Abstract
Helminth-derived products, either released into the circulation during the course of the infection or isolated after in vitro cultivation of the parasite and applied by the injection, are able to suppress the host immune response to autoantigens and allergens, but mechanisms could differ. Prophylactic application of Trichinella spiralis excretory–secretory muscle larvae (ES L1) products ameliorates experimental autoimmune encephalomyelitis (EAE) with the same success as infection did. However, a shift to the Th2-type response in the periphery and in the central nervous system, accompanied by activation of regulatory mechanisms, had a striking, new feature of increased proportion of unconventional CD4+CD25−Foxp3+ regulatory cells both in the periphery and in the central nervous system of animals treated with ES L1 before the induction of EAE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the relationship between infectious agents and autoimmunity still remains a key issue in immunology. Despite the large body of evidence supporting the contention that infections can cause autoimmunity, numerous epidemiological and experimental studies have suggested that exposure to pathogens early in life could ensure proper development of immunoregulatory mechanisms and hence decreases the risk of autoimmune diseases [1, 2]. Throughout evolution, the immune system has evolved in the continuous presence of a vast number of microorganisms and those organisms became essential for its proper development. The impact of pathogens made the immune system capable of mounting appropriate defensive responses, while the presence of organisms such as helminths or microbiota influenced the development of immunoregulatory mechanisms essential for immune tolerance. Nowadays, in economically developed countries, with a high level of health care and sanitation, the immune system is deprived of contact with some organisms that have shaped it during coevolution, which might cause dysregulation leading to the development of inflammatory disorders such as autoimmune diseases [3].
Infections with parasitic helminths usually are not life threatening, and host “tolerates” the presence of parasites in such way that clinical symptoms fade during the infection course and become either restricted or disappear. During coevolution, both helminth parasites and their hosts developed specific adaptations that enabled each participant in the complex relationship to survive. The key adaptation mechanism is immunomodulation that is beneficial to both the host and the parasite, since this can protect the host from unnecessary damage caused by excessive inflammatory responses and at the same time protect the parasites from elimination. This modulation of the host immune response does not refer only to helminth antigens, but to nonrelated antigens as well [4]. The beneficial effect of helminth infections on the outcome of different autoimmune diseases such as multiple sclerosis (MS) [5], inflammatory bowel disease [6] and type 1 diabetes mellitus (T1D) [7] has been already demonstrated. Results from experimental models led to a series of clinical trials and studies of live parasitic infestations in patients with Crohn’s disease, ulcerative colitis and MS [3, 8]. Parasite-driven protection from autoimmune diseases was associated with induction of a Th2 response and a network consisting of regulatory T cells (Tregs) (either Foxp3+ or Foxp3−), CD8+ Tregs, regulatory B cells, alternatively activated macrophages and regulatory/anti-inflammatory cytokines such as IL-10 and TGF-β [9]. Regulatory T cells (Tregs) are, however, key components of the immune regulatory network capable of suppressing not only the specific response against the parasite but also toward autoantigens [10].
Experimental autoimmune encephalomyelitis (EAE) has been widely used as an animal model for the human disease MS [11, 12]. The inflammatory process in both MS and EAE has been considered to be a consequence of activation of a Th1/Th17 cytokine cascade [13]. Th1 and Th17 cells can be suppressed by Tregs but their activity seems to be impaired in patients with MS [14]. Parasites, especially helminths, might be very helpful in restoring homeostasis, since they are able to promote normal immune regulation and suppress immunopathology during autoimmune diseases [3, 15]. Experimental models have shown that infection with Schistosoma mansoni [16], Trypanosoma cruzi [17] or Trichinella spiralis (T. spiralis) [18, 19] suppressed the development of EAE. However, since treatment with live worms carries certain risks, investigations have focused on identifying helminth-derived molecules with immunomodulatory capacities that could be safely administered to patients [20]. One of the most studied helminth-derived products is ES-62, a molecule from Acantocheilonema viteae that possesses the capacity to modulate both Th1- and Th2-mediated diseases [21]. Excretory–secretory products (ES L1) released from T. spiralis muscle larvae during the chronic phase of the infection are most likely to be important for communication with the host organism. Besides their participation in establishing parasitism [22], these molecules are undoubtedly involved in the modulation of the host immune response, thereby creating an environment suitable for the survival of both the parasite and the host organism. Here, we investigated the potential of T. spiralis ES L1 products to promote immune regulation and control the development of EAE. We have provided evidence for the immunomodulatory capacity of T. spiralis muscle larvae ES L1 products, represented by their beneficial effect on the outcome of EAE. We also explored mechanisms by which ES L1 acts to alleviate the disease.
Materials and methods
Animals
Dark Agouti (DA) rats were bred and housed at the Military Medical Academy in Belgrade. Animals were used at 12–14 weeks of age, and they had free access to food and water. All animal experiments were performed according to institutional guidelines and were approved by the local Institutional Animal Care and Use Committee.
Antigen preparation
Trichinella spiralis strain (ISS 161) was maintained and recovered from muscles of infected Wistar rats by digestion of the carcasses in prewarmed gastric juice [23]. Muscle larvae were kept under controlled conditions (37 °C, 5 % CO2) in complete Dulbecco’s modified Eagle’s medium (DMEM) (Sigma, St Louis, MO), for 18 h [24]. Excretory–secretory products of the muscle larvae (ES L1) were obtained by dialysis and concentration of culture supernatants and were kept at −20 °C until further use.
EAE induction and evaluation
EAE was induced in DA rats by a single intradermal injection (0.1 ml), in the right hind footpad, with an encephalitogenic emulsion prepared by mixing rat spinal cord (SC) tissue homogenate (50 % w/v in saline) emulsified in an equal amount of complete Freund’s adjuvant (CFA; Difco, Detroit, MI) containing 4 mg/ml Mycobacterium tuberculosis (Difco). Starting from day 8 postimmunization (pi), rats were weighed and assessed for signs of the disease daily as follows: 0 = no clinical signs; 1 = flaccid tail; 2 = hind limb paresis; 3 = complete bilateral hind limb paralysis often associated with incontinence; and 4 = moribund state or death. Intermediate scores were assigned if neurological signs were of lower severity than observed typically. Several disease parameters were examined to evaluate the severity of EAE: incidence, mean day of onset, duration of illness, mean maximal severity score (the mean of the maximal clinical score that each rat in a group developed over the course of the experiment) and cumulative disease index (the sum of the daily mean clinical scores for a group over a given number of days). Animals were observed for 25 days after the immunization.
In vivo treatment with ES L1 antigens
DA rats were divided into five groups (5 animals/group), and each group was subjected to EAE induction. Prior to this, the control group (marked EAE) received a PBS injection, while the experimental groups were injected intraperitoneally (i.p.) with different amounts of T. spiralis ES L1 products in PBS: Dose of 150 µg was administered once (ES L1/150) 7 days before EAE induction, or twice (ES L1/2x150) 14 and 7 days before EAE induction; 250 µg dose was administered once (ES L1/250) 7 days before EAE induction, or twice (ES L1/2x250) 14 and 7 days before EAE induction.
For the analyses of cytokine production and the presence of regulatory T cells at different phases of EAE development, ES L1-treated or untreated EAE-induced animals were killed during the inductive (day 8 pi), effector (day 15 pi) and recovery phases (day 25 pi) and cells were harvested from spleens and CNS.
Cells
Spinal-cord-infiltrating cells were obtained from the SC of rats perfused with sterile PBS. They were homogenized, adjusted to 30 % Percoll (Sigma) and overlaid on a 40 %/70 % Percoll gradient. Following centrifugation at 700×g for 20 min, mononuclear cells were recovered from the 40/70 % Percoll interface and washed in RPMI medium (Sigma). The cells obtained from spleens and SC were used for assessment of cytokine production and stained for markers of Tregs, CD4, CD25 and Foxp3.
Bone marrow-derived DCs (BMDCs) were generated from male DA rats 16–18 weeks old according to the procedure described previously [25]. DCs were obtained by culturing bone marrow cells from DA rats in RPMI-1640 supplemented with 10 % FCS, 2 mM l-glutamine, 1 mM Na-pyruvate, 10 mMHepes, 50 µM 2-ME (Sigma), 50 U/ml gentamycin (Galenika, Belgrade, Serbia) and growth factors: 25 ng/ml GM-CSF, 25 ng/ml IL-4 and 25 ng/ml Flt-3 ligand (all from Biosource Invitrogen, Camarillo, CA). Cells were maintained at 37 °C in a humidified CO2 incubator, and fresh medium was added on days 3 and 6. On day 8, BMDCs (5 × 106cells/well) were pulsed for 48 h with T. spiralis ES L1 antigens (50 μg/ml), myelin oligodendrocyte glycoprotein (MOG)63–87 peptide (10 μg/ml) (a kind gift from Prof Dr M. Lukic, Center for Molecular Medicine and Stem Cell Research, Faculty of Medical Sciences, University of Kragujevac, Serbia) and the combination of MOG peptide and ES L1 (except that ES L1 was added for the final 24 h), or left unstimulated by cultivation in medium alone. At day 10, supernatants were collected for measuring cytokine levels, while unstimulated and stimulated DCs were co-cultured with T lymphocytes.
T lymphocytes were isolated from popliteal draining lymph nodes (DLN) of EAE-immunized animals on day 8 pi, using Pan T isolation microbeads on a MiniMACS separation column (Miltenuyi Biotech, Auburn, CA) according to the manufacturer’s instructions.
Cell cultures
Spleen cells were seeded in 24-well plates (2 × 106/well) and cultivated at 5 % CO2 and 37 °C in RPMI-1640 medium (PAA Laboratories, Pasching, Austria) supplemented with antibiotics (Galenika, Belgrade, Serbia), 5 % FCS and in the presence of concanavalin A (2.5 µg/ml) (ConA, INEP, Belgrade, Serbia) or in medium alone for 48 h.
SC-infiltrating cells were cultured in 96-well plates (5 × 105/ml) at 5 % CO2 and 37 °C in RPMI-1640 medium supplemented with antibiotics, 2 % rat serum, without stimulation, for 72 h.
Culture supernatants from spleens and SC were collected and analyzed for cytokines.
Purified T cells obtained from DLN of EAE-immunized animals were plated in round-bottomed 96-well plates at 1 × 105/well, and for determination of proliferation capacity, cells were co-cultivated with unstimulated and stimulated DCs (1 × 104, 0.5 × 104, 0.25 × 104/well) for 2 days. In some DC/T cell co-cultures, T cells were stimulated with MOG peptide (10 μg/ml). They were pulsed with 1 μCi/well 3H thymidine (Amersham, Amersham, UK) for an additional 18 h and measured for 3H thymidine incorporation. For determination of cytokine production, purified T cells (2.5 × 106/well) were co-cultivated with stimulated or unstimulated DCs (5 × 105/well) in 24-well plates in a final volume of 1 ml for 48 h. Again, in some DC/T cell co-cultures, T cells from EAE-immunized animals were stimulated with MOG peptide (10 μg/ml). Culture supernatants were collected and stored at −20 °C for cytokine analyses.
Cytokine ELISAs
Cytokines (IL-12p70, IL-4, IL-10, IL-17, IFN-γ and TGF-β) were measured in culture supernatants using commercially available ELISA kits (for IL-4, IL-10, IFN-γ BD Biosciences, San Jose, CA; for IL-12p70 and IL-17 PeproTech, Rocky Hill, NJ; for TGF-β R&D Systems, Minneapolis, MN). Cell culture supernatants were analyzed according to the manufacturer’s instructions.
Flow cytometry analysis
Spleen and SC cells from each animal were prepared for phenotype analysis and intracellular staining. For surface antigen staining, cells were incubated with the following anti-rat antibodies: FITC-labeled anti-CD4 and PE-labeled anti-CD25 (both purchased from eBioscience, San Diego, CA). After washing, intracellular staining was performed using a Foxp3 Staining Buffer Set and PECy5-labeled anti-rat Foxp3 antibody according to the manufacturer’s protocol (eBioscience). Stained cells were analyzed using the CyFlowCL (Partec, Munster, Germany). For characterization of lymphocytes, at least 10 000 events were collected. Data were analyzed with FlowJo software (TreeStar, Ashland, OR).
Statistical analysis
Statistical comparisons between groups were made using the Student’s unpaired or paired t test and the Mann–Whitney test. The results are presented as mean ± SD. Differences with probability (p) values less than 0.05 were considered statistically significant.
Results
Application of ES L1 antigens ameliorates EAE
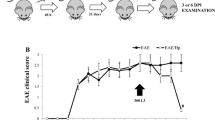
Previously Sofronic et al. [26] demonstrated that T. spiralis ES L1 antigens, acting via DCs, possess immunomodulatory capacity, which was indicated by alleviation of EAE in DA rats. This study was focused on the investigation whether direct application of isolated ES L1 to DA rats could ameliorate EAE. The first task was to determine whether chosen doses of ES L1 in applied time intervals influence the course of the disease. Groups of DA rats injected with ES L1 as indicated in Materials and Methods, as well as the control PBS-injected group, were subjected to EAE induction and further monitored for the development of clinical signs and body weight loss. The changes in average body weight were not significantly different between the groups injected with ES L1 or PBS (data not shown). DA rats that had received ES L1 antigens exhibited milder signs of EAE compared to the control EAE group (Fig. 1a, b), with reduced clinical parameters (except maximal clinical score for ES L1/2x150, Fig. 1c) and significantly lower cumulative indices (except for ES L1/150, Fig. 1d). The onset of disease was not altered, but the duration of illness was significantly shorter in all ES L1-treated animals than in control rats (Fig. 1c). Moreover, according to the obtained results, the dose that showed the most convincing effect on the course of EAE was 250 µg given twice at weekly intervals, i.e., 14 and 7 days before EAE induction. This dose was applied in further studies related to the mechanisms underlying the observed immunomodulation. Rats injected twice i.p. with 250 µg of ES L1 antigens or PBS were subjected to EAE induction and analyzed for T cell response at different time points during the course of EAE, i.e., at induction (day 8 pi), effector (day 15 pi) and recovery phase (day 25 pi) of the disease. The groups of ES L1-treated and untreated, EAE-immunized DA rats that were intended to be analyzed in the recovery phase were also monitored for disease course. EAE was reduced in severity in animals treated with ES L1 antigens prior to EAE induction (Fig. 2). The cumulative index for the ES L1-treated group was 0.32 ± 0.08, while for the control group, it was 0.97 ± 0.18 (Fig. 2b). The difference clearly shows that treatment with ES L1, performed as described above, had a significant impact on disease development.
ES L1 ameliorates EAE in DA rats. Groups designated ES L1/150 + EAE and ES L1/250 + EAE were injected once i.p. with 150 and 250 µg of ES L1, respectively, 7 days before EAE induction. Groups marked ES L1/2x150 + EAE and ES L1/2x250 + EAE were injected i.p. with the indicated amounts of ES L1 antigens twice, 14 and 7 days before EAE induction. Animals treated with PBS at the same times as indicated for ES L1 treatment were used as the control group. Clinical score on each day of the disease if the 150-µg ES L1 dose was applied once or twice before EAE induction (a); clinical score on each day of the disease if the 250-µg ES L1 dose was applied once or twice before EAE induction (b); parameters of illness: incidence, mean day of onset, duration of illness, and mean maximal clinical score (c); cumulative index of the disease for each group (d). Data represent the mean ± SD of results from two independent experiments. *p < 0.05; **p < 0.01
Course of EAE in ES L1-treated and untreated DA rats used for in vitro examination. Rats injected i.p. with 250 μg of ES L1 antigens or PBS twice, 14 and 7 days before EAE induction, were divided into groups (5 animals/group) and killed at the indicated time points (arrows). The presented results concern groups analyzed at the end of the observation period (day 25 pi, recovery phase). The following parameters were monitored: clinical score on each day of the disease (a), cumulative index of the disease (b) and incidence, mean day of onset, duration of illness, mean maximal clinical score (c). Data represent the mean ± SD of results from two independent experiments. *p < 0.05; **p < 0.01
Production of cytokines by SC-infiltrating cells
SC-infiltrating cells were isolated in the inductive (8 day pi), effector (15 day pi) and recovery (25 day pi) phase during EAE from ES L1-treated and control animals. The production of cytokines IL-4, IL-10, IL-17, IFN-γ and TGF-β was determined in cells cultivated in medium alone. The release of IFN-γ by SC-infiltrating cells from treated animals was lower than in control groups during all phases of EAE but the difference reached statistical significance only in the inductive phase of the disease (Fig. 3a). IL-17 production presented the same profile as IFN-γ, characterized by diminished production throughout the disease in ES L1-treated animals. Significantly lower levels of IL-17 were detected in the inductive and recovery phase of EAE, but not at the peak of the disease (Fig. 3b). During the course of EAE, release of Th2 and regulatory cytokines was higher in treated animals compared to control rats. Thus, IL-4 release was significantly elevated in ES L1-treated rats with values almost twice as high as in EAE animals (Fig. 3c). IL-10 production could not be detected in the inductive phase of the disease in the culture supernatants of SC-infiltrating cells. Nevertheless, release of this cytokine was greatly enhanced as the disease progressed and peaked in the effector phase, with reduced, but still significantly elevated levels in the recovery phase (Fig. 3d). On the other hand, there was no significant difference in the production of TGF-β between ES L1-treated and untreated groups during the inductive and effector phases (Fig. 3e). Only in the recovery phase of EAE, did production of TGF-β by SC-infiltrating cells from ES L1-treated animals attain a significant increase when compared to the control.
Effect of in vivo ES L1 treatment on cytokine production by SC-infiltrating cells during EAE. Cytokine production by SC-infiltrating cells was measured in the inductive (8 day pi), effector (15 day pi) and recovery (25 day pi) phase during EAE from ES L1 (EAE/ES L1)-treated and control (EAE) animals. Data represent the mean ± SD of results from two independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001
Production of cytokines by spleen cells
Spleen cells harvested at the indicated time points throughout the disease were cultivated in medium alone or stimulated with T cell mitogen ConA. Supernatants were analyzed for cytokine content as an indicator of the immune response at the systemic level during the course of EAE. Figure 4 displays production of cytokines by splenocytes from treated and untreated, EAE-induced rats cultivated in medium alone. Production of IFN-γ in ES L1-treated animals was reduced compared to untreated EAE animals, during the course of the disease, but the difference reached statistical significance only in the inductive and recovery phases (Fig. 4a). Release of IL-17 declined throughout the disease in animals treated with ES L1, when compared to controls also leading to a statistically significant difference in the inductive and recovery phases of EAE (Fig. 4b). Thus, ES L1 treatment of DA rats markedly suppressed the release of IFN-γ and IL-17, mediators of disease induction and progression. On the other hand, splenocytes from ES L1-treated, EAE-immunized animals produced greatly elevated levels of the Th2 cytokine IL-4 in the inductive and effector phases of EAE, when compared to control EAE rats (Fig. 4c). Production of the regulatory cytokines IL-10 and TGF-β was hardly affected by treatment with ES L1. Only at the peak of the disease did spleen cells from ES L1-treated animals release somewhat more IL-10 than controls, but without statistical significance (Fig. 4d), while levels of TGF-β were slightly higher in treated than in untreated animals in all EAE phases, especially during recovery, but the differences were not statistically significant (Fig. 4e). ConA stimulation provoked higher levels of all secreted cytokines but did not change the pattern of release (data not shown).
Effect of in vivo ES L1 treatment on cytokine production by spleen cells during EAE. Cytokine production by spleen cells was measured in the inductive (8 day pi), effector (15 day p.) and recovery (25 day pi) phase during EAE from ES L1 (EAE/ES L1)-treated and control (EAE) animals. Data represent the mean ± SD of results from two independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001
Quantification of Foxp3+ T cells in SC-infiltrating cells and spleen
SC-infiltrating and spleen cells were analyzed for the presence of CD4+CD25+Foxp3+ cells during the course of EAE, at day 8, 15 and 25 pi. Flow cytometric analysis of SC-infiltrating cells revealed that the percentage of CD4+CD25+Foxp3+ T cells was higher in the ES L1-treated, EAE-immunized group than in controls throughout the disease, and the difference reached statistical significance during the inductive and the recovery phases of EAE (Fig. 5b). Surprisingly, a very high percentage of CD4+ Foxp3+ cells that did not express CD25 were found among SC-infiltrating cells from ES L1-treated animals, on day 15 pi (Fig. 5b). An increased proportion of CD4+CD25−Foxp3+ cells were still present on 25 day pi in ES L1-treated compared with untreated, EAE-induced animals. The number of CD4+CD25−Foxp3+ cells was low in the control EAE-immunized group throughout the disease (Fig. 5b).
Foxp3+ expression in SC-infiltrating cells during the course of EAE. Representative plots for each time point from one of two experiments present CD25 versus Foxp3, gated on CD4+ T cells (a); the percentage of different T regulatory cell populations, CD4+CD25+Foxp3+ (left panel) and CD4+CD25−Foxp3+ (right panel) in the inductive (8 day pi), effector (15 day pi) and recovery (25 day pi) phase during EAE from ES L1 (EAE/ES L1)-treated and control (EAE) animals (b). Data represent the mean ± SD of results from two independent experiments. *p < 0.05; ***p < 0.001
Spleen cells were also analyzed for the presence of Foxp3+ T cells during the course of EAE. At day 8 pi, the percentage of CD4+CD25+Foxp3+ cells was low in both ES L1-treated and untreated animals (Fig. 6b). CD4+CD25+Foxp3+ T cells showed expansion at the effector and the recovery phases in ES L1-treated, EAE-induced rats, but when compared to controls, the increase was significant only on day 15 pi, in the effector phase of the disease (Fig. 6b). In the control EAE group, the number of CD4+CD25+Foxp3+ cells was low in the inductive and effector phases of the disease, and only started to rise in the recovery phase (Fig. 6b). Again, the striking feature of ES L1 treatment was the appearance of CD4+CD25−Foxp3+cells among spleen cells isolated these EAE-immunized rats (Fig. 6b). This cell population could only be seen at day 15 pi, in a number that greatly exceeded that found in control animals. Unlike the situation with SC-infiltrating cells, CD4+CD25−Foxp3+ cells could not be detected among spleen cells from ES L1-treated animals in the recovery phase.
Foxp3+ expression in spleen cells during the course of EAE. Representative plots for each time point from one of two experiments show CD25 versus Foxp3, gated on CD4+ T cells (a); the percentage of different T regulatory cell populations, CD4+CD25+Foxp3+ (left panel) and CD4+CD25−Foxp3+ (right panel) in the inductive (8 day pi), effector (15 day pi) and recovery (25 day pi) phase during EAE from ES L1 (EAE/ES L1)-treated and control (EAE) animals (b). Data represent the mean ± SD of results from two independent experiments. *p < 0.05; ***p < 0.001
Induction of CD4+CD25−Foxp3+ cells in healthy rats treated with ES L1
It was obvious that application of ES L1 antigens before EAE induction provoked a significant expansion of CD4+CD25−Foxp3+ cells at the periphery and in the target organ, since these cells were absent from the spleen and SC infiltrates in untreated animals. Support to this finding was provided by the results obtained when healthy DA rats were injected with the same amount of ES L1 antigens at the same time intervals and were analyzed for the presence of CD4+CD25−Foxp3+ and CD4+CD25+Foxp3+ among spleen cells obtained at different time points corresponding with EAE induction (day 0), and days 8, 15 and 25 pi. Control rats were treated with PBS. The percentage of both CD4+CD25−Foxp3+ and CD4+CD25+Foxp3+ cells in control rats was very low (Fig. 7a,b). However, ES L1 treatment provoked expansion of both types of cells (Fig. 7a,b). The significant increase of CD4+CD25+Foxp3+ cells was detected as soon as 7 days after antigen application (time that corresponds to day 0 of EAE induction) and persisted thereafter (Fig. 7a). CD4+CD25−Foxp3+ cells were detected at day 8 (time that corresponds to day 8 after EAE induction), in a number five times greater than the control value (Fig. 7b). The relative number of these cells slowly declined till the end of the observation period, but remained significantly higher than in the controls.
Induction of CD4+CD25−Foxp3+ cells in healthy rats treated with ES L1. Percentage of CD4+CD25+Foxp3+ (a) and CD4+CD25−Foxp3+ (b) at different time points corresponding with EAE induction (day 0), and days 8, 15 and 25 pi. PBS-treated animals served as controls. Data represent the mean ± SD of results from two independent experiments. **p < 0.01; ***p < 0.001
The impact of ES L1 products on DC and T cell activity in vitro
Results presented in this paper point out that ES L1, applied before EAE induction, could successfully alleviate this autoimmune disease. The impact of ES L1, applied intraperitoneally, is most likely mediated through DCs, key cells for initiation, progression and regulation of the immune response. It was already shown that stimulation of DCs with ES L1 antigens in vitro provoked the release of IL-10 and lowered that of IL12p70 from DCs [27]. Here, we are proving that ES L1 could alter the existing phenotype of DCs that had already been in contact with encephalitogenic antigens, like MOG peptide. DCs stimulated with MOG peptide (DC/MOG), ES L1 antigens (DC/ES L1) and a combination of MOG and ES L1 (DC/48hMOG24hESL1) were examined for cytokine production. It was observed that ES L1, added to the culture of MOG-pulsed DCs, significantly reduced the release of IL-12p70 and increased that of IL-10, compared to DC/MOG alone (Fig. 8a). It can be concluded that ES L1 products possess the capacity to modify cytokine secretion from DCs already primed with MOG.
Impact of ES L1 on DC and T cells in vitro. DCs were stimulated with ES L1 (DC/ES L1), MOG (DC/MOG), MOG for the whole 48 h and ES L1 for the final 24 h (DC/48hMOG/24hES L1) or were cultivated in medium alone (unstimulated; DC/med). The production of IL-10 and IL-12p70 from DCs (a); cytokine profile of encephalitogenic T cells isolated from DLN of EAE-induced animals and co-cultivated with stimulated or unstimulated DCs (b); the impact of ES L1-stimulated DCs on cytokine production of encephalitogenic T cells restimulated with MOG (c). Data represent the mean ± SD of results from two independent experiments. #Statistical significance obtained when results were compared with DC/MOG. ### p < 0.001. *Statistical significance obtained when results were compared to DC/med *p < 0.05; **p < 0.01; ***p < 0.001
To explore whether ES L1-provoked change in DC activation is reflected on autoreactive T cells, stimulated and unstimulated DCs were co-cultivated with T cells isolated from DLN of EAE-immunized animals. T cells, cultivated in vitro with DC/MOG, produced elevated levels of IL-17 compared to DC/med (Fig. 8b). ES L1-stimulated MOG-pulsed DCs altered the cytokine profile of T cells by causing significant reduction in IL-17 secretion and to a lesser extent IFN-γ in comparison with DC/MOG (Fig. 8b). On the other hand, the presence of ES L1-stimulated MOG-pulsed DCs provoked strong release of IL-4 and IL-10 from T cells, compared to DC/MOG. The impact of ES L1 on MOG-pulsed DCs was also confirmed by reduced proliferation of T cells isolated from EAE-immunized animals compared to co-cultures with DC/MOG (3.375 ± 177 for DC/48hMOG24h ESL1 vs. 5.823 ± 183 for DC/MOG, p < 0.001).
The influence of ES L1 products on the activity of T cells in EAE-induced rats was assessed by co-cultivation of ES L1-stimulated DCs with autoreactive T cells, restimulated with MOG. ES L1-stimulated DCs significantly suppressed the production of IL-17 and IFN-γ (Fig. 8c), compared with T cells cultivated with unstimulated DCs (DC/med). On the other hand, DC/ES L1 provoked eightfold greater release of IL-4 from autoreactive T cells stimulated with MOG, accompanied by increased levels of IL-10 and TGF-β, in comparison with T cells co-cultivated with DC/med. ES L1-stimulated DCs affected cell proliferation as well, significantly reducing the proliferative potential of T cells originated from EAE-induced rats (2.750 ± 196 for DC/ES L1 vs. 4.918 ± 167 for DC/med, p < 0.001).
Discussion
The causal relationship between the incidence of inflammatory disorders, such as autoimmune diseases, and helminth infections, as explained by the “Hygiene hypothesis,” ranks these organisms among those important for the proper development of the immune system [6]. Many studies are focused on understanding the complex cellular and molecular mechanisms that regulate the host immune response during parasitic infections, especially those that strive to define the nature and role of parasite-derived products responsible for protection from immunopathology. Among other helminthes, T. spiralis demonstrated capacity to improve the outcome of EAE. Namely, chronic infection with this parasite significantly ameliorated EAE [19]. The chronic phase of the infection is characterized by the presence of encapsulated larvae (cysts) in infected muscles that continually communicate with the host through ES L1 products, which influence the host immune system. Therefore, amelioration of EAE by T. spiralis infection could be ascribed to these products, but the direct impact of ES L1 on the disease course has not been explored so far. Our research was set up to investigate the immunomodulatory capacities of T. spiralis ES L1 antigens prophylactically applied to animals before EAE induction. ES L1 antigens provided significant amelioration from EAE, as reflected in the lower maximal clinical score and cumulative index and reduced duration of illness in ES L1-treated animals. This indicated that the applied parasite antigens could influence the course of CNS autoimmunity. The capacity of T. spiralis ES L1 antigens to modulate the immune response in EAE-induced animals was demonstrated by a shift to the Th2-type response in the periphery and CNS and activation of regulatory mechanisms. Kuijk et al. [28] demonstrated successful amelioration of EAE using worm antigens, but unlike us they applied soluble extracts of T. suis and T. spiralis larvae, without addressing mechanisms underlying observed phenomenon. Motomura and coworkers [29] also used T. spiralis larval crude muscle antigens for prophylactic treatment of experimentally induced colitis in mice. The results were satisfactory, since the severity of the disease and mortality rate were significantly reduced. Our results also correlate with the study on treatment with Shistosoma egg antigen (SEA) antigens from Shistosoma japonicum before EAE induction or during the preclinical phase. This treatment resulted in amelioration of the severity and progression of EAE [30]. Moreover, exposure to the phosphorylcholine containing glycoprotein ES-62, secreted by the filarial nematode, Acanthochelionema vitae, prevented initiation and progression of collagen-induced arthritis in a murine model [31].
The effect of ES L1 was most prominent on the production of the Th2-type cytokine IL-4, both at the periphery and in the target organ, which could suppress the release of Th1 cytokines such as IFN-γ. Indeed, significant reduction in the levels of IFN-γ and IL-17, cytokines crucial for the induction and progression of the disease, was observed in EAE animals treated with ES L1. This treatment resulted in high-level production of IL-4 throughout the disease. We assume that the ES L1-induced switch of the immune response to Th2 type and downregulation of the Th1/Th17 response was partly responsible for mitigation of the severity of EAE. The suppressive effect of ES L1 antigens on pro-inflammatory cytokines could also be explained through impact of the anti-inflammatory cytokine IL-10, abundantly produced in the CNS during the course of EAE in ES L1-treated animals. The influence of ES L1 on TGF-β production of was negligible, indicating that immunomodulation provoked by ES L1 is not dependent of TGF-β. Prophylactic treatment with ES L1 antigens creates a cytokine milieu that prevents the disease from developing to the extent seen in untreated EAE rats. In a model of EAE treatment with S. japonicum egg antigens amelioration of the disease was accompanied by down-modulation of IFN-γ production and the induction of IL-4 [30]. Moreover, intraperitoneal injection of soluble proteins isolated from Schistosoma mansoni eggs (SEA) inhibited the development of type 1 diabetes (T1D) in NOD mice by inducing Th2/regulatory type of response [32]. On the other hand, ES-62 reduced the severity of developing collagen-induced arthritis by suppression of antigen-specific T helper (Th)1-type cytokine production and collagen-specific immunoglobulin levels, with no increase in Th2 responses [21]. It is clear that the impact of helminth infections or their products on the development of various inflammatory disorders differs, depending on the helminth species, the nature of their products and on the host organism.
ES L1 antigens may also act on autoreactive T cells through local DCs, altering their maturation status. Recently published results demonstrated that DCs are rendered tolerant by exposure to T. spiralis ES L1 antigens, suggesting that the observed suppression could be promoted by DCs/ES L1 [27]. Indeed, DC/ES L1 revealed suppressive activity on the response of T cells from EAE-immunized animals stimulated with MOG peptide, which was manifested in reduced cell proliferation and IFN-γ and IL-17 secretion. On the other hand, DC/ES L1 provoked abundant production of IL-4 and increased secretion of anti-inflammatory cytokines from T cells originating from EAE-induced rats, which could indicate their capacity to modify the function of existing autoreactive T cells. ES L1 antigens could also act by altering the maturation status of already existing DCs in EAE-induced animals. We have demonstrated that ES L1 possess the capacity to modulate cytokine production of DCs previously pulsed with MOG (DC/48hMOG24hESL1), by suppressing IL-12 and enhancing IL-10 release. DCs treated this way had the potential to modify the pattern of cytokine secretion of T cells from EAE-induced animals, when compared to co-cultures with DC/MOG. These results, although obtained in vitro, are promising, since they could indicate the potential of ES L1 antigens to change the course of an autoimmune disease by modulating the activity of existing immune cells, both innate and adaptive in nature.
ES L1 also facilitated induction/expansion of CD4CD25Foxp3-positive T cells, which persisted in increased proportions in both the CNS and spleen of treated rats throughout the disease. The enhanced presence of these cells in the spinal cord of DC/ES L1 recipients correlated with significant reduction in EAE severity. It was already emphasized that Treg cells are actively involved in diminishing the severity and in resolution of the disease [33]. Depletion of Treg cells prior to active immunization increased the severity of EAE, accompanied by enhanced IFN-γ and IL-17 production, indicating the importance of Tregs in restraining inflammatory responses and autoreactive effector cells [34, 35]. Key mediators of Treg suppressive activity are IL-10 and TGF-β [36]; IL-10 being recognized as a crucial component in Treg mediated suppression of EAE [37]. Evidence from investigations with different parasite antigens revealed that they promote the host regulatory network and stimulate activation of regulatory CD4+CD25+Foxp3+ T cells responsible for suppression of autoreactive effector T cells [38–40]. What was intriguing about our findings was the large proportion of unconventional CD4+ T cells in the spleen and spinal cords of EAE-induced rats that received ES L1 treatment. These cells were CD25 negative but they expressed the transcriptional factor Foxp3. Neither infection with T. spiralis [19] nor treatment with ES L1-stimulated DCs [25, 27] gave rise to the population of CD4+CD25−Foxp3+cells, so we assumed that this phenomenon must be the consequence of application of isolated ES L1 antigens. When we assessed the percentage of CD4+CD25+Foxp3+ and CD4+CD25−Foxp3+ T cells in spleens of DA rats treated with ES L1 antigens, we found increased numbers of both subtypes, with dominance of CD4+CD25−Foxp3+T cells. Why these cells are induced with purified antigens and not by live infection or by DCs stimulated with the same product remains to be investigated. What should be emphasized at this point is that, albeit application of isolated ES L1 products exerted a beneficial effect on the outcome of EAE, it did not provoke the same immune response as live infection. Although attempts to replace live infection with more appropriate treatments are understandable and most welcome, it is possible that isolated products could not replace the evolutionarily created relationship between the host and the parasite. However, administered in a right amount, time and route, maybe they could generate immunomodulation sufficient to overcome this kind of disease, and this is why these products and their effects are extensively investigated.
Based on present knowledge, suppressor activity is not confined to CD25+ T cells, and those within the CD4+CD25− T cell population, CD8+ cells and natural killer (NK) T cells have also been shown to exert an immunosuppressive function in vitro and in vivo [41–43]. CD4+CD25−Foxp3+ T cells were found in both rodents and humans, and it was shown that they possess suppressive activity [44, 45]. Fontenot and coworkers [44] even concluded that Foxp3 expression, and not CD25 expression, is essential for Treg activity in a mice model. The regulatory function of CD25− cells was demonstrated in a study of the protective effect of various populations of CD4+ T cells on diabetes development [46]. Other authors have confirmed the existence of CD4+ regulatory T cells that do not express CD25 and their regulatory function in animal models of autoimmune encephalomyelitis [47, 48], and inflammatory bowel disease [49]. In another model system of bacterial superantigen-induced tolerance, CD4+CD25− T cells exerted their regulatory activity by suppressing staphylococcal enterotoxin B responsiveness [50]. The suppression was mediated by large amounts of secreted IL-10. In our model, spinal-cord-infiltrating cells from ES L1-injected animals produced significantly more IL-10 than cells from untreated EAE animals, and the level of this cytokine correlated with the relative number of CD4+CD25−Foxp3+ T cells within spinal cord infiltrates of these animals. Whether IL-10 originated from CD4+CD25−Foxp3+ T cells or not remains to be elucidated. Immunoregulatory role of CD4+CD25−Foxp3+ T cells was demonstrated in mice infected with Bordetella pertussis [51]. The authors found that this type of cell is the dominant Treg population in the lung, gut and liver and that they act through IL-10 to control the immune response to infective agents and to mediate tolerance. It has been proposed that CD4+CD25−Foxp3+ T cells represent a peripheral reservoir of CD4+CD25+Foxp3+ Treg cells, which could be recruited when needed, e.g., in autoimmune diseases [52]. Several groups have reported increased proportions of CD4+CD25−Foxp3+ T cells in the blood of patients with systemic lupus erythematosus [53, 54]. In a study of multiple sclerosis, patients in remission were shown to have normal levels of CD4+CD25+Foxp3+ Treg cells, but relapsing patients had an increased proportion of systemic CD4+CD25−Foxp3+ Treg cells, and these Treg cells were able to suppress effector T cells in vitro [55].
In conclusion, we have shown that ES L1 antigens possess the capacity to modulate the outcome of the autoimmune disease, EAE, by prophylactic administration. Modulation of EAE with these antigens was achieved by triggering and maintaining a Th2 and anti-inflammatory response and presumably by the induction of Tregs that do not express CD25. Furthermore, it was shown that ES L1 antigens, in vitro, provoked bias in the response of T cells isolated from EAE-induced rats from Th1/Th17 toward the Th2/regulatory type. These antigens also possess the capacity to modulate the function of MOG-stimulated DCs. The results obtained from in vitro experiments gave us only a hint of what effects ES L1 might have if they were applied after EAE induction. This represents a great challenge and sets the task for the future to explore the full immunomodulatory potential of ES L1 antigens in a model of autoimmune disease.
References
Maizels RM, Yazdanbakhsh M. Regulation of the immune response by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–4.
Bach JF. Infections and autoimmune diseases. J Autoimmun. 2005;25:74–80.
Rook GA. Hygiene hypothesis and autoimmune diseases. Clin Rev Allerg Immunol. 2011;42:5–15.
Jackson JA, Friberg IM, Little S, Bradley JE. Immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies? Immunology. 2008;126:18–27.
Fleming JO. Helminth therapy and multiple sclerosis. Int J Parasitol. 2013;43:259–74.
Elliott DE, Weinstock JV. Where are we on worms? Curr Opin Gastroenterol. 2012;28:551–6.
Zaccone P, Cooke A. Helminth mediated modulation of type 1 diabetes (T1D). Int J Parasitol. 2013;43:311–8.
Khan AR, Fallon PG. Helminth therapies: translating the unknown unknowns to known knowns. Int J Parasitol. 2013;43:293–9.
McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev. 2012;25:585–608.
McSorley HJ, Hewitson JP, Maizels RM. Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol. 2013;43:301–10.
Swanborg RH. Experimental autoimmune encephalomyelitis in rodents as a model for human demyelinating disease. Clin Immunol Immunopathol. 1995;77:4–13.
Handel AE, Lincoln MR, Ramagopalan SV. Of mice and men: experimental autoimmune encephalitis and multiple sclerosis. Eur J Clin Invest. 2011;41:1254–8.
Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–42.
Haas J, Hug A, Viehöver A, Fritzsching B, Falk CS, Filser A, et al. Reduced suppressive effect of CD4+CD25 high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343–52.
Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor M, Allen JE. Helminth parasites: masters of regulation. Immunol Rev. 2004;201:89–116.
La Flamme AC, Ruddenklau K, Backstrom T. Shistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect Immun. 2003;71:4996–5004.
Tadokoro CE, Vallochi AL, Rios LS, Martins GA, Schlesinger D, Mosca T, Kuchroo VK, Rizzo LV, Abrahamsohn IA. Experimental autoimmune encephalomyelitis can be prevented and cured by infection with Trypanosoma cruzi. J Autoimmun. 2004;23:103–15.
Gruden-Movsesijan A, Ilic N, Mostarica-Stojkovic M, Stosic-Grujicic S, Milic M, Sofronic-Milosavljevic L. Trichinella spiralis: modulation of experimental autoimmune encephalomyelitis in DA rats. Exp Parasitol. 2008;118:641–7.
Gruden-Movsesijan A, Ilic N, Mostarica-Stojkovic M, Stosic-Grujicic S, Milic M, Sofronic-Milosavljevic L. Mechanisms of modulation of experimental autoimmune encephalomyelitis by chronic Trichinella spiralis infection in dark agouti rats. Parasite Immunol. 2010;32:450–9.
Harnett W, Harnett MM. Helminth-derived immunomodulators: can understanding the worm produce the pill? Nat Rev Immunol. 2010;10:278–84.
Harnett MM, Melendez AJ, Harnett W. The therapeutic potential of the filarial nematode-derived immunodulator, ES-62 in inflammatory disease. Clin Exp Immunol. 2010;159:256–67.
Nagano I, Wu Z, Takahashi Y. Functional genes and proteins of Trichinella spp. Parasitol Res. 2009;104:197–207.
Gamble HR, Bessonov AS, Cuperlovic K, Gajadhar AA, van Knapen F, Noeckler K, et al. International commission on trichinellosis: recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. Vet Parasitol. 2000;93:393–408.
Ilic N, Worthington JJ, Gruden-Movsesijan A, Travis MA, Sofronic-Milosavljevic L, Grencis RK. Trichinella spiralis antigens prime mixed Th1/Th2 response but do not induce de novo generation of Foxp3+ T cells in vitro. Parasite Immunol. 2011;33:572–82.
Ilic N, Colic M, Gruden-Movsesijan A, Vasilev S, Sofronic-Milosavljevic L. Characterization of rat bone marrow dendritic cells initially primed by Trichinella spiralis antigens. Parasite Immunol. 2008;30:491–5.
Sofronic-Milosavljevic L, Radovic I, Ilic N, Majstorovic I, Cvetkovic J, Gruden-Movsesijan A. Application of dendritic cells stimulated with Trichinella spiralis excretory–secretory antigens alleviates experimental autoimmune encephalomyelitis. Med Microbiol Immunol. 2013;202:239–49.
Gruden-Movsesijan A, Ilic N, Colic M, Majstorovic I, Vasilev S, Radovic I, et al. The impact of Trichinella spiralis excretory–secretory products on dendritic cells. Comp Immunol Microbiol Infect Dis. 2011;34:429–39.
Kuijk LM, Klaver EJ, Kooij G, van der Pol SM, Heijnen P, Bruijns SC, et al. Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol Immunol. 2012;51:210–8.
Motomura Y, Wang H, Deng Y, El-Sharkawy RT, Verdu EF, Khan WI. Helminth antigen-based strategy to ameliorate inflammation in an experimental model of colitis. Clin Exp Immunol. 2009;155:88–95.
Zheng X, Hu X, Zhou G, Lu Z, Qiu W, Bao J, et al. Soluble egg antigen from Schistosoma japonicum modulates the progression of chronic progressive experimental autoimmune encephalomyelitis via Th2-shift response. J Neuroimmunol. 2008;194:107–14.
Harnett MM, Kean DE, Boitelle A, McGuiness S, Thalhamer T, Steiger CN, et al. The phosphorylcholine moiety of the filarial nematode immunomodulator ES-62 is responsible for its anti-inflammatory action in arthritis. Ann Rheum Dis. 2008;67:518–23.
Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol. 2009;39:1098–107.
O’Connor RA, Anderton SM. Foxp3+ regulatory T cells in the control of experimental CNS autoimmune disease. J Neuroimmunol. 2008;193:1–11.
McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–32.
Reddy J, Waldner H, Zhang X, Illes Z, Wucherpfennig KW, Sobel RA, et al. Cutting edge: CD4+CD25+ regulatory T cells contribute to gender differences insusceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2005;175:5591–5.
Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta 1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–7.
Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, et al. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:249–56.
Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–78.
Doetze A, Satoguina J, Burchard G, Rau T, Löliger C, Fleischer B, et al. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int Immunol. 2000;12:623–30.
Metenou S, Dembele B, Konate S, Dolo H, Coulibaly SY, Coulibaly YI, et al. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J Immunol. 2010;184:5375–82.
Garba ML, Pilcher CD, Bingham AL, Eron J, Frelinger JA. HIV antigens can induce TGF-β1-producing immunoregulatory CD8+ T cells. J Immunol. 2002;168:2247–54.
Sonoda KH, Faunce DE, Taniguchi M, Exley M, Balk S, Stein-Streilein J. NK T cell-derived IL-10 is essential for the differentiation of antigen-specific T regulatory cells in systemic tolerance. J Immunol. 2001;166:42–50.
Haynes LM, Vanderlugt CL, Dal Canto MC, Melvold RW, Miller SD. CD8+ T cells from Theiler’s virus-resistant BALB/cByJ mice downregulate pathogenic virus-specific CD4+ T cells. J Neuroimmunol. 2000;106:43–52.
Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41.
Ono M, Shimizu J, Miyachi Y, Sakaguchi S. Control of autoimmune myocarditis and multiorgan inflammation by glucocorticoid-induced TNF receptor family-related protein (high), Foxp3-expressing CD25+ and CD25− regulatory T cells. J Immunol. 2006;176:4748–56.
Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25− subpopulations. J Immunol. 2000;165:3105–10.
Olivares-Villagomez D, Wensky AK, Wang Y, Lafaille JJ. Repertoire requirements of CD4+ T cells that prevent spontaneous autoimmune encephalomyelitis. J Immunol. 2000;164:5499–507.
Furtado GC, Olivares-Villagomez D, Curotto de Lafaille MA, Wensky AK, Latkowski JA, Lafaille JJ. Regulatory T cells in spontaneous autoimmune encephalomyelitis. Immunol Rev. 2001;182:122–34.
Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. CD25+CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–18.
Feunou P, Poulin L, Habran C, Le Moine A, Goldman M, Braun MY. CD4+CD25+ and CD4+CD25− T cells act respectively as inducer and effector T suppressor cells in superantigen-induced tolerance. J Immunol. 2003;171:3475–84.
Coleman MM, Finlay CM, Moran B, Keane J, Dunne PJ, Mills KH. The immunoregulatory role of CD4+ FoxP3+ CD25− regulatory T cells in lungs of mice infected with Bordetella pertussis. FEMS Immunol Med Microbiol. 2012;64:413–24.
Zelenay S, Lopes-Carvalho T, Caramalho I, Moraes-Fontes MF, Rebelo M, Demengeot J. Foxp3+ CD25− CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. PNAS. 2005;102:4091–6.
Lin SC, Chen KH, Lin CH, Kuo CC, Ling QD, Chan CH. The quantitative analysis of peripheral blood FOXP3-expressing T cells in systemic lupus erythematosus and rheumatoid arthritis patients. Eur J Clin Invest. 2007;37:987–96.
Bonelli M, Savitskaya A, Steiner CW, Rath E, Smolen JS, Scheinecker C. Phenotypic and functional analysis of CD4+ CD25− Foxp3+ T cells in patients with systemic lupus erythematosus. J Immunol. 2009;182:1689–95.
Fransson M, Burman J, Lindqvist C, Atterby C, Fagius J, Loskog A. T regulatory cells lacking CD25 are increased in MS during relapse. Autoimmunity. 2010;43:590–7.
Acknowledgments
We wish to thank Prof Dr Marija Mostarica-Stojkovic (Institute of Microbiology and Immunology, School of Medicine, University of Belgrade) for critical reading and valuable suggestions during the preparation of this manuscript. This work was supported by the Ministry of Education, Science and Technological Development, Republic of Serbia (Project 173047).
Conflict of interest
Authors: Ivana Radovic, Alisa Gruden-Movsesijan, Natasa Ilic, Jelena Cvetkovic, Slavko Mojsilovic, Marija Devic and Ljiljana Sofronic-Milosavljevic declare that they have no conflict of interest.
Ethical standard
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radovic, I., Gruden-Movsesijan, A., Ilic, N. et al. Immunomodulatory effects of Trichinella spiralis-derived excretory–secretory antigens. Immunol Res 61, 312–325 (2015). https://doi.org/10.1007/s12026-015-8626-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-015-8626-4