Abstract
Severe combined immunodeficiency (SCID) is a group of syndromes resulting from genetic defects causing absence in T-cell and B-cell function, leading to serious and life-threatening infections. SCID is often fatal in the first 2 years of life if not identified and properly treated. While additional laboratory methods are being developed, the current T-cell receptor excision circle assay has proven to have outstanding specificity and sensitivity to accurately identify infants with SCID and other T-cell lymphopenia. The Jeffrey Modell Foundation (JMF) has a long history of advocacy and continues to promote newborn screening for SCID to be implemented in the United States and worldwide. Based on reports provided by California, New York, Texas, and Wisconsin on the results of their population based newborn screening programs, the overall incidence of SCID averaged 1:33,000 and T-cell lymphopenia averaged 1:6,600. JMF has developed a working algorithm or “decision tree”, validated by peer-reviewed scientific literature, to be used by Public Health Departments and Health Ministries in states, countries, and regions throughout the world. This decision tool allows for local or regional data to be applied to measure the threshold and economic impact of implementing newborn screening for SCID and T-cell lymphopenia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Severe combined immunodeficiency (SCID) is a group of syndromes resulting from genetic defects causing absence in T-cell and B-cell function. These conditions are serious and cause life-threatening infections [1–3]. SCID is often fatal in the first 2 years of life if not identified and properly treated [1–3].
SCID and related conditions can be detected in newborns by a simple screening test, the T-cell receptor excision circle (TREC) assay, using the same dried blood spot samples already collected from newborns to screen for other genetic disorders [4]. The TREC assay facilitates the earliest possible identification in non-familial cases of SCID before irreversible organ damage or death, thus allowing for the possibility of curative treatment through hematopoietic stem cell transplant. Infants receiving hematopoietic stem cell transplantation in the first few months of life, after being identified through screening, have a high probability of survival, (95–100 %) along with lower morbidity [1–6].
While additional laboratory methods are being developed, the current TREC assay has proven to have outstanding specificity and sensitivity to accurately identify infants with SCID (the primary targets) as well as additional infants with other T-cell lymphopenia (secondary targets) [1–5]. The screening test is nearly 100 % sensitive [7].
In the period of 2001–2007, the Jeffrey Modell Foundation advocated before the United States Congress to consider adding SCID to the National Newborn Screening Core Panel. In 2008, JMF funded the first pilot program, in the state of Wisconsin, to screen 10,000 newborns using the TREC assay [8]. In this initial pilot program, a newborn with a combined immunodeficiency was identified and was successfully treated with a cord blood transplant.
In 2010, soon after the implementation of this pilot program, JMF advocated before the US Secretary of Health and Human Services’ Advisory Committee on Heritable Disorders in Newborns and Children, making an impassioned plea to add SCID to the National Newborn Screening Core Panel. It was unanimously recommended that all newborns be screened for SCID and T-cell lymphopenia, characterizing SCID screening using the TREC assay as “The National Standard for Newborn Screening Programs” [9].
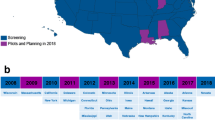
In 2012, JMF announced that it would provide funding to every state for every baby to be screened for SCID, a total of approximately 4.1 million newborns. Currently, there are 16 states screening infants for SCID (see Fig. 1) [10]. Based on state population provided from the most recent census, a total of over 2.2 million newborns are screened per year in the US. From 2010 to 2013 more than 6 million newborns have been screened for SCID. However, 1.78 million newborns per year are born in states awaiting legislation to make this screening possible. “Appendix 1: SCID newborn screening status by state” provides a detailed description of screening status of states and their annual birth population.
Map of newborn screening of SCID in the United States. Figure displays a map of the status of newborn screening of SCID in the United States, as of September 2013. Currently, 16 states are actively screening for SCID, 2 states are screening select populations in Navajo Nation, and 27 states are prepared to begin screening for SCID in 2014
The Jeffrey Modell Foundation has collaborated and provided funding for several state health departments to implement newborn screening of SCID. Four of the states, California, New York, Texas and Wisconsin, reported back to JMF and separately published the results of their population based newborn screening program.
A total of 993,724 newborns were screened in California, while 243,895, 381,625, and 207,696 newborns were screened in New York, Texas, and Wisconsin, respectively. Newborns identified with SCID were classified as typical SCID, leaky SCID or Omenn syndrome, or variant SCID. These newborns had absent or non-functional T-cells with a TREC count <25, with a CD3 cell count of <1,500 (normal range is 2,550–5,000). The following defects and deficiencies were identified:
-
IL-2R
-
JAK3
-
IL-7R
-
CD45
-
RAG1
-
RAG2
-
Artemis
-
ADA
-
CD3
-
CD8
-
Ataxia-Telangiectasia (A-T)
The overall incidence of SCID reported in the four states averaged 1:33,000. However, a number of other disorders, which present with abnormal TREC counts, were identified through newborn screening for SCID. These disorders include Jacobsen syndrome, trisomy 18, trisomy 21, DiGeorge spectrum, chylothorax, and pulmonary hypoplasia with other anomalies, as well as degenerative neuromuscular disease, cardiac anomalies, congenital heart defects, and multiple congenital anomalies. Table 1 shows the incidence of significant T-cell lymphopenia reported in California, New York, Texas, and Wisconsin.
SCID is life threatening and requires emergency immune restorative treatment. It is vital that patients identified with typical SCID, leaky SCID or Omenn syndrome, or variant SCID receive hematopoietic stem cell transplantation (HSCT) drawn from cord blood, matched related cells or matched unrelated cells, gene therapy, or enzyme therapy. Additionally, it is important that newborns identified with T-cell lymphopenia through this program avoid all live vaccines, receive appropriate prophylactic antibiotics, and receive appropriate interventional therapies if needed, such as bone marrow transplantation, gene therapy, enzyme therapy, thymus transplantation or heart surgery.
To evaluate the cost-effectiveness of newborn screening of SCID, the JMF developed a working algorithm or “decision tree”, validated by peer-reviewed scientific literature, and harmonized for application to be used by Public Health Departments and Health Ministries. This decision tool allows for local or regional data to be applied to measure the threshold and economic impact of implementing or not implementing newborn screening for SCID and T-cell lymphopenia. The “decision tree” (see Fig. 2) provides a usable tool and understandable formula that assists in deciding upon the willingness to pay for additional years of life utilizing criteria and costs specifically relevant to the locality.
Decision tool for implementation of newborn screening. Figure displays a “decision tree”, or working algorithm developed by the Jeffrey Modell Foundation, validated by peer-reviewed scientific literature, and harmonized for application to be used by Public Health Departments and Health Ministries in states, countries, and regions throughout the world. The “decision tree” provides a usable tool and understandable formula that assists in deciding upon the willingness to pay for additional years of life utilizing criteria and costs specifically relevant to the locality
The decision to implement newborn screening for SCID and related T-cell lymphopenia depends largely on the cost and effectiveness of the screening test, the incidence of SCID and related T-cell lymphopenia within a population, the cost ratio of the intervention, and the benefit of earliest possible treatment [11, 12]. As an example, we make the assumption that the number of births within a region is 100,000 per year, and the incidence of SCID or related T-cell lymphopenia is approximately 1:33,000 newborns [13]. This decision tree projects three cases per year.
The cost of the test is approximately $4.25 per infant, which includes equipment usage, labor and reagents. Thus, the cost to screen 100,000 newborns totals $425,000 [11]. The cost to transplant one newborn is $120,000 [14, 15]. The cost of post-transplant care over the next 5 years may be as much as $200,000 for one newborn. Therefore, the cost to screen 100,000 newborns and treatment of one patient would be approximately $745,000. The cost to screen 100,000 newborns and treat three patients totals $1,385,000.
If newborns are not screened at birth, they will sustain overwhelming infections and hospitalizations, averaging costs estimated to be at least $2 million in the first year of life [16, 17]. Given the incidence and population, the total costs of care for the predicted three affected newborns would amount to at least $6 million in healthcare costs [16, 17].
In a previous analysis, Chan et al. [11] found that the incremental cost-effective ratio (ICER) was $27,907 per quality of adjusted life year (QALY), given 70 years of life saved. This ratio is highly favorable and also compares closely with other metabolic diseases currently screened [11]. Additionally, this analysis stated that assuming society is willing to pay $50,000 per QALY, preference for screening occurred if incidence of SCID was at least 1:250,000 [11].
In 2011, three US federal agencies estimated the value of one life saved to be $7.7 million [18]. This estimate is an average provided by the Environmental Protection Agency ($9.1 million), Food and Drug Administration ($7.9 million), and the Transportation Department ($6.1 million) [18]. Given this economic information, a newborn baby with SCID or T-cell lymphopenia that is screened and treated in the first 3.5 months of life, generates a contribution to society that is at least 15 times greater than the cost of screening and curative treatment.
The TREC assay is inexpensive, highly sensitive, and has been effectively integrated into public health programs (e.g., Wisconsin, California, New York, Ontario) [7, 13, 19]. SCID is a fatal disease that causes accrual of exorbitant healthcare costs in just 1 year of life [16, 17]. The cost of care for just one infant with SCID could be more than the cost of screening for an entire regional population [17]. Implementation of screening through the TREC assay will provide the earliest possible identification and allow for intervention of early transplantation before infants suffer from severe infections, organ damage, and ultimately death [5]. Newborn screening for SCID and related T-cell lymphopenia is cost-effective, and most importantly, it is lifesaving and allows children with SCID the opportunity to live a healthy life. It is important that data-driven analyses supporting newborn screening, and a united front of advocacy continues, so that 1 day universal screening can be achieved worldwide. The complete summary of the JMF study to evaluate the cost-effectiveness of newborn screening of SCID can be found in “Appendix 2: JMF economic analysis”, which is included to be distributed to Public Health Departments and Health Ministries in states, countries, and regions throughout the world.
In addition to expansion of awareness and education of PI, the successful public health intervention of newborn screening for life-threatening severe combined immunodeficiencies must continue to be adopted across the United States and worldwide. In July 2013, preeminent physicians from 78 countries were brought together by the Jeffrey Modell Foundation for a 3 day global summit in Berlin, Germany. These expert physicians represent the world’s leadership and are authorities in the diagnosis, treatment, and management of primary immunodeficiencies. At the conclusion of the symposium, 215 physicians signed the “Berlin Declaration” calling for the immediate implementation of TRECs screening in order to identify, treat, and cure newborn babies born with SCID and related T-cell lymphopenia.
Following the global summit, JMF developed the economic analysis to provide government agencies with a usable tool to assist in the decision making process to include screening for SCID using the TREC assay into newborn screening programs. SCID is a fatal disease that causes an exorbitant amount in healthcare costs. Implementation of screening is not only cost effective, but also allows for the earliest possible lifesaving treatment.
References
Buckley RH, Schiff SE, Schif RI, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340(7):508–16.
Buckley RH. Transplantation of hematopoietic stem cells in human severe combined immunodeficiency: longterm outcomes. Immunol Res. 2011;49:25–43.
Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99:872–8.
Patel NC, et al. Outcomes of severe combined immunodeficiency patients treated with hematopoietic stem cell transplantation with and without pre-conditioning. J Allergy Clin Immunol. 2009;124(5):1062-9.e1–4.
Puck JM. Laboratory technology for population-based screening for severe combined immunodeficiency in neonates: the winner is T-cell receptor exision circles. In: Boyce JA, Finkelman F, Shearer WT, Vercelli D, editors. J Allergy Clin Immunol. 2012;129:607–16.
Buckley RH. The multiple causes of human SCID. J Clin Investig. 2004;114:1409–11.
Baker M. Universal newborn screening for severe combined immunodeficiency (SCID). [PowerPoint]. Atlanta, GA: APHL CDC Newborn Screening Molecular Workshop; 2012.
Routes JM, Grossman WJ, Verbsky J, Laessig RH, Hoffman GL, Brokopp CD, Baker MW. Statewide newborn screening for severe T-cell lymphopenia. JAMA. 2009;302(22):2465–70.
Sebelius, K. (2010, May 21). Letter to the committee chairperson for the Secretary’s advisory committee on heritable disorders in newborns and children US Department of Health and Human Services. http://tinyurl.com/l9baeng.
The University of Texas Health Science Center at San Antonio (UTHSCSA). National newborn screening status report. November 21, 2011. http://genes-r-us.uthscsa.edu.
Chan K, Davis J, Pai SY, Bonilla FA, Puck JM, Apkon M. A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID). Mol Genet Metab. 2011;104(3):383–9.
Lipstein EA, et al. Systematic evidence review of newborn screening and treatment of severe combined immunodeficiency. Pediatrics. 2010;125:e1226–35. doi:10.1542/peds.2009-1567.
Kwan A, Church JA, Cowan MJ, Agarwal R, Kapoor N, Kohn DB, Lewis DB, McGhee SA, Moore TB, Stiehm ER, Porteus M, Aznar CP, Currier R, Lorey F, Puck JM. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: results of the first 2 years. J Allergy Clin Immunol. 2013;132(1):140–50.
Hospital Cost and Utilization Project (HCUP), Nationwide Inpatient Database under the auspices of the Agency for Healthcare Research and Quality (AHRQ). ICD-9 CM Principal Diagnosis Code for HSCT.
Centers for Medicare and Medicaid Services, Hospital Accounting Records, April 28, 2010.
Caggana M, Brower A, Baker M, Comeau AM, Lorey F. National SCID Pilot Study. http://preview.tinyurl.com/lsanbqh.
Kuehn BM. State, federal efforts under way to identify children with “Bubble Boy Syndrome”. JAMA. 2010;304(16):1771–3.
Appelbaum B. As US Agencies put more value on a life, businesses fret [Newspaper]. New York Times, February 16, 2011. http://tinyurl.com/mlynth7. Accessed 16 Aug 2013.
CBCNews Canada. ‘Bubble boy’ welcomes new Ontario screening test. Published online Aug 20, 2013. http://www.cbc.ca/news/canada/story/2013/08/20/newborn-screening-bubble-boy-scid.html. Accessed 21 Aug 2013.
Antoine C, Muller S, Cant A, et al. Long-term survival and transplantation of hemopoietic stem cells for immunodeficiencies; report of the European experience 1986–1999. Lancet. 2003;361(9357):553–60.
Hassan A, Booth C, Brightwell A, et al. Outcome of hematopoietic stem cell transplantation for adenosine deaminase-deficient severe combined immunodeficiency. Blood. 2012;120(17):3615–24.
Modell V, Gee B, Lewis DB, Orange JS, Roifman CM, Routes JM, Sorensen RU, Notarangelo LD, Modell F. Global study of primary immunodeficiency diseases (PI)—diagnosis, treatment, and economic impact: an updated report from the Jeffrey Modell Foundation. Immunol Res. 2011;51:61–70.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: SCID newborn screening status by state

Total live births in the United States = 4,007,105
State | Live births |
|---|---|
Total Live Births in the United States = 4,007,105 | |
Currently screening | |
Arizona | 1,650a |
California | 510,980 |
Colorado | 66,822 |
Connecticut | 38,539 |
Delaware | 11,682 |
Florida | 214,962 |
Iowa | 38,574 |
Massachusetts | 73,275 |
Michigan | 113,509 |
Minnesota | 68,269 |
Mississippi | 39,177 |
New Mexico | 1,065a |
New York | 246,081 |
Ohio | 139,858 |
Pennsylvania | 142,724 |
Texas | 392,764 |
Utah | 53,395 |
Wisconsin | 67,719 |
Total | 2,221,045 |
Prepared for implementation | |
Alaska | 11,366 |
Arizona | 86,440 |
District of Columbia | 13,790 |
Georgia | 135,411 |
Hawaii | 18,948 |
Idaho | 22,799 |
Illinois | 161,758 |
Indiana | 84,794 |
Louisiana | 62,531 |
Maine | 12,814 |
Maryland | 71,739 |
Missouri | 77,588 |
Nebraska | 26,242 |
Nevada | 35,671 |
New Hampshire | 13,032 |
New Jersey | 103,932 |
New Mexico | 25,956 |
North Carolina | 123,468 |
North Dakota | 10,470 |
Oklahoma | 52,347 |
Oregon | 45,904 |
Rhode Island | 11,843 |
South Dakota | 12,382 |
Tennessee | 84,533 |
Vermont | 5,775 |
Virginia | 101,202 |
Washington | 86,507 |
West Virginia | 20,757 |
Wyoming | 6,914 |
Total | 1,526,913 |
Currently no plans for implementation | |
Alabama | 58,783 |
Arkansas | 37,536 |
Kansas | 41,598 |
Kentucky | 53,565 |
Montana | 12,066 |
South Carolina | 55,599 |
Total | 259,147 |
Appendix 2: JMF economic analysis

Objectives of the study
-
1.
This study develops a working algorithm or “decision tree” that is validated by peer-reviewed scientific literature, and harmonized for application to be used by Public Health Departments and Health Ministries in states, countries, and regions throughout the world.
-
2.
Local or regional data can be applied to measure the threshold and economic impact of implementing or not implementing newborn screening for SCID.
-
3.
This decision tree will provide the appropriate agency with a usable tool and understandable formula that will assist in deciding upon the willingness to pay for additional years of life utilizing criteria and costs specifically relevant to the locality.
Ten important facts to know
-
1.
Infants born with severe combined immunodeficiency (SCID), as well as related conditions with T-cell lymphopenia, suffer from serious, life-threatening infections, and will likely not survive their first year of life without specific therapy to protect them from infections and restore their immune function [1, 5, 8].
-
2.
SCID and related conditions can be detected by a simple screening test (TREC assay) using the same dried blood spot samples already collected from newborns to screen for other genetic disorders [5, 8].
-
3.
The TREC assay provides earliest possible identification before irreversible organ damage or death. Infants receiving hematopoietic stem cell transplantation in the first few months of life, after being identified through screening, have a high probability of survival, and will have the chance to grow up and live a healthy life [1–5, 20, 21].
-
4.
While additional laboratory methods are being developed, the current TREC assay has proven to have outstanding specificity and sensitivity to accurately identify all infants affected with SCID (the primary targets) as well as additional infants with other T-cell lymphopenia (secondary targets) [5, 8].
-
5.
The screening test is 100 % sensitive [7]. There has not been a single missed case of SCID since the program began 5 years ago [7].
-
6.
The US Secretary of Health and Human Services has recommended that all newborns be screened for SCID and T-cell lymphopenia, characterizing SCID screening using the TREC assay as “The National Standard for Newborn Screening Programs” [9, 10].
-
7.
There are established, dedicated, and specialized treatment centers for affected patients to receive care [22].
-
8.
The cost of the screen is $4–5 per infant. This includes equipment usage, labor and reagents [11]. More than 2.5 million babies have already been screened [8, 21]. The actual incidence of SCID was found to be approximately 1:66,000 and T-cell lymphopenia 1:20,000. The average was approximately 1:33,000 [13].
-
9.
There is a 95–100 % success rate of survivorship for babies transplanted in the first 3 months of life [2–4]. However, the survival rate sharply declines with time [10]. SCID is fatal in infancy if not treated, and as more serious infections develop, it is more difficult to successfully transplant [1–4, 6, 20, 21].
-
10.
Preeminent physicians from 78 countries were brought together by the Jeffrey Modell Foundation for a 3 day Global Summit in Berlin, Germany. These expert physicians represent the world’s leadership and are authorities in the diagnosis, treatment, and management of primary immunodeficiencies. At the conclusion of the symposium, the physicians signed the “Berlin Declaration” calling for the immediate implementation of TRECs screening in order to identify, treat, and cure newborn babies born with SCID and related T-cell lymphopenia.
Economic analysis
The decision to implement newborn screening for SCID and related T-cell Lymphopenia will depend on the cost and effectiveness of the screening test, the incidence of SCID and related T-cell lymphopenia within a population, the cost ratio of the intervention, and the benefit of earliest possible treatment [11, 12]. If we make an assumption that the number of births within a region is 100,000 per year, and the incidence of SCID or related T-cell lymphopenia is approximately 1:33,000 newborns, this decision tree projects three cases per year (see Fig. 1).
The cost to screen 100,000 newborns, at $4.25 per patient, totals $425,000 [11]. The cost to transplant one newborn is $120,000 [14, 15]. The cost of post-transplant care over the next 5 years may be as much as $200,000 for one newborn. Therefore, the cost to screen 100,000 newborns and treatment of one patient would be approximately $745,000. The cost to screen 100,000 newborns and treat three patients totals $1,385,000.
If newborns are not screened at birth, they will sustain overwhelming infections and hospitalizations, averaging costs estimated to be at least $2 million in the first year of life [16, 17]. Given the incidence and population, the total costs of care for the predicted three affected newborns will amount to $6 million in healthcare costs [16, 17].
In a previous analysis, Chan et al. [11] found that the incremental cost-effective ratio (ICER) was $27,907 per quality of adjusted life year (QALY), given 70 years of life saved [11]. This ratio is highly favorable and also compares closely with other metabolic diseases currently screened. Additionally, this analysis stated that assuming society is willing to pay $50,000 per QALY, preference for screening occurred if incidence of SCID was at least 1:250,000 [11].
In 2011, three US federal agencies estimated the value of one life saved to be $7.7 million [18]. This estimate is an average provided by the Environmental Protection Agency ($9.1 million), Food and Drug Administration ($7.9 million), and the Transportation Department ($6.1 million) [18]. Given this economic information, a newborn baby with SCID or T-cell Lymphopenia that is screened and treated in the first 3.5 months of life, generates a contribution to society that is at least 15 times greater than the cost of screening and curative treatment.
The TREC assay is inexpensive, highly sensitive, and has been effectively integrated into public health programs (e.g. Wisconsin, California, New York, Ontario) [8, 13, 19]. SCID is a fatal disease that causes accrual of exorbitant healthcare costs in just 1 year of life [16, 17]. The cost of care for just one infant with SCID could be more than the cost of screening for an entire regional population [17]. Implementation of screening through the TREC assay will provide the earliest possible identification and allow for intervention of early transplantation before infants suffer from severe infections, organ damage, and ultimately death [5]. Newborn screening for SCID and related T-cell lymphopenia is cost-effective, and most importantly, it is lifesaving and allows children with SCID the opportunity to live a healthy life.
Rights and permissions
About this article
Cite this article
Modell, V., Knaus, M. & Modell, F. An analysis and decision tool to measure cost benefit of newborn screening for severe combined immunodeficiency (SCID) and related T-cell lymphopenia. Immunol Res 60, 145–152 (2014). https://doi.org/10.1007/s12026-014-8485-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-014-8485-4