), IIA in 31 (19.9 %), IIB in 21 (13.5 %), IIIA in 42 (26.9 %) and IIIB in 3 (1.9 %). Moreover, locations of tumor were 37 (23.7 %) in left upper lobe, 36 (23.1 %) in left lower lobe, 43 (27.6 %) in right upper lobe, 12 (7.7 %) in right middle lobe and 28 (17.9 %) in right lower lobe. Furthermore, pathological analysis reported 7 patients (4.5 %) with well differentiated, 42 (26.9 %) with moderately differentiated and 107 (68.6 %) with poorly differentiated.
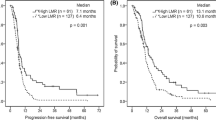
The mean follow-up for survivors as of December 2014 was 47.43 months (range 0.53–92.67 months, median 52.52 months). Besides, mean OS was 2163 days, and 23.1 % of the patients were alive without disease, 48.1 % were alive with disease and 28.8 % dies of disease. The overall 1-, 3- and 5-year OS rates were 97.3, 85.3 and 64.7 %, respectively (Fig. 1).
PD-L1 expressed on tumor cells. Among all these 156 specimens of squamous NSCLC, PD-L1 expressed in either one or both of the cell membrane and cytoplasm, in a focal or scattered pattern (Fig. 2). Accordingly, PD-1 expressed on tumor-infiltrating lymphocytes (TILs) (Fig. 3). PD-1-positive TILs were counted and recorded.
There was no significant difference between the training (n = 104) and validation (n = 52) cohorts in patients’ sex, age, smoking habit, tumor size, tumor location, differentiation, pathological stage, follow-up, PD-L1 immunostaining score, PD-1-positive TILs counts, monocyte ratio and neutrophils to lymphocytes ratio (NLR) ratio (P > 0.05) (Table 1).
Class prediction analysis
Based on training cohorts, four independent predictors of OS were identified by using Fisher’s linear discriminant analysis (FLDA) with stepwise variant selection. All other potential predictors were analyzed but not associated with OS and therefore were not included in the final model. The clinical classifying model was described by the following equation: Y = − 1.212 + 0.211 × NLR ratio + 0.437 × monocyte ratio − 0.390 × PD-L1 + 0.035 × PD-1 (eigenvalue 0.673, canonical correlation 0.634, P < 0.001). In this equation, PD-L1 represented PD-L1 immunostaining score; PD-1 represented PD-1-positive TILs counts.
Group centroids for OS ≤ 5 years and OS > 5 years were 0.505 and −1.307, respectively. Next, a cut score halfway between the two centroids was determined: cut score = (−1.307 + 0.505)/2 = −0.401. When the discriminant score Y was calculated to be >−0.401, the case was predicted to be an OS ≤ 5 years case; otherwise, the case was classified as an OS > 5 years. For the training set of 104 leave-one-out cross-validated cases, 27 of 29 OS > 5 years (93.1 % sensitivity) and 61 of 75 OS ≤ 5 years (81.3 % specificity) were correctly classified with an overall accuracy of 84.6 % (88 of 104) and an area under the curve (AUC) of 0.938 [P < 0.001, 95 % confidence interval (CI) 0.864–1] (Table 2, Fig. 4a, b).
Receiver operating characteristic curve analysis of the discriminant model with NLR ratio, monocyte ratio, PD-L1 and PD-1 for discriminate OS ≤ 5 years and OS > 5 years on training (a) and validation (c) samples. Box and Whisker plot showing the distributions of the discriminant scores of OS ≤ 5 years and OS > 5 years in training (b) and validation (d) samples. OS overall survival
Next, the predicting model consisting of the four predictors (NLR ratio, monocyte ratio, PD-L1 and PD-1) was applied to the validation set of 52 patients (14 OS > 5 years and 38 OS ≤ 5 years) (Table 2). A survival prediction for 38 of the 52 patients (73.1 %) with an AUC of 0.908 (P < 0.001, 95 % CI 0.806–1) was achieved (Table 2, Fig. 4c, d). Also, 12 of 14 OS > 5 years (85.7 % sensitivity) and 26 of 38 OS ≤ 5 years (68.4 % specificity) were correctly identified (Table 2).
Discussion
Co-development of novel appropriate biomarkers, which could be used in predicting overall survival, is emerging its significance in cancer research [23]. A growing body of evidence suggested that tumor cells evade host immune surveillance through regulating the expression of soluble ligands and cytokines [24]. Furthermore, in murine models and possibly patients, several strategies were shown to be associated with immune suppression and poor prognosis, including down-regulation of cell surface major histocompatibility complex class I molecules, [25, 26] secretion of immunosuppressive factors, [27] and expression of death ligands [28]. As a member of the B7 superfamily, PD-L1 was more broadly expressed than the other members in B7 superfamily [6]. Recently, expression of PD-L1 on cancer cells could induce apoptosis of human T cell clones, whereas blockading PD-L1 has been shown the ability in directly down-regulating immune responses and host immune evading by PD-1 receptor on activated T and B cells in vitro and in vivo, which meant PD-L1 could function as a negative regulator of T cell-mediated anti-tumor immunity [29, 30]. Consequently, effector immune cells inactivation through PD-1 receptor signaling could finally result in disease progression [31].
Interaction of PD-1 and PD-L1 has been recognized as key mechanism for immune evasion in NSCLC; the clinical efficacy of anti-PD-1 and PD-L1 blocking antibodies has been proved though a serial of clinical trials [8, 9]. Although preliminary results suggested that PD-L1 expression might be associated with higher response rate to PD-1 blockade treatment, this association remains inconsistent, because not all PD-L1-positive tumors were sensitive [32]. Evidence also illustrated the contribution of PD-L1 expression on tumor cells to down-regulate immune responses in NSCLC [33]. Accordingly, PD-L1 blockade has recently been proved to improve anti-tumor immunity [34, 35]. Although PD-1 and PD-L1 have been well studied in lung adenocarcinomas, their prognostic roles remained controversial whether squamous NSCLC shared the same characters. Importantly, studies have presented the different expression of PD-L1 in different histological types [13]. In current study, comprehensive and concurrent analyses of PD-1 and PD-L1 were performed in a large cohort of patients with squamous NSCLC, aiming to illustrate the prognosis role of PD-1 and PD-L1 in squamous NSCLC. Moreover, the inconsistent prognostic role of PD-L1 and PD-1 in NSCLC might be caused by heterogeneous patient population; thus, our study focused only on one subgroup of NSCLC to figure out the prognosis role of immunological markers, including PD-L1 and PD-1.
Publications have already shown that both the intrinsic characteristics and environment of tumor would stimulate the invaded and metastatic ability [36]. Abnormal tumor phenotype could stimulate inflammatory cells flowing into tissues around the tumor. In addition, generalized and nonspecific inflammatory response could be triggered by generalized and nonspecific inflammatory response and following tissue destruction and disruption [37]. Evidence indicated an association among systemic inflammatory response, progressive nutritional and functional decline in cancer victims and poor prognosis, which could be partly interpreted by insidious cancer progression activating innate immunity [14, 38]. A correlation between the increasing monocytes and neutrophils, decreasing lymphocytes, and inflammation-induced tumor growth and progression via various growth and pro-angiogenic cytokines has been observed and proved, although immunosuppression was common in the cancer population [39, 40]. Studies have showed peripheral blood monocytes count, low blood neutrophil count and high lymphocyte count to be independent prognosis factors in patients with neck and head, biliary, cervix, liver, stomach and colon cancers [41–44]. In addition, various evidences indicated elevating NLR accompanying poor prognosis in different types of cancer [45, 46]. Investigations revealed that this association might be caused by suppressed anti-tumor cellular immune activity of natural killer cells and lymphocyte by increased neutrophils [47]. Previous studies on circulating leukocyte influenced us concerning the association between clinical circulating monocytes, neutrophils, lymphocytes and tumor prognosis. Differently, relative large number of patients with squamous NSCLC was enrolled in current study. Furthermore, monocyte and NLR ratio, instead of cells counts, were used in our analysis, which could assess the impact of response to treatment. Neutrophils and lymphocytes increasing tumor infiltration suggested strong anti-tumor immune responses.
Several limitations remain in this study. First, all the data were retrospectively collected; thus, clinical and survival comparison might be influenced by selection bias due to its retrospective nature. Second, the monocyte phenotype or molecular information was not analyzed, which was caused by lack of this information in our retrospective data. Third, other systemic inflammatory immune indexes, such as C-reactive protein or albumin, which were known as prognostic factors [48], also were absent in our retrospective data.
In conclusion, our analyses demonstrated that the analysis of a set of immunological markers could effectively and reproducibly classify patients with squamous NSCLC according to their overall survival. Further prospective validation in larger independent cohorts of patients with similar or different regimens is warranted to fully assess its predictive power. However, the 4-immunological-marker model offers a novel tool for survival prediction and could have important clinical implications for the consideration of differential treatment strategies in patients with squamous NSCLC, thus providing a framework for future individualized therapy.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi:10.3322/caac.21208.
Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117(2):294–9. doi:10.1002/ijc.21183.
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–94. doi:10.4065/83.5.584.
Yasumoto K, Hanagiri T, Takenoyama M. Lung cancer-associated tumor antigens and the present status of immunotherapy against non-small-cell lung cancer. Gen Thorac Cardiovasc Surg. 2009;57(9):449–57. doi:10.1007/s11748-008-0433-6.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi:10.1038/nrc3239.
Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–9. doi:10.1038/70932.
Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8(3):239–45. doi:10.1038/ni1443.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi:10.1056/NEJMoa1200690.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi:10.1056/NEJMoa1200694.
Boland JM, Kwon ED, Harrington SM, Wampfler JA, Tang H, Yang P, et al. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin Lung Cancer. 2013;14(2):157–63. doi:10.1016/j.cllc.2012.05.006.
Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012;98(6):751–5. doi:10.1700/1217.13499.
Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094–100. doi:10.1158/1078-0432.CCR-04-0428.
Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28(3):682–8. doi:10.1007/s12032-010-9515-2.
McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–6. doi:10.1097/MCO.0b013e32832a7902.
Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–63. doi:10.2217/fon.09.136.
Satomi A, Murakami S, Ishida K, Mastuki M, Hashimoto T, Sonoda M. Significance of increased neutrophils in patients with advanced colorectal cancer. Acta Oncol. 1995;34(1):69–73.
Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137(2):425–8. doi:10.1016/j.jtcvs.2008.05.046.
Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32(8):1757–62. doi:10.1007/s00268-008-9552-6.
Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. Prognostic value of preoperative peripheral blood monocyte count in patients with hepatocellular carcinoma. Surgery. 2006;139(6):755–64. doi:10.1016/j.surg.2005.10.009.
Lee YY, Choi CH, Sung CO, Do IG, Huh S, Song T, et al. Prognostic value of pre-treatment circulating monocyte count in patients with cervical cancer: comparison with SCC-Ag level. Gynecol Oncol. 2012;124(1):92–7. doi:10.1016/j.ygyno.2011.09.034.
Zielinski C, Knapp S, Mascaux C, Hirsch F. Rationale for targeting the immune system through checkpoint molecule blockade in the treatment of non-small-cell lung cancer. Ann Oncol. 2013;24(5):1170–9. doi:10.1093/annonc/mds647.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi:10.1016/j.cell.2011.02.013.
Waki K, Yamada T, Yoshiyama K, Terazaki Y, Sakamoto S, Matsueda S, et al. PD-1 expression on peripheral blood T-cell subsets correlates with prognosis in non-small cell lung cancer. Cancer Sci. 2014;105(10):1229–35. doi:10.1111/cas.12502.
Shang B, Zhang G, Pan Y, Zhou Q. Deciphering the key features of malignant tumor microenvironment for anti-cancer therapy. Cancer Microenviron. 2012;5(3):211–23. doi:10.1007/s12307-012-0108-9.
Yamazaki K, Spruill G, Rhoderick J, Spielman J, Savaraj N, Podack ER. Small cell lung carcinomas express shared and private tumor antigens presented by HLA-A1 or HLA-A2. Cancer Res. 1999;59(18):4642–50.
Redondo M, Concha A, Oldiviela R, Cueto A, Gonzalez A, Garrido F, et al. Expression of HLA class I and II antigens in bronchogenic carcinomas: its relationship to cellular DNA content and clinical-pathological parameters. Cancer Res. 1991;51(18):4948–54.
Samuels V, Barrett JM, Bockman S, Pantazis CG, Allen MJ. Immunocytochemical study of transforming growth factor expression in benign and malignant gliomas. Am J Pathol. 1989;134(4):894–902.
Oizumi S, Yamazaki K, Nakashima M, Watanabe T, Hommura F, Ogura S, et al. RCAS1 expression: a potential prognostic marker for adenocarcinomas of the lung. Oncology-Basel. 2002;62(4):333–9. doi:10.1159/000065065.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34.
Chen C, Qu QX, Huang JA, Zhu YB, Ge Y, Wang Q, et al. Expression of programmed-death receptor ligands 1 and 2 may contribute to the poor stimulatory potential of murine immature dendritic cells. Immunobiology. 2007;212(3):159–65. doi:10.1016/j.imbio.2007.01.004.
Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1). J Thorac Oncol. 2013;8(6):803–5. doi:10.1097/JTO.0b013e318292be18.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi:10.1038/nm730.
Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63(19):6501–5.
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99(19):12293–7. doi:10.1073/pnas.192461099.
Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9(5):562–7. doi:10.1038/nm863.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi:10.1038/nature01322.
Nagtegaal ID, Marijnen CA, Kranenbarg EK, Mulder-Stapel A, Hermans J, van de Velde CJ, et al. Local and distant recurrences in rectal cancer patients are predicted by the nonspecific immune response; specific immune response has only a systemic effect–a histopathological and immunohistochemical study. BMC Cancer. 2001;1:7.
Droin N, Hendra JB, Ducoroy P, Solary E. Human defensins as cancer biomarkers and antitumour molecules. J Proteomics. 2009;72(6):918–27. doi:10.1016/j.jprot.2009.01.002.
Wong CP, Bray TM, Ho E. Induction of proinflammatory response in prostate cancer epithelial cells by activated macrophages. Cancer Lett. 2009;276(1):38–46. doi:10.1016/j.canlet.2008.10.025.
Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Okumura H, Matsumoto M, et al. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res. 2003;23(5A):4079–83.
Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, Tominaga M, et al. Prognostic value of preoperative peripheral blood monocyte count in patients with colorectal liver metastasis after liver resection. J Gastrointest Surg. 2007;11(5):596–602. doi:10.1007/s11605-007-0140-0.
Graziosi L, Marino E, De Angelis V, Rebonato A, Cavazzoni E, Donini A. Prognostic value of preoperative neutrophils to lymphocytes ratio in patients resected for gastric cancer. Am J Surg. 2015;209(2):333–7. doi:10.1016/j.amjsurg.2014.06.014.
Subimerb C, Pinlaor S, Lulitanond V, Khuntikeo N, Okada S, McGrath MS, et al. Circulating CD14(+) CD16(+) monocyte levels predict tissue invasive character of cholangiocarcinoma. Clin Exp Immunol. 2010;161(3):471–9. doi:10.1111/j.1365-2249.2010.04200.x.
Shamamian P, Schwartz JD, Pocock BJ, Monea S, Whiting D, Marcus SG, et al. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol. 2001;189(2):197–206. doi:10.1002/jcp.10014.
Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58(1):15–23. doi:10.1007/s00262-008-0516-3.
Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology-Basel. 2007;73(3–4):215–20. doi:10.1159/000127412.
Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol. 1985;134(1):230–4.
Crozier JE, McKee RF, McArdle CS, Angerson WJ, Anderson JH, Horgan PG, et al. Preoperative but not postoperative systemic inflammatory response correlates with survival in colorectal cancer. Br J Surg. 2007;94(8):1028–32. doi:10.1002/bjs.5706.
Conflict of interest
The authors declare no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Long Jiang, Zerui Zhao and Shanshan Jiang have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Jiang, L., Zhao, Z., Jiang, S. et al. Immunological markers predict the prognosis of patients with squamous non-small cell lung cancer. Immunol Res 62, 316–324 (2015). https://doi.org/10.1007/s12026-015-8662-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-015-8662-0