Abstract
Objective
This study was to perform a meta-analysis to assess the relationship between hypertriglyceridemic-waist (HTW) phenotype and abnormal glucose metabolism.
Methods
The data sources were PubMed and EMBASE up to June 2018. Studies providing the relationship between HTW phenotype and abnormal glucose metabolism were included.
Results
In total, 48 eligible studies that evaluated 2,42,879 subjects were included in the meta-analysis. In the general population, the pooled odds ratios (ORs) for elevated blood glucose and diabetes related to HTW phenotype was 2.32 (95% confidence interval (CI): 1.98–2.71) and 2.69 (95% CI: 2.40–3.01), respectively. In cohort studies, the pooled OR for diabetes related to HTW phenotype was 2.89 (95% CI: 1.97–4.25) in subjects without diabetes. The levels of homeostasis model assessment of insulin resistance (HOMA-IR) in the HTW population were increased with values of mean differences (MD) 1.12 (95% CI: 0.81–1.43. P < 0.00001, I2 = 99%) in the general population and 0.89 (95% CI: 0.75–1.04, P < 0.00001, I2 = 67%) in subjects without diabetes.
Conclusion
HTW phenotype was closely associated with increased risk of abnormal glucose metabolism. There was also a significant correlation between HTW phenotype and insulin resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a chronic metabolic disease. Long-term hyperglycemia is the factor that promotes vascular lesions and dysfunction, leading to a variety of complications of diabetes mellitus. The incidence of diabetes is increasing globally. Now, diabetes is a global health burden due to its high prevalence, deaths attributable, and healthcare expenditure [1, 2].
The hypertriglyceridemic-waist (HTW) phenotype is represented by the simultaneous presence of elevated serum triglyceride (TG) and increased waist circumference (WC). Lemieux et al. suggested that the simultaneous measurement and interpretation of WC and fasting TG could be used as inexpensive screening tools to identify men characterized by the atherogenic metabolic triad and at high risk for coronary artery disease [3].
A meta-analysis has shown that HTW phenotype was significantly associated with risk of type 2 diabetes worldwide [4]. However, this meta-analysis only analyzed the relationship between HTW phenotype and diabetes in cross-sectional studies. As we know, the cross-sectional design could not identify the causal relationship. Cohort studies have confirmed the association between HTW phenotype and risk of future diabetes in subjects without diabetes. The predictive power of HTW phenotype was similar to metabolic syndrome. For simpler and fewer components, HTW phenotype might be more practical than metabolic syndrome, and it might be recommended in most clinical practices [5]. Insulin resistance and islet beta cell dysfunction are the main pathogenesis of type 2 diabetes. The comprehensive assessment and management of cardiovascular risk factors were also very important in patients with type 2 diabetes. In recent years, the applications of HTW phenotype in the fields of abnormal glucose metabolism have attracted more and more attention, including insulin resistance [6, 7], islet cell dysfunction [8] and cardiometabolic abnormality [9], and diabetic complications [10] in subjects with diabetes. This systematic review aimed to meta-analyze the relationship between hypertriglyceridemic-waist phenotype and each phase of abnormal glucose metabolism.
Methods
Search strategy and eligibility criteria
A systematic review of the published articles was performed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (http://www.prisma-statement.org/). The predefined inclusion criteria for study selection were as follows: the study must have (1) prospective cohort study, case–control study or cross-sectional study; (2) the exposure was hypertriglyceridemic-waist phenotype; (3) the outcome was abnormal glucose metabolism, including prevalence or incidence of elevated blood glucose and diabetes, insulin resistance, islet cell function. Metabolic abnormality and diabetic complications in diabetic patients were also included. Studies were excluded if (1) the paper were presented without necessary data or other information that allowed calculation of necessary data; (2) letters, reviews, and abstracts from conference proceedings were excluded.
A systematic search was conducted in the bibliographic databases, including PubMed and Embase from inception to June 17, 2018. First, a categorical search using the following keywords was conducted (i) hypertriglyceridemic-waist phenotype or their synonyms (e.g., hypertriglyceridemic waist or HTW); (ii) elevated blood glucose, diabetes, insulin resistance, islet β cell function, metabolic abnormality, and diabetic complications or their synonyms (e.g., impaired fasting glucose or impaired glucose tolerance). Second, categories ‘i’ to ‘ii’ were combined using “and”, and the duplicates were removed. Also, Reference lists of retrieved articles were manually scanned for all relevant additional studies and review articles. The study selection was limited to English language and studies on humans only.
Study selection and quality assessment
Irrelevant articles were eliminated from the primary search based on the information in the title and abstract. Once the studies were identified by the information in the titles and abstracts, full papers were obtained for all relevant studies. Two reviewers (C-M.M. and X-L.L.) independently read the full papers obtained from the search for relevance. All studies that did not meet the inclusion criteria or that met the exclusion criteria were removed. Disagreements between the two reviewers regarding study inclusion were resolved through face-to-face discussion of the full-text assessment. Eligible studies were further reviewed.
The methodological quality of case–control studies and cohort studies was assessed using a checklist based on the Newcastle–Ottawa Quality Assessment Scale (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). The methodological quality of cross-sectional studies was assessed using a checklist based on the Agency for Healthcare Research and Quality (https://www.ncbi.nlm.nih.gov/books/NBK35156/). These enabled the reviewers to evaluate the quality of the studies. Disagreements between the reviewers on individual items were resolved through consensus meeting with a third reviewer (F.-Z.Y.).
Data extraction
We extracted the following information about the studies: study characteristics (study name, authors, publication year, journal, study country, study type, follow-up years, and number of participants) and participants’ characteristics (mean age or age range, sex).
The diagnostic criteria of HTW phenotype were extracted. There are three grouping methods according to the status of WC and TG. First, four groups: (i) normal WC and normal TG level (NWNT); (ii) normal WC and high TG level (NWHT); (iii) enlarged WC and normal TG level (EWNT); and (iv) enlarged WC and high TG level (HTW phenotype). Second, three groups: (i) NWNT; (ii) sole high TG or enlarged WC (including NWHT and EWNT); (iii) HTW phenotype. Third, two groups: (i) non-HTW phenotype (including NWNT, NWHT, and EWNT); (ii) HTW phenotype. The data were extracted according to original grouping methods and then the data were combined into two groups, non-HW phenotype and HW phenotype.
Disease outcomes include four parts: (i) elevated blood glucose or diabetes: dichotomous data, number of participants and cases were extracted; (ii) insulin resistance and islet cell function: continuous data, mean (standard deviation (SD), standard errors (SE) and 95% confidence interval (CI)), median (interquartile range (IQR)) and geometric mean (95% CI) were extracted. The median or geometric mean was considered equivalent to mean. The 95% CI and SE were converted to SD by the calculator from cochrane (http://training.cochrane.org/sites/training.cochrane.org/files/public/uploads/resources/embedded_resources/online_learning_modules/Analysing_and_Presenting_Data/AD_continuous/files/Finding_SDs.xls). The width of the interquartile range was considered as 1.35 SD. The mean and SD were combined into two groups by our own calculator; (iii) metabolic abnormality in subjects with diabetes, including fasting plasma glucose (FPG), glycosylated hemoglobin A1c (HbA1c), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoprotein B (Apo B), systolic blood pressure (SBP), and diastolic blood pressure (DBP): continuous data, mean (SD) or median (IQR) were extracted. The data was handled as the same way with insulin resistance; (iv) diabetic complications: dichotomous data, number of participants and cases were extracted.
If the information was unavailable or not clear from a published report, we collected relevant data by corresponding with the authors. The data were extracted from primary studies by two reviewers (C.-M.M. and X.-L.L.) independently.
Data synthesis and statistical analysis
We used Review Manager 5.3 for data merging and statistical analysis. For dichotomous data, the statistical model is based on Mantel–Haenszel, the effect index is odds ratio (OR) with 95% CI, and the analysis model is the random-effects model. For continuous data, we used the random-effects model and the inverse variance (IV) method to interpolate the mean differences (MD) of each study. The results are reported with a 95% CI. Heterogeneity across studies was evaluated by the I2 statistic (ranges from 0 to 100% with lower values representing less heterogeneity). The possibility of publication bias was evaluated using the Egger test and visual inspection of a funnel plot. Moreover, stratified analyses and sensitivity analyses were performed to evaluate the influences of selected study and participant characteristics on the results. Analyses were performed using the STATA version 12.0 software (STATA Corporation, TX, USA).
Results
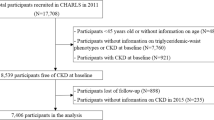
Figure 1 summarizes the selection process of the studies. In total, 249 references were obtained using PubMed and Embase. Additional six full-text articles were included after scanning the related literature and reference lists of the studies selected for inclusion. Eventually, a total of 48 studies were included in this review [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. In these studies, 33 studies were analysis of elevated blood glucose and/or diabetes [5, 7, 8, 11, 13, 15, 18,19,20, 22,23,24, 26, 28,29,30,31,32,33,34,35,36, 39,40,41,42, 46,47,48,49,50,51,52], 18 studies were analysis of insulin resistance and/or islet cell function [6,7,8, 12, 14, 16, 17, 23, 25, 27,28,29, 36, 38, 41, 45, 46, 49], 5 studies were analysis of metabolic abnormality [9, 10, 21, 37, 43], and 4 studies were for analysis of diabetic complications [10, 21, 44, 48] (Table 1). Several studies included more than one disease outcome. The quality of the included articles is also summarized in Table 1.
Supplementary Table 1 shows the characteristics of articles about the risk of elevated blood glucose and/or diabetes included in the meta-analysis. Elevated bood glucose include subjects with prediabetes or diabetes at the same time. The definition of elevated blood glucose was FPG ≥ 5.6 mmol/L and/or OGTT 2-h plasma glucose ≥ 7.8 mmol/L and/or HbA1c ≥ 5.7% and/or had been diagnosed with diabetes. The definitions of elevated blood glucose were not the same among each studies. The definitions of elevated blood glucose and diabetes were shown in Supplementary Table 1. The risk of diabetes in the HTW phenotype was increased. The pooled OR for diabetes related to HTW phenotype was 2.69 (95% CI: 2.40–3.01, P < 0.00001, I2 = 86%) in overall [5, 11, 18, 22,23,24, 26, 28,29,30,31, 33,34,35,36, 39,40,41,42, 47,48,49,50,51,52], 2.65 (95% CI: 2.17–3.25, P < 0.00001, I2 = 72%) for males [23, 29, 31, 33,34,35, 39,40,41, 49, 51], and 3.16 (95% CI: 2.51–3.97, P < 0.00001, I2 = 84%) for females [7, 23, 29, 31, 34, 39,40,41, 49, 51], respectively (Fig. 2). In these studies, 20 studies were cross-sectional studies [7, 18, 22,23,24, 26, 29, 31, 33,34,35,36, 39,40,41, 47,48,49,50, 52] and 5 studies were cohort studies [5, 28, 30, 41, 51]. Subgroup analyses were done, and the results were similar between two research methods. In cross-sectional studies, the pooled OR for diabetes related to HTW phenotype was 2.66 (95% CI: 2.35–3.01, P < 0.00001, I2 = 87%). In cohort studies, the pooled OR for diabetes related to HTW phenotype was 2.89 (95% CI: 1.97–4.25, P < 0.00001, I2 = 83%). In 13 studies [23, 24, 28, 30, 31, 35, 40, 42, 47,48,49,50,51], the samples were devided into four groups, NWNT, NWHT, EWNT, and HTW phenotype. Compared with NWNT, the pooled OR for diabetes was 2.37 (95% CI: 2.04–2.75, P < 0.00001, I2 = 65%) in NWHT, 2.66 (95% CI: 2.11–3.34, P < 0.00001, I2 = 91%) in EWNT and 4.66 (95% CI: 3.60–6.03, P < 0.00001, I2 = 94%) in HTW phenotype. Compared with NWHT and EWNT, the pooled ORs for diabetes related to HTW phenotype was 1.88 (95% CI: 1.57–2.25, P < 0.00001, I2 = 79%) and 1.76 (95% CI: 1.53–2.02, P < 0.00001, I2 = 80%), respectively (Fig. 3).
Risk of diabetes according to the status of waist circumference and triglyceride. NWNT normal WC and normal TG level, NWHT normal WC and high TG level, EWNT enlarged WC and normal TG level, HTW enlarged WC and high TG level, WC waist circumference, TG triglyceride. a NWNT vs. NWHT, b NWNT vs. EWNT, c NWNT vs. HTW, d NWHT vs. HTW, e EWNT vs. HTW
The risk of elevated blood glucose in the HTW phenotype was also increased. The pooled OR for elevated blood glucose related to HTW phenotype was 2.32 (95% CI: 1.98–2.71, P < 0.00001, I2 = 80%) in overall [8, 11, 13, 15, 18,19,20, 22,23,24, 32, 39, 46, 48, 49, 51], 2.35 (95% CI: 1.79–3.09, P < 0.00001, I2 = 66%) for males [8, 23, 32, 39, 49, 51], and 2.89 (95% CI: 2.25–3.72, P < 0.00001, I2 = 70%) for females [8, 15, 23, 32, 39, 49, 51], respectively. In these studies, 12 studies were about adults [8, 13, 15, 18, 22,23,24, 39, 46, 48, 49, 51] and 4 studies were about children [11, 19, 20, 32]. In adults, the pooled OR for elevated blood glucose related to HTW phenotype was 2.45 (95% CI: 2.08–2.88, P < 0.00001, I2 = 82%). In children, the pooled OR for elevated blood glucose related to HTW phenotype was 1.66 (95% CI: 1.02–2.69, P = 0.04, I2 = 47%). In seven studies [13, 23, 24, 46, 48, 49, 51], the samples were devided into four groups, NWNT, NWHT, EWNT, and HTW phenotype. Compared with NWNT, the pooled OR for elevated blood glucose was 2.06 (95% CI: 1.80–2.35, P < 0.00001, I2 = 36%) in NWHT, 2.23 (95% CI: 1.86–2.67, P < 0.00001, I2 = 79%) in EWNT and 3.67 (95% CI: 2.68–5.03, P < 0.00001, I2 = 91%) in HTW phenotype. Compared with NWHT and EWNT, the pooled ORs for elevated blood glucose related to HTW phenotype was 1.70 (95% CI: 1.41–2.06, P < 0.00001, I2 = 54%) and 1.61 (95% CI: 1.35–1.91, P < 0.00001, I2 = 68%), respectively.
Overall, 18 studies analyzed the relationship between HTW phenotype and insulin resistance (Supplementary Table 2). In these studies, insulin resistance was evaluated by homeostasis model assessment of insulin resistance (HOMA-IR) in 16 studies [6, 7, 12, 14, 16, 23, 25, 27,28,29, 36, 38, 41, 45, 46, 49], by HOMA2-IR in one study [8] and by euglycaemic–hyperinsulinaemic clamp technique in one study [17]. Due to only one study, the study of HOMA2-IR and euglycaemic–hyperinsulinaemic clamp technique was not involved in the quantitative synthesis. The results were statistically different from non-HTW phenotype to HTW phenotype with values of MD 1.12 (95% CI: 0.81–1.43, P < 0.00001, I2 = 99%) (Fig. 4a). The levels of HOMA-IR in the HTW population were increased for both men and women. The results were statistically different from non-HTW phenotype to HTW phenotype with values of MD 1.18 (95% CI: 0.91–1.44, P < 0.00001, I2 = 77%) for males [12, 23, 29, 41, 45] and MD 1.01 (95% CI: 0.82–1.21, P < 0.00001, I2 = 75%) for females [7, 23, 25, 29, 41, 45], respectively. In these 16 studies, the data were described as mean (SD, 95% CI, or SE) and geometric mean (95% CI) in 11 studies [6, 7, 12, 14, 16, 25, 27, 28, 38, 45, 49] and median (interquartile range) in 6 studies [23, 29, 36, 41, 46, 49]. Subgroup analyses were done, and the results were similar between two description methods. HTW phenotype was also worse insulin resistance than non-HTW phenotype in five studies without diabetes patients with an MD 0.89 (95% CI: 0.75–1.04, P < 0.00001, I2 = 67%) [25, 28, 41, 46, 49]. In five studies [23, 28, 38, 46, 49], the samples were devided into four groups, NWNT, NWHT, EWNT, and HTW phenotypes. The results were statistically different from NWNT to NWHT (MD 0.57, 95% CI: 0.44–0.70, P < 0.00001, I2 = 86%), EWNT (MD 0.76, 95% CI: 0.57–0.94, P < 0.00001, I2 = 96%), and HTW phenotype (MD 1.49, 95% CI: 1.24–1.73, P < 0.00001, I2 = 95%), respectively. The results were also statistically different from NWHT to HTW phenotype (MD 0.87, 95% CI: 0.72–1.02, P < 0.00001, I2 = 76%) and EWNT to HTW phenotype (MD 0.73, 95% CI: 0.60–0.85, P < 0.00001, I2 = 74%), respectively (Fig. 5). Three studies analyzed the relationship between HTW phenotype and islet β cell function. In these studies, islet β cell function was evaluated by homeostasis model assessment of β cell function (HOMA-β) in two studies [12, 41], by HOMA2-β in one study [8]. In two studies of HOMA-β, the results were not statistically different from non-HTW phenotype to HTW phenotype with values of MD 33.88 (95% CI: −13.37−81.13. P = 0.16, I2 = 91%) (Fig. 4b).
The levels of insulin resistance according to the status of waist circumference and triglyceride. NWNT normal WC and normal TG level, NWHT normal WC and high TG level, EWNT enlarged WC and normal TG level, HTW enlarged WC and high TG level, WC waist circumference, TG triglyceride. a NWNT vs. NWHT, b NWNT vs. EWNT, c NWNT vs. HTW, d NWHT vs. HTW, e EWNT vs. HTW
Five studies analyzed the metabolic abnormality (Supplementary Table 3). Overall, the results were statistically different from non-HTW phenotype to HTW phenotype with values of MD 1.18 (95% CI: 0.24–2.12, P = 0.01, I2 = 84%) for FPG [9, 10, 37], MD 0.47 (95% CI: 0.30–0.64, P < 0.00001, I2 = 25%) for HbA1c [9, 10, 21, 37, 43], MD 0.98 (95% CI: 0.73–1.23, P < 0.00001, I2 = 0%) for TC [9, 21], MD 0.36 (95% CI: 0.19–0.52, P < 0.0001, I2 = 0%) for LDL [9, 21, 43], MD −0.18 (95% CI: −0.27 to −0.08, P = 0.0003, I2 = 73%) for HDL [9, 10, 21, 43], MD 20.27 (95% CI: 14.78–25.75, P < 0.00001, I2 = 0%) for Apo B [21, 43], MD 6.33 (95% CI: 1.52–11.15, P = 0.010, I2 = 71%) for SBP [9, 10, 21], and MD 5.30 (95% CI: 1.70–8.90, P = 0.004, I2 = 84%) for DBP [9, 10, 21], respectively. Diabetic complications were analyzed in four studies, one about ischemic stroke [48], two about coronary artery disease [21, 44], and one about early diabetic nephropathy [10]. Due to a limited number of studies, the results of these studies were only shown in Supplementary Table 4.
We performed a sensitivity analysis by omitting one study at a time to estimate the pooled ORs of elevated blood glucose and diabetes, and MD of HOMA-IR. No individual study affected the ultimate conclusion. Visual inspection of the funnel plot revealed symmetry for elevated blood glucose and diabetes, and the Egger’s test was not significant (elevated blood glucose P = 0.883, diabetes P = 0.605). Visual inspection of the funnel plot revealed asymmetry for HOMA-IR (Supplementary Figs. 1–3).
Discussion
Our study is the first systematic review with meta-analysis to evaluate the application value of HTW phenotype in each phase of abnormal glucose metabolism. A previous study focused on diabetes [4]. HTW phenotype had an association with abnormal glucose metabolism regardless of gender, age, and the definition of abnormal glucose metabolism. This study provides evidence that subjects with HTW phenotype were 2.32-fold and 2.69-fold as likely to have elevated blood glucose and diabetes as those with non-HTW phenotype. The results were similar between genders. Unlike adult researches, the results of children were not uniform [11, 19, 20, 32]. Pooled analysis shown that HTW phenotype also correlated with abnormal glucose metabolism in children.
A lot of researches have shown that abdominal obesity and hypertriglyceridemia are independent risk factors for abnormal glucose metabolism [53,54,55,56]. Compared with NWNT subjects, NWHT and EWNT have higher risk of abnormal glucose metabolism and the risk further increased in subjects with HTW phenotype. These results highlight the importance of the simultaneous analysis of WC and TG in clinical screening of individuals at risk for abnormal glucose metabolism.
The results from cross-sectional studies and cohort studies were consistent. Whether in cross-sectional studies or in cohort studies, HTW phenotype is all related to diabetes. The results suggest that the prevalence of diabetes was higher in subjects with HTW phenotype. Subjects with HTW phenotype have already existed abnormal glucose metabolism. Even in HTW subjects without diabetes, the incidence of diabetes was higher in the future. Subjects with HTW phenotype, all should screen diabetes in the baseline and in the future. HTW phenotype is an important cause of diabetes. Insulin resistance and islet β cell dysfunction may be the biological mechanism linking HTW phenotype and abnormal glucose metabolism.
Insulin plays a critical role in balancing glucose metabolism in the body. Glucose metabolism is normally regulated by a feedback loop, including islet β cells and insulin-sensitive tissues. If insulin resistance is present, β cells maintain normal glucose tolerance by increasing insulin output. Only when β cells cannot release sufficient insulin in the presence of insulin resistance do glucose concentrations rise [57]. HTW phenotype was closely related to insulin resistance, even in subjects without diabetes. Compared with sole high TG or enlarged WC, HTW phenotype has more serious insulin resistance. Our study shown overstimulation of β-cell function in subjects without diabetes, although there is no significant statistical difference. This may be due to insulin secretion increases to maintain normal glucose levels in the face of insulin resistance resulting from abdominal obesity and hypertriglyceridemia. Bardini et al. found that β-cell function declined in impaired glucose tolerance (IGT) subjects with HTW phenotype [8]. With the progress of abnormal glucose metabolism, β-cell function declined earlier in HTW phenotype than non-HTW phenotype.
The UK Prospective Diabetes Study (UKPDS) has demonstrated that blood glucose control decreases the risk of microvascular complications in patients with type 2 diabetes [58]. Diabetic patients often accompany with cardiovascular risk factors, such as elevated blood pressure, dyslipidemia, etc., and higher incidence of cardiovascular diseases [59, 60]. Multiple risk-factor control may lower the increased risks for mortality and cardiovascular events in people with diabetes [61]. Our study shows that subjects with HTW phenotype have inappropriate blood glucose control and worse cardiovascular risk factor in diabetic patients.
The burden of diabetes is associated primarily with increased risks of macrovascular and microvascular diseases. HTW phenotype was associated with an increased risk for coronary artery disease and chronic kidney disease [36, 62]. The study about diabetic complications is fewer. The existing researches have found that HTW phenotype was associated with coronary artery disease and early diabetic nephropathy in type 2 diabetes [10, 21]. Interestingly, HTW phenotype is also associated with an increased risk of ischemic stroke in subjects with normal glucose levels, but not in subjects with impaired fasting glucose and diabetes [48]. When HTW phenotype is used to screening diabetic complications, further work will be necessary to confirm the performance of HTW phenotype.
There are some strengths in our meta-analysis. First, the systematic search was conducted from inception to February 2014 in previous meta-analysis [4]. In our studies, a systematic search was conducted from inception to June 17, 2018. Therefore, many new studies were included in our study. A total of 48 studies were included in our review, and 23 (47.9%) of them were published from 2015 to 2018. Second, the prevention of diabetes is of paramount importance. HTW phenotype can be used for screening high-risk groups for future diabetes in subjects without diabetes. Third, to our knowledge, this is the first meta-analysis to evaluate the relationship between HTW phenotype and insulin resistance. Subjects with HTW phenotype have more serious insulin resistance, even among subjects without diabetes. Fourth, our meta-analysis found that HTW phenotype can be used for evaluating the cardiovascular risk factors in type 2 diabetes. Fifth, HTW phenotype was proposed because HTW phenotype has more serious atherogenic metabolic abnormalities compared with NWHT and EWNT [3]. In our meta-analysis, some data were analyzed according to four groups: NWNT, NWHT, EWNT, and HTW phenotypes. HTW phenotype has higher risk of abnormal glucose metabolism compared with subjects with NWNT, NWHT, and EWNT.
There are some limitations in our meta-analysis. First, we found heterogeneity in the meta-analyses, and the heterogeneity still remained in many subgroup analysis. Second, the definition of HTW phenotype varied across studies. It is well known that WC was different in different races. Population- and country-specific definition of WC was recommended [63]. Third, abnormal glucose metabolism in most studies were evaluated by FPG only, not OGTT. Fourth, median (IQR) was transformed to mean (SD) in the studies of HOMA indexes. The distribution of HOMA indexes are skewed in these studies. This conversion might not be appropriate. So subgroup analyses were done, and the results were similar between two description methods, median (IQR) and mean (SD).
Prospect
The studies about HTW phenotype and abnormal glucose metabolism were observational study. As compared with the consistent HTW phenotype, the risk of developing type 2 diabetes was greatly reduced in subjects recovering to normal TG level and/or normal WC [41]. The intervention study should be done in HTW phenotype to prove whether appropriate intervention can improve insulin resistance or prevent future diabetes and diabetic complications.
Conclusion
HTW phenotype was closely associated with increased risk of abnormal glucose metabolism in the general population. There was also a significant correlation between HTW phenotype and insulin resistance. With the progress of abnormal glucose metabolism, β-cell function declines earlier in HTW phenotype. HTW phenotype has inappropriate blood glucose control and worse cardiovascular risk factor. The relationship between HTW phenotype and diabetic complications should be further confirmed.
Study Highlights
What is already known about this subject?
-
Diabetes is a global health burden due to its high prevalence, deaths attributable and healthcare expenditure.
-
HTW phenotype was significantly associated with risk of type 2 diabetes.
What does this study add?
-
HTW phenotype can predict future diabetes in subjects without diabetes.
-
Subjects with HTW phenotype have more serious insulin resistance, even among subjects without diabetes.
-
HTW phenotype has inappropriate blood glucose control and worse cardiovascular risk factor in type 2 diabetes.
References
L. Guariguata, D.R. Whiting, I. Hambleton, J. Beagley, U. Linnenkamp, J.E. Shaw, Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 103(2), 137–149 (2014)
N.H. Cho, J.E. Shaw, S. Karuranga, Y. Huang, J.D. da Rocha Fernandes, A.W. Ohlrogge et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138, 271–281 (2018)
I. Lemieux, A. Pascot, C. Couillard, B. Lamarche, A. Tchernof, N. Almeras et al. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation 102(2), 179–184 (2000)
Y. Ren, X. Luo, C. Wang, L. Yin, C. Pang, T. Feng et al. Prevalence of hypertriglyceridemic waist and association with risk of type 2 diabetes mellitus: a meta-analysis. Diabetes Metab. Res. Rev. 32(4), 405–412 (2016)
S. He, Y. Zheng, Y. Shu, J. He, Y. Wang, X. Chen, Hypertriglyceridemic waist might be an alternative to metabolic syndrome for predicting future diabetes mellitus. PLoS ONE 8(9), e73292 (2013)
F. Abbasi, A. Mathur, G.M. Reaven, C.R. Molina, Cardiometabolic risk in South Asian inhabitants of California: hypertriglyceridemic waist vs hypertriglyceridemic body mass index. Ethn. Dis. 26(2), 191–196 (2016)
P. Blackburn, I. Lemieux, B. Lamarche, J. Bergeron, P. Perron, G. Tremblay et al. Hypertriglyceridemic waist: a simple clinical phenotype associated with coronary artery disease in women. Metabolism 61(1), 56–64 (2012)
G. Bardini, I. Dicembrini, L. Pala, B. Cresci, C.M. Rotella, Hypertriglyceridaemic waist phenotype and beta-cell function in subjects with normal and impaired glucose tolerance. Diabet. Med. 28(10), 1229–1233 (2011)
S.P. Radenkovic, R.D. Kocic, M.M. Pesic, D.N. Dimic, M.D. Golubovic, D.B. Radojkovic et al. The hypertriglyceridemic waist phenotype and metabolic syndrome by differing criteria in type 2 diabetic patients and their relation to lipids and blood glucose control. Endokrynol. Pol. 62(4), 316–323 (2011)
C.M. Ma, R. Wang, X.L. Liu, N. Lu, Q. Lu, F.Z. Yin, The relationship between hypertriglyceridemic waist phenotype and early diabetic nephropathy in type 2 diabetes. Cardiorenal Med. 7(4), 295–300 (2017)
D.P. Bailey, L.A. Savory, S.J. Denton, B.R. Davies, C.J. Kerr, The hypertriglyceridemic waist, waist-to-height ratio, and cardiometabolic risk. J. Pediatr. 162(4), 746–752 (2013)
P. Blackburn, B. Lamarche, C. Couillard, A. Pascot, N. Bergeron, D. Prud’homme et al. Postprandial hyperlipidemia: another correlate of the “hypertriglyceridemic waist” phenotype in men. Atherosclerosis 171(2), 327–336 (2003)
D. Brisson, P. Perron, S.P. Guay, D. Gaudet, L. Bouchard, The “hypertriglyceridemic waist” phenotype and glucose intolerance in pregnancy. CMAJ: Can. Med. Assoc. journal = journal de. l’Assoc. Med. Can. 182(15), E722–E725 (2010)
D.S. Buchan, L.M. Boddy, J.P. Despres, F.M. Grace, N. Sculthorpe, C. Mahoney et al. Utility of the hypertriglyceridemic waist phenotype in the cardiometabolic risk assessment of youth stratified by body mass index. Pedia. Obes. 11(4), 292–298 (2016)
N.A. Cabral, V.S. Ribeiro, A.K. Franca, J.V. Salgado, A.M. Santos, N. Salgado Filho et al. Hypertriglyceridemic waist and cardiometabolic risk in hypertensive women. Rev. da Assoc. Med. Bras. (1992) 58(5), 568–573 (2012)
A.L. Cabral Rocha, P. Feliciano Pereira, M. Cristine Pessoa, C. Goncalves Alfenas Rde, W. Segheto, D.C. da Silva et al. Hypertriglyceridemic waist phenotype and cardiometabolic alterations in Brazilian adults. Nutr. Hosp. 32(3), 1099–1106 (2015)
A.C. Carlsson, U. Riserus, J. Arnlov, Hypertriglyceridemic waist phenotype is associated with decreased insulin sensitivity and incident diabetes in elderly men. Obes. (Silver Spring, Md) 22(2), 526–529 (2014)
S. Chen, X. Guo, S. Yu, G. Sun, Z. Li, Y. Sun, Association between the hypertriglyceridemic waist phenotype, prediabetes, and diabetes mellitus in rural Chinese population: a cross-sectional study. Int. J. Environ. Res. Public. Health 13(4), 368 (2016)
M.E. Conceicao-Machado, L.R. Silva, M.L. Santana, E.J. Pinto, C. Silva Rde, L.T. Moraes et al. Hypertriglyceridemic waist phenotype: association with metabolic abnormalities in adolescents. J. Pediatr. (Rio J.) 89(1), 56–63 (2013)
P.R.F. Costa, A.M.O. Assis, C.M. Cunha, E.M. Pereira, G.S. De Jesus, L.E.M. Da Silva et al. Hypertriglyceridemic waist phenotype and changes in the fasting glycemia and blood pressure in children and adolescents over one-year follow-up period. Arquivos Brasileiros de. Cardiologia 109(1), 47–53 (2017)
F.R. de Graaf, J.D. Schuijf, A.J. Scholte, R. Djaberi, J.E. van Velzen, C.J. Roos et al. Usefulness of hypertriglyceridemic waist phenotype in type 2 diabetes mellitus to predict the presence of coronary artery disease as assessed by computed tomographic coronary angiography. Am. J. Cardiol. 106(12), 1747–1753 (2010)
M.V. Diaz-Santana, E.L. Suarez Perez, A.P. Ortiz Martinez, M. Guzman Serrano, C.M. Perez Cardona, Association between the hypertriglyceridemic waist phenotype, prediabetes, and diabetes mellitus among adults in Puerto Rico. J. Immigr. Minor. Health 18(1), 102–109 (2016)
T. Du, X. Sun, R. Huo, X. Yu, Visceral adiposity index, hypertriglyceridemic waist and risk of diabetes: the China Health and Nutrition Survey 2009. Int. J. Obes. (Lond.). 38(6), 840–847 (2014)
G.M. Egeland, Z. Cao, T.K. Young, Hypertriglyceridemic-waist phenotype and glucose intolerance among Canadian Inuit: the International Polar Year Inuit Health Survey for Adults 2007-2008. CMAJ: Can. Med. Assoc. journal = journal de. l’Assoc. Med. Can. 183(9), E553–E558 (2011)
I.F. Gazi, H.J. Milionis, T.D. Filippatos, V. Tsimihodimos, M.S. Kostapanos, M. Doumas et al. Hypertriglyceridaemic waist phenotype criteria and prevalent metabolic triad in women. Diabetes Metab. Res. Rev. 24(3), 223–230 (2008)
R. Gomez-Huelgas, M.R. Bernal-Lopez, A. Villalobos, J. Mancera-Romero, A.J. Baca-Osorio, S. Jansen et al. Hypertriglyceridemic waist: an alternative to the metabolic syndrome? Results of the IMAP study (multidisciplinary intervention in primary care). Int. J. Obes. (Lond.). 35(2), 292–299 (2011)
V.L. Guattini, C.H. Piovesan, E. Wittke, A. Marcadenti, Hypertriglyceridemic waist (Ewet), glycidic and lipid profile in patients with newly diagnosed heart attack. Nutr. Hosp. 32(3), 1004–1008 (2015).
K.J. Han, S.Y. Lee, N.H. Kim, H.B. Chae, T.H. Lee, C.M. Jang et al. Increased risk of diabetes development in subjects with the hypertriglyceridemic waist phenotype: a 4-year longitudinal study. Endocrinol. Metab. (Seoul., Korea) 29(4), 514–521 (2014)
J. Huang, C. Zhou, Y. Li, S. Zhu, A. Liu, X. Shao et al. Visceral adiposity index, hypertriglyceridemic waist phenotype and chronic kidney disease in a southern Chinese population: a cross-sectional study. Int. Urol. Nephrol. 47(8), 1387–1396 (2015)
M. Janghorbani, M. Amini, Utility of hypertriglyceridemic waist phenotype for predicting incident type 2 diabetes: The Isfahan Diabetes Prevention Study. J. Diabetes Investig. 7(6), 860–866 (2016)
A. Karagoz, A. Onat, M. Aydin, G. Can, B. Simsek, M. Yuksel, Distinction of hypertriglyceridemic waist phenotype from simple abdominal obesity: interaction with sex hormone-binding globulin levels to confer high coronary risk. Postgrad. Med. 129(2), 288–295 (2017)
R. Kelishadi, F. Jamshidi, M. Qorbani, M.E. Motlagh, R. Heshmat, G. Ardalan et al. Association of hypertriglyceridemic-waist phenotype with liver enzymes and cardiometabolic risk factors in adolescents: the CASPIAN-III study. J. Pediatr. (Rio J.) 92(5), 512–520 (2016)
S. LeBlanc, F. Coulombe, O.F. Bertrand, K. Bibeau, P. Pibarot, A. Marette, et al. Hypertriglyceridemic waist: a simple marker of high-risk atherosclerosis features associated with excess visceral adiposity/ectopic fat. J. Am. Heart Assoc 7(8), e008139 (2018). https://doi.org/10.1161/JAHA.117.008139
B.J. Lee, J.Y. Kim, Identification of type 2 diabetes risk factors using phenotypes consisting of anthropometry and triglycerides based on machine learning. IEEE J. Biomed. Health Inf. 20(1), 39–46 (2016)
I. Lemieux, N. Almeras, P. Mauriege, C. Blanchet, E. Dewailly, J. Bergeron et al. Prevalence of ‘hypertriglyceridemic waist’ in men who participated in the Quebec Health Survey: association with atherogenic and diabetogenic metabolic risk factors. Can. J. Cardiol. 18(7), 725–732 (2002)
Y. Li, C. Zhou, X. Shao, X. Liu, J. Guo, Y. Zhang et al. Hypertriglyceridemic waist phenotype and chronic kidney disease in a Chinese population aged 40 years and older. PLoS ONE 9(3), e92322 (2014)
O. Mayer, J. Seidlerova, J. Bruthans, K. Timoracka, P. Vagovicova, J. Vanek et al. Hypertriglyceridemic waist increased risk of inappropriate glucose control in patients with coronary heart disease. Clin. Lipidol. 9(5), 515–522 (2014)
B.S. Moon, H.J. Park, M.K. Lee, W.S. Jeon, S.E. Park, C.Y. Park et al. Increased association of coronary artery calcification in apparently healthy Korean adults with hypertriglyceridemic waist phenotype: The Kangbuk Samsung Health Study. Int. J. Cardiol. 194, 78–82 (2015)
R.L. Pollex, A.J. Hanley, B. Zinman, S.B. Harris, R.A. Hegele, Clinical and genetic associations with hypertriglyceridemic waist in a Canadian aboriginal population. Int. J. Obes. (Lond.). 30(3), 484–491 (2006)
A. Ramezankhani, F. Azizi, A. Ghanbarian, D. Parizadeh, F. Hadaegh, The hypertriglyceridemic waist and waist-to-height ratio phenotypes and chronic kidney disease: cross-sectional and prospective investigations. Obes. Res Clin. Pr. 11(5), 585–596 (2017)
Y. Ren, Y. Liu, X. Sun, K. Deng, C. Wang, L. Li et al. Hypertriglyceridemia-waist and risk of developing type 2 diabetes: The Rural Chinese Cohort Study. Sci. Rep. 7(1), 9072 (2017)
Y. Ren, M. Zhang, J. Zhao, C. Wang, X. Luo, J. Zhang et al. Association of the hypertriglyceridemic waist phenotype and type 2 diabetes mellitus among adults in China. J. Diabetes Investig. 7(5), 689–694 (2016)
S. Sam, S. Haffner, M.H. Davidson, R.B. D’Agostino Sr., S. Feinstein, G. Kondos et al. Hypertriglyceridemic waist phenotype predicts increased visceral fat in subjects with type 2 diabetes. Diabetes care 32(10), 1916–1920 (2009)
J. St-Pierre, I. Lemieux, P. Perron, D. Brisson, M. Santure, M.C. Vohl et al. Relation of the “hypertriglyceridemic waist” phenotype to earlier manifestations of coronary artery disease in patients with glucose intolerance and type 2 diabetes mellitus. Am. J. Cardiol. 99(3), 369–373 (2007)
H. Vaverkova, D. Karasek, D. Novotny, M. Halenka, J. Orsag, L. Slavik, Hypertriglyceridemic waist - a simple clinical tool to detect cardiometabolic risk: comparison with harmonized definition of metabolic syndrome. Physiol. Res. 64(Suppl 3), S385–S394 (2015)
H. von Bibra, S. Saha, A. Hapfelmeier, G. Muller, P.E.H. Schwarz, Impact of the triglyceride/high-density lipoprotein cholesterol ratio and the hypertriglyceremic-waist phenotype to predict the metabolic syndrome and insulin resistance. Horm. Metab. research = Hormon- und Stoffwechselforschung = Hormones et. Metab. 49(7), 542–549 (2017)
A. Wang, Z. Li, Y. Zhou, C. Wang, Y. Luo, X. Liu et al. Hypertriglyceridemic waist phenotype and risk of cardiovascular diseases in China: results from the Kailuan Study. Int. J. Cardiol. 174(1), 106–109 (2014)
W. Wang, C. Shen, H. Zhao, W. Tang, S. Yang, J. Li et al. A prospective study of the hypertriglyceridemic waist phenotype and risk of incident ischemic stroke in a Chinese rural population. Acta Neurol. Scand. 138(2), 156–162 (2018)
Z. Yu, L. Sun, Q. Qi, H. Wu, L. Lu, C. Liu et al. Hypertriglyceridemic waist, cytokines and hyperglycaemia in Chinese. Eur. J. Clin. Invest. 42(10), 1100–1111 (2012)
J. Zeng, M. Liu, L. Wu, J. Wang, S. Yang, Y. Wang et al. The Association of hypertriglyceridemic waist phenotype with chronic kidney disease and its sex difference: a cross-sectional study in an urban Chinese elderly population. Int. J. Environ. Res. Public Health 13(12), E1233 (2016)
M. Zhang, Y. Gao, H. Chang, X. Wang, D. Liu, Z. Zhu et al. Hypertriglyceridemic-waist phenotype predicts diabetes: a cohort study in Chinese urban adults. Bmc. Public. Health 12, 1081 (2012)
C. Zhou, Y. Li, X. Shao, H. Zou, Identification of chronic kidney disease risk in relatively lean Southern Chinese: the hypertriglyceridemic waist phenotype vs. anthropometric indexes. Eat Weight Disord.: EWD 23(6), 885–892 (2018). https://doi.org/10.1007/s40519-017-0476-8
H. Xue, C. Wang, Y. Li, J. Chen, L. Yu, X. Liu et al. Incidence of type 2 diabetes and number of events attributable to abdominal obesity in China: A cohort study. J. Diabetes 8(2), 190–198 (2016)
S.H. Ryu, S.H. Beck, Y.S. Chang, D.I. Kim, B.S. Suh, W.S. Kim et al. Abdominal obesity in relation to the incidence of type 2 diabetes mellitus and impaired fasting glucose among some Korean adults: A Retrospective Cohort Study. J. Prev. Med. Public Health 37(4), 359–365 (2004)
J.P. Reis, A.L. Hankinson, C.M. Loria, C.E. Lewis, T. Powell-Wiley, G.S. Wei et al. Duration of abdominal obesity beginning in young adulthood and incident diabetes through middle age: the CARDIA study. Diabetes Care 36(5), 1241–1247 (2013)
F. Guerrero-Romero, M. Rodriguez-Moran, Hypertriglyceridemia is associated with development of metabolic glucose disorders, irrespective of glucose and insulin levels: a 15-year follow-up study. Eur. J. Intern. Med. 25(3), 265–269 (2014)
S.E. Kahn, M.E. Cooper, S. Del Prato, Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383(9922), 1068–1083 (2014)
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33), UK Prospective Diabetes Study (UKPDS) Group. Lancet 352(9131), 837–853 (1998).
N.D. Wong, Y. Zhao, R. Patel, C. Patao, S. Malik, A.G. Bertoni et al. Cardiovascular risk factor targets and cardiovascular disease event risk in diabetes: A Pooling Project of the Atherosclerosis Risk in Communities Study, Multi-Ethnic Study of Atherosclerosis, and Jackson Heart Study. Diabetes Care 39(5), 668–676 (2016)
E.Y.F. Wan, C.S.C. Fung, E.Y.T. Yu, W.Y. Chin, D.Y.T. Fong, A.K.C. Chan, et al. Effect of multifactorial treatment targets and relative importance of hemoglobin A1c, blood pressure, and low-density lipoprotein-cholesterol on cardiovascular diseases in chinese primary care patients with type 2 diabetes mellitus: A Population-Based Retrospective Cohort Study. J. Am. Heart Assoc 6(8), e006400 (2017). https://doi.org/10.1161/JAHA.117.006400
S. Hamada, M.C. Gulliford, Multiple risk factor control, mortality and cardiovascular events in type 2 diabetes and chronic kidney disease: a population-based cohort study. BMJ Open 8(5), e019950 (2018)
B.J. Arsenault, I. Lemieux, J.P. Despres, N.J. Wareham, J.J. Kastelein, K.T. Khaw et al. The hypertriglyceridemic-waist phenotype and the risk of coronary artery disease: results from the EPIC-Norfolk prospective population study. CMAJ: Can. Med. Assoc. journal = journal de. l’Assoc. Med. Can. 182(13), 1427–1432 (2010)
K.G. Alberti, R.H. Eckel, S.M. Grundy, P.Z. Zimmet, J.I. Cleeman, K.A. Donato et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16), 1640–1645 (2009)
Funding
The funding of this study was self-financing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
This paper is a system review and meta-analysis. No informed consent was obtained.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ma, CM., Liu, XL., Lu, N. et al. Hypertriglyceridemic waist phenotype and abnormal glucose metabolism: a system review and meta-analysis. Endocrine 64, 469–485 (2019). https://doi.org/10.1007/s12020-019-01945-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-01945-6