Abstract
Metabolic reprogramming is a cellular process contributing to carcinogenesis. However, it remains poorly understood in adrenal cortical carcinoma (ACC), an aggressive malignancy with overall poor prognosis and limited therapeutic options. We characterized the metabolic phenotype of ACC, by examining the immunoprofile of key proteins involved in glucose metabolism, hexokinase (HK1), pyruvate kinase (PKM1, PKM2), succinate dehydrogenase (SDHB), and phospho-S6 ribosomal protein (pS6), in a tissue microarray of 137 adrenal cortical tissue samples. Protein expression was compared between ACC (n = 42), adrenal cortical adenoma (ACA; n = 50), and normal adrenal cortical tissue samples (n = 45). Cytoplasmic expression of HK1 and PKM2 was significantly higher in ACC than in ACA (p < 0.001 and p = 0.014, respectively) or normal adrenal cortical tissue samples (p < 0.001 and p < 0.001, respectively). Expression of HK1 and PKM2 was also higher in ACA than in normal adrenal cortical tissue samples (p < 0.001 and p < 0.001, respectively). PKM1 expression was overall low in ACC, ACA, and normal samples, although expression of PKM1 was higher in ACC than in ACA (p = 0.027). There was no loss of cytoplasmic granular SDHB expression in our cohort of adrenal cortical tumors, and cytoplasmic expression of pS6 was lower in ACC than in ACA (p = 0.003) or normal adrenal cortical tissue samples (p = 0.008). Significantly, HK1 expression correlated with pyruvate kinase isoform (PKM2 and PKM1) expression (p < 0.001 and p = 0.007, respectively). Although functional validation was not performed, this study provides further evidence that metabolic reprogramming and altered glucose metabolism may occur in a subset of ACC through overexpression of intracellular glycolytic enzymes, notably HK1 and PKM2. The possibility of utilizing the reprogrammed glucose metabolism in ACC for novel therapeutic strategies should be explored in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adrenal cortical carcinoma (ACC) is a rare endocrine malignancy with frequent aggressive biology and overall poor prognosis [1,2,3]. Although our understanding of this disease has progressed tremendously over the last decade, our knowledge of ACC pathogenesis remains incomplete and new therapeutic avenues are needed [1,2,3,4,5,6,7].
Recently, several groups have begun to scrutinize the role of metabolic reprogramming in the pathogenesis of ACC [8,9,10]. Altered glucose metabolism has been described in many cancers and is thought to be a necessary step to fulfill the anabolic demands of proliferating tumor cells [11,12,13,14]. First described over 80 years ago, aerobic glycolysis (also known as the “Warburg” effect) refers to a propensity for proliferating tumor cells to acquire a hyperglycolytic phenotype, whereby glucose is taken up and preferentially converted into lactate, even in the presence of oxygen and functioning mitochondria [11,12,13,14,15,16] (Fig. 1). Although this adaptive phenomenon has been observed in many cancers, it remains poorly understood in ACC [8,9,10,11, 17, 18]. A deeper understanding of ACC metabolism may allow for more effective targeted therapeutic strategies to benefit patients in the future [12, 14, 19].
Glycolysis is a central pathway of cell metabolism, in which glucose is converted to pyruvate through a series of enzymatic reactions (a). The energy derived from this process is used to generate ATP molecules. In normal tissue, pyruvate is usually metabolized through the citric acid cycle when oxygen is present, and alternatively ferments to lactate when oxygen is lacking. A hallmark of cancer cells is an increase in glucose consumption followed by accelerated fermentation to lactate, even in the presence of oxygen and fully functioning mitochondria (b). This metabolic reprogramming phenomenon, known as aerobic glycolysis or the “Warburg effect,” is thought to be a required step to fulfill the anabolic demands of proliferating tumor cells, and has been described in various malignancies. Promotion of mTOR signaling and loss of p53 are two changes in cancers that impact glycolysis. GLUT, glucose transporter; HK1, hexokinase; PKM1, pyruvate kinase M1 isoform; PKM2, pyruvate kinase M2 isoform; MCT, monocarboxylate transporter; TCA cycle, citric acid cycle; SDHB, succinate dehydrogenase
In order to characterize the metabolic phenotype of ACC, we investigated the immunoprofile of key proteins involved in glucose energy metabolism. We developed an immunohistochemical panel that would allow for screening of key markers to provide a snapshot of metabolism in the stained tissues. These proteins were designed to cover the glycolytic enzyme hexokinase I (HK1), and pyruvate kinase M1 and M2 isoforms (PKM1 and PKM2), as well as mitochondrial proteins involved in the citric acid cycle using staining for succinate dehydrogenase (SDHB); while SDHB expression is well known to reflect the genetic integrity of the various members of the SDH complex, in this study, we used it as a biomarker that can reflect SDH-related pseudohypoxia. The panel also included phospho-S6 ribosomal protein (pS6), a marker of growth response to anabolic orchestrators like mammalian target of rapamycin (mTOR). These markers were assessed in a surgical series of benign and malignant adrenal cortical neoplasms, as well as normal adrenal cortical tissue.
Material and Methods
Study Group and Histology
We evaluated a series of tissue microarrays (TMA), constructed at the University Health Network (UHN), as reported in a previous study [7]. Briefly, after obtaining the approval of the institutional Research Ethics Board, a total of 137 surgical specimens (42 adrenal cortical carcinomas (ACC), 50 adrenal cortical adenomas (ACA), and 45 normal adrenal cortical tissue samples) were selected for the construction of TMA. All cases were retrospectively reviewed by 3 endocrine pathologists (O.M., H.G., and S.L.A.) to ensure the appropriate inclusion of the diagnostic categories.
Tissue Microarray Construction
Reference hematoxylin and eosin-stained slides for each patient were reviewed and marked with at least 3 circles (each measuring 1 mm in diameter) to select regions without necrosis. Three cores of 1 mm diameter were taken from the corresponding paraffin block matching the circled regions. The cores were then mounted in linear arrays in recipient paraffin blocks using a manual tissue arrayer. At least 80% viable cells were obtained for each sample. A character map was constructed delineating the various cases and their order in the TMA.
Immunohistochemistry
Formalin-fixed paraffin-embedded sections (4 μm) were dewaxed in 5 changes of xylene and rehydrated through graded alcohols. The details of the antibodies used are summarized in Table 1. Negative and positive control tissues were selected based on manufacturer recommendations as well as previous publications where these antibodies were applied. Multiple control experiments were undertaken to optimize each antibody. Endogenous peroxidase was blocked with 3% hydrogen peroxide. The detection system used was MACH4 universal HRP polymer system (Inter Medico; cat #BC-M4U534). Color development was performed with freshly prepared DAB (DAKO; cat #K3468). Sections were counterstained lightly with Mayer’s hematoxylin, dehydrated in alcohols, cleared in xylene, and mounted with Permount mounting medium (Fisher; cat #SP15-500). Individual cores were scored by multiplying the percent positive cells by intensity scores (1 to 3) and each case received an average score (max 300) and an overall staining intensity (max 3) for HK1, PKM1, PKM2, and pS6. Levels of cytoplasmic granular staining of SDHB expression were recorded.
Statistical Analysis
The metabolic biomarker (HK1, PKM2, PKM1, pS6) expression scores and SDHB expression were compared between ACCs, ACAs, and normal adrenal cortical tissue samples. The available data on mitotic tumor grade and Ki67 labeling indices [7] were also correlated with metabolic biomarkers in ACCs. Statistical analyses were performed using the SPSS statistical software program (SPSS for Windows release 19.0; SPSS Inc., Chicago, IL).
Results
Expression Profile of Proteins Involved in Glycolysis
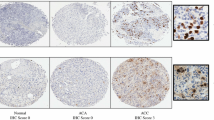
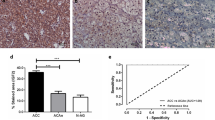
The expression scores are summarized in Table 2. Overall, HK1 and PKM2 expression differed significantly between ACC (n = 42), ACA (n = 50), and normal adrenal cortical tissue samples (n = 45) (p < 0.001 and p < 0.001, respectively). Intermediate-to-high intensity cytoplasmic HK1 expression was seen in 62% of ACC versus 20% of ACA versus 13% of normal adrenal cortical tissue samples (p < 0.001). The mean HK1 expression scores were 146.20 in ACC versus 58.62 in ACAs versus 22.52 in normal adrenal cortical tissue samples. Cytoplasmic expression of HK1 was significantly higher in ACC than in ACA (p < 0.001) or normal adrenal cortical tissue samples (p < 0.001) (Fig. 2). Expression of HK1 was also higher in ACA than in normal adrenal cortical tissue samples (p < 0.001). Similarly, intermediate-to-high intensity cytoplasmic PKM2 expression was seen in 79% of ACC versus 60% of ACA versus 47% of normal adrenal cortical tissue samples (p = 0.001). The mean PKM2 expression scores were 203.24 in ACC versus 156.56 in ACAs versus 100.20 in normal adrenal cortical tissue samples. Cytoplasmic expression of PKM2 was significantly higher in ACC than in ACA (p = 0.014) or normal adrenal cortical tissue samples (p < 0.001) (Fig. 2). Expression of PKM2 was also higher in ACA than in normal adrenal cortical tissue samples (p < 0.001). In contrast, PKM1 expression was overall low in ACC, ACA, and normal adrenal cortical tissue samples. Intermediate-to-high intensity cytoplasmic PKM1 expression was seen in 7% of ACC versus 0% of ACA versus 13% of normal adrenal cortical tissue samples, while the mean PKM1 expression scores were 22.44 in ACC versus 2.82 in ACAs versus 17.26 in normal adrenal cortical tissue samples. Cytoplasmic expression of PKM1 was higher in ACC than in ACA (p = 0.027) but not statistically different when compared with normal adrenal cortical tissue samples (p = 0.85) (Fig. 3). Significantly, HK1 expression correlated with pyruvate kinase M2 and M1 isoforms (PKM2 and PKM1) expression (r = 0.5; p < 0.001)(r = 0.2; p = 0.007), with no association seen between PKM2 and PKM1 expression (p = 0.28).
Hexokinase I (HK1) and pyruvate kinase isoform M2 (PKM2) expression patterns in adrenal cortical neoplasms and non-tumorous adrenal cortex. Cytoplasmic expression of HK1 was significantly higher in ACC (a) than in ACA (b) or normal adrenal cortical tissue samples (c). Similarly, cytoplasmic expression of PKM2 was significantly higher in ACC (d) than in ACA (e) or normal adrenal cortical tissue samples (f). Significantly, HK1 expression patterns correlated with PKM2 expression
Pyruvate kinase isoform M1 (PKM1) and phosphorylated S6 ribosomal protein (pS6) expression patterns in adrenal cortical neoplasms and non-tumorous adrenal cortex. Although higher in ACC (a), cytoplasmic expression of PKM1 expression (a–c) was overall low in ACC (a), ACA (b), and adrenal cortical tissue samples (c). Cytoplasmic expression of phosphorylated S6 ribosomal protein (pS6), a downstream target of the PI3k-AKT-mTOR pathway, was not overexpressed in ACC (d) compared with ACA (e) and normal adrenal cortical tissue samples (f)
Expression Profile of Succinate Dehydrogenase
None of the 40 ACCs (2 of 42 ACCs technically failed) and 50 ACAs in our study cohort showed loss of succinate dehydrogenase (SDHB) expression by immunohistochemistry. Levels were similar in most tumors with the exception of a small number of neoplasms enriched in oncocytic cells that showed more intense reactivity.
Expression Profile of PI3k Pathway-Related Phospho-S6 Ribosomal Protein
Intermediate-to-high intensity cytoplasmic phospho-S6 ribosomal protein (pS6) expression was seen in 10% of ACC versus 34% of ACA versus 29% of normal adrenal cortical tissue samples (p < 0.001). The mean pS6 expression scores were 26.59 in ACC versus 72.89 in ACAs versus 60.23 in normal adrenal cortical tissue samples (Table 2). Cytoplasmic expression of pS6 was lower in ACC than in ACA (p = 0.003) or normal adrenal cortical tissue samples (p = 0.008), while no difference in pS6 expression was seen between ACA and normal tissue samples (p = 0.60) (Fig. 3). Expression of pS6 did not correlate with HK1, PKM2, and PKM1 expression.
Correlation of Metabolic Biomarkers with Tumor Mitotic Grade and Ki67 Labeling Index in Adrenal Cortical Carcinomas
Although none of the metabolic biomarkers correlated with the tumor mitotic grade (low grade vs high grade; based on the cutoff of 20 per 50 high power fields), HK1 expression was associated with Ki67 tumor proliferation index (r = 0.34; p = 0.03). None of the other metabolic biomarkers were associated with Ki67 tumor proliferation index.
Discussion
Metabolic reprogramming is a phenomenon with well-recognized contribution to carcinogenesis yet it remains poorly understood in adrenal cortical carcinoma [2, 8,9,10,11, 17, 18]. We characterized the metabolic phenotype of a large series of ACC, ACA, and normal adrenal cortical tissue samples, and demonstrated that HK1 and PKM2, two important glycolytic enzymes, are differentially expressed in malignant versus benign adrenal cortical tumors, as well as in neoplastic versus non-neoplastic adrenal cortical tissue.
Glycolysis is a central pathway of cell metabolism, in which glucose is converted to pyruvate through a series of reversible and irreversible enzymatic reactions [11, 14]. The energy derived from this process is used to generate ATP molecules, a process that is essential for mammalian life [11, 14]. In normal tissue, pyruvate is usually metabolized through the citric acid cycle when oxygen is present, and alternatively ferments to lactate when oxygen is lacking [11, 14]. A hallmark of cancer cells is an increase in glucose consumption followed by accelerated fermentation to lactate, even in the presence of oxygen and fully functioning mitochondria [11, 14, 20]. This metabolic reprogramming phenomenon, known as aerobic glycolysis or the “Warburg effect,” is thought to be a required step to fulfill the anabolic demands of proliferating tumor cells, and has been described in various malignancies, including glioblastoma, colorectal cancer, gastric cancer, pancreatic cancer, and breast cancer [11, 14, 21,22,23,24,25]. Prior imaging data using positron emission tomography (PET) and glucose analogue tracer 18fluorodeoxyglucose (FDG) have shown a high level of glucose consumption in adrenal cortical carcinoma [8, 9, 26,27,28]. As a result, several groups have recently scrutinized the role of altered glucose metabolism in ACC pathogenesis and found that the glucose transporter (GLUT1) and monocarboxylate transporters (MCT4), two membranous transporters involved in glycolysis, are overexpressed in ACC when compared with ACA [8,9,10]. Glucose transporters are thought to play a crucial role in accelerated glycolysis, as cancer cells are only able to depend on the glycolytic pathway for energy production if the demands for increased glucose uptake by cancer cells are met [8, 15, 19]. Similarly, monocarboxylate transporters are also thought to be important for the “Warburg effect” as they are responsible for lactate transport outside the cell to drive the glycolysis reaction forward [8, 10, 14]. Both Fenske et al. and Pinheiro et al. found higher GLUT1 expression in ACC compared with ACA [8, 10]. Similarly, MCT4 expression was higher in ACC compared with ACA [8]. Taken together, these findings suggest that metabolic reprogramming to a hyperglycolytic phenotype may occur in a subset of ACC through overexpression of membrane transporters involved in glycolysis.
However, the contribution of intracellular glycolytic enzymes in metabolic reprogramming of ACC remains unclear. A recent proteomics study revealed that aldolase A, an enzyme responsible for a reversible step in glycolysis, is overexpressed in malignant compared with benign adrenal cortical tumors [17]. Our study findings add to the growing evidence that intracellular glycolytic enzymes may play a role in ACC pathogenesis, by demonstrating that HK1 and PKM2 are also differentially expressed in malignant versus benign adrenal cortical tumors, as well as in neoplastic versus non-neoplastic adrenal cortical tissue. Hexokinase catalyzes the first irreversible step of glycolysis, phosphorylating glucose to produce glucose-6-phosphate (G6P) [14]. This reaction is an important step in glucose energy metabolism because it traps glucose within cells by converting it to G6P, a convergence point of many important metabolic pathways including the pentose phosphate pathway (PPP), the hexosamine pathway, and glycogenesis [14]. By binding to the outer mitochondrial membrane, hexokinase preferentially use ATP derived from mitochondria to phosphorylate glucose, thereby coupling oxidative phosphorylation (OXPHO) with glycolysis [14, 29]. Cancer cells often facilitate this first irreversible step of glycolysis by inducing high level expression of hexokinase [14], and this was also observed in our study, as HK1 expression was significantly higher in ACC than in ACA or normal adrenal cortical tissue, suggesting perhaps that the first irreversible step of glycolysis may be aberrantly activated in a subset of ACC. In addition to hexokinase, we found that pyruvate kinase isoform M2 (PKM2) expression was also higher in ACC than in ACA or normal adrenal cortical tissue. Pyruvate kinase catalyzes the third and final irreversible step of glycolysis, converting phosphoenolpyruvate (PEP) into pyruvate [14]. Contrary to other steps, the last committed step is attenuated in cancer cells, allowing metabolites to be diverted into branching pathways, such as the PPP and the serine biosynthesis pathway to generate sufficient metabolic intermediates and augment the anabolic reactions required for tumor growth and proliferation [14]. This attenuation can be achieved by favoring the use of low-affinity PKM2 to catalyze this reaction instead of the high-affinity PKM1 [14, 30], and our study found PKM2 expression to be significantly higher in ACC than in ACA or normal adrenal cortical tissue. Furthermore, PKM2 expression was associated with HK1 expression in ACC, suggesting perhaps that metabolic remodeling towards a hyperglycolytic immunophenotype may occur in a subset of ACC through overexpression of intracellular enzymes involved in glycolysis.
In addition to the glycolysis pathway, we also examined the expression of the succinate dehydrogenase (SDH) complex in adrenal cortical tumors. The SDH complex plays a pivotal role in cellular energy metabolism, as it links the Krebs cycle to oxidative phosphorylation [18, 31]. Loss-of-function of SDH is thought to interrupt the Krebs cycle, leading to a state of cellular pseudohypoxia, which is described as one the most common causes of hereditary endocrine tumors [14, 18, 31,32,33]. While SDH mutations have not been widely implicated in adrenal cortical tumorigenesis, a recent study examined the rare occurrence of SDH mutations in 4 unrelated individuals with ACC and germline SDHx mutations [18]. In this study, we evaluated the immunohistochemical expression of SDHB and found that none of the adrenal cortical tumors in our cohort showed loss of SDHB expression, suggesting that SDH loss-of-function may not be a salient feature of adrenal cortical neoplasia. We also used SDHB expression as a surrogate biomarker of SDHx-related hypoxia and found no alteration in expression levels in the various tissues examined.
Recently, the metabolism of cancer cells has been the focus of much attention with the realization that many oncogenic drivers, including the PI3k-AKT-mTOR (mTOR) pathway, modify glucose metabolism [14]. A transgenic animal model of ACC showed that mTOR activation is an early step in tumorigenesis [34]. Furthermore, the TCGA data identified singleton gene fusions (EXOSC10-MTOR fusion) in ACC correlating with high phospho-mTOR levels [2]. Variable expression of HK1, PKM, and RPS6 is also seen in the TCGA data of ACCs [2]. Nevertheless, the contribution of mTOR signaling to adrenal cortical carcinogenesis remains controversial [7, 35, 36]. A previous study by our group showed that adrenal cortical carcinomas and adenomas were no different with respect to PTEN and phospho-mTOR immunoexpression scores [7]. Similarly, De Martino and colleagues did not find any difference in mRNA levels of mTOR between ACC and ACA [35]. In this study, we found that the expression of phosphorylated S6 ribosomal protein (pS6), a downstream target of the PI3k-AKT-mTOR pathway, was not overexpressed in ACC compared with ACA and normal adrenal cortical tissue. Similar findings were previously reported [35], suggesting that aberrant mTOR activation may be infrequent in ACC.
Conclusion
Variable expression of HK1, PKM, and RPS6 was noted in the TCGA data of ACCs [2]. Our study provides further evidence that intracellular glycolytic enzymes, notably HK1 and PKM2, are differentially expressed in malignant versus benign adrenal cortical tumors, as well as in neoplastic versus non-neoplastic adrenal cortical tissue. Furthermore, our finding of glycolytic enzyme overexpression in ACC, which was largely unrelated to proliferative variables, adds to the growing evidence that metabolic remodeling towards a hyperglycolytic phenotype may occur in a subset of ACC, similar to that described in other types of cancer. The possibility of utilizing the reprogrammed glucose metabolism in ACC as a potential tumor biomarker or for novel therapeutic strategies should be explored in future studies.
References
Duan K, Giordano TJ, Mete O (2016) Adrenal cortical proliferations. In: Mete O, Asa SL, eds. Endocrine Pathology. UK: Cambridge University Press; 602–627.
Zheng S, Cherniack AD, Dewal N, et al (2016) Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell 29:723–736
Libè R, Fratticci A, Bertherat J (2007) Adrenocortical cancer: pathophysiology and clinical management. Endocr Relat Cancer 14:13–28
Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J, Sanders D, Thomas DG, Doherty G, Hammer G (2009) Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res Off J Am Assoc Cancer Res 15:668–676
de Reyniès A, Assié G, Rickman DS, Tissier F, Groussin L, René-Corail F, Dousset B, Bertagna X, Clauser E, Bertherat J (2009) Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol Off J Am Soc Clin Oncol 27:1108–1115
Assié G, Letouzé E, Fassnacht M, et al (2014) Integrated genomic characterization of adrenocortical carcinoma. Nat Genet 46:607–612
Mete O, Gucer H, Kefeli M, Asa SL (2018) Diagnostic and Prognostic Biomarkers of Adrenal Cortical Carcinoma. Am J Surg Pathol 42:201–213
Pinheiro C, Granja S, Longatto-Filho A, Faria AM, Fragoso MCBV, Lovisolo SM, Lerário AM, Almeida MQ, Baltazar F, Zerbini MCN (2015) Metabolic reprogramming: a new relevant pathway in adult adrenocortical tumors. Oncotarget 6:44403–44421
Pinheiro C, Granja S, Longatto-Filho A, et al (2017) GLUT1 expression in pediatric adrenocortical tumors: a promising candidate to predict clinical behavior. Oncotarget. https://doi.org/10.18632/oncotarget.19135
Fenske W, Völker H-U, Adam P, et al (2009) Glucose transporter GLUT1 expression is an stage-independent predictor of clinical outcome in adrenocortical carcinoma. Endocr Relat Cancer 16:919–928
Liberti MV, Locasale JW (2016) The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci 41:211–218
Vander Heiden MG, DeBerardinis RJ (2017) Understanding the Intersections between Metabolism and Cancer Biology. Cell 168:657–669
Sullivan LB, Gui DY, Heiden MGV (2016) Altered metabolite levels in cancer: implications for tumour biology and cancer therapy. Nat Rev Cancer 16:680–693
Hay N (2016) Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer 16:635–649
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033
Vander Heiden MG, Locasale JW, Swanson KD, et al (2010) Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science 329:1492–1499
Kjellin H, Johansson H, Höög A, Lehtiö J, Jakobsson P-J, Kjellman M (2014) Differentially Expressed Proteins in Malignant and Benign Adrenocortical Tumors. PLOS ONE 9:e87951
Else T, Lerario AM, Everett J, et al (2017) Adrenocortical carcinoma and succinate dehydrogenase gene mutations. Eur J Endocrinol EJE-17-0358
Ganapathy-Kanniappan S, Geschwind J-FH (2013) Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer 12:152
Warburg O (1956) On the origin of cancer cells. Science 123:309–314
Reske SN, Grillenberger KG, Glatting G, Port M, Hildebrandt M, Gansauge F, Beger HG (1997) Overexpression of glucose transporter 1 and increased FDG uptake in pancreatic carcinoma. J Nucl Med Off Publ Soc Nucl Med 38:1344–1348
Gao Y, Xu D, Yu G, Liang J (2015) Overexpression of metabolic markers HK1 and PKM2 contributes to lymphatic metastasis and adverse prognosis in Chinese gastric cancer. Int J Clin Exp Pathol 8:9264–9271
Wincewicz A, Sulkowska M, Koda M, Sulkowski S (2007) Clinicopathological significance and linkage of the distribution of HIF-1α and GLUT-1 in human primary colorectal cancer. Pathol Oncol Res 13:15
Grover-McKay M, Walsh SA, Seftor EA, Thomas PA, Hendrix MJ (1998) Role for glucose transporter 1 protein in human breast cancer. Pathol Oncol Res POR 4:115–120
Agnihotri S, Zadeh G (2016) Metabolic reprogramming in glioblastoma: the influence of cancer metabolism on epigenetics and unanswered questions. Neuro-Oncol 18:160–172
Takeuchi S, Balachandran A, Habra MA, Phan AT, Bassett RL, Macapinlac HA, Chuang HH (2014) Impact of 18F-FDG PET/CT on the management of adrenocortical carcinoma: analysis of 106 patients. Eur J Nucl Med Mol Imaging 41:2066–2073
Becherer A, Vierhapper H, Pötzi C, Karanikas G, Kurtaran A, Schmaljohann J, Staudenherz A, Dudczak R, Kletter K (2001) FDG-PET in adrenocortical carcinoma. Cancer Biother Radiopharm 16:289–295
Deandreis D, Leboulleux S, Caramella C, Schlumberger M, Baudin E (2011) FDG PET in the management of patients with adrenal masses and adrenocortical carcinoma. Horm Cancer 2:354–362
Robey RB, Hay N (2006) Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 25:4683–4696
Israelsen WJ, Vander Heiden MG (2015) Pyruvate kinase: Function, regulation and role in cancer. Semin Cell Dev Biol 43:43–51
Jochmanova I, Pacak K (2016) Pheochromocytoma: The First Metabolic Endocrine Cancer. Clin Cancer Res 22:5001–5011
Duan K, Mete O (2017) Familial endocrine tumor syndromes: Clinical and predictive roles of molecular histopathology. AJSP: Reviews and Reports; 22:246–268.
Pacak K, Wimalawansa SJ (2015) Pheochromocytoma and paraganglioma. Endocr Pract 21:406–412
Batisse-Lignier M, Sahut-Barnola I, Tissier F, et al (2017) P53/Rb inhibition induces metastatic adrenocortical carcinomas in a preclinical transgenic model. Oncogene. https://doi.org/10.1038/onc.2017.54
De Martino MC, Feelders RA, de Herder WW, et al (2014) Characterization of the mTOR pathway in human normal adrenal and adrenocortical tumors. Endocr Relat Cancer 21:601–613
Ross JS, Wang K, Rand JV, et al (2014) Next-generation sequencing of adrenocortical carcinoma reveals new routes to targeted therapies. J Clin Pathol 67:968–973
Acknowledgements
The authors would like to thank both Drs. Thomas Giordano and Antonio Marcondes Lerario (University of Michigan Hospital, Michigan Medicine), for assisting the authors to obtain the expression status of HK1, PKM and RPS6 from the TCGA database.
Funding
This work was funded in part by a Canadian Institutes of Health Research (CIHR) New Investigator Foundation Grant FDN-148385 (D.A.W.). D.A.W. holds an Ontario Ministry of Innovation Early Researcher Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duan, K., Gucer, H., Kefeli, M. et al. Immunohistochemical Analysis of the Metabolic Phenotype of Adrenal Cortical Carcinoma. Endocr Pathol 31, 231–238 (2020). https://doi.org/10.1007/s12022-020-09624-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-020-09624-3