Abstract
Purpose
Many different treatments are suggested by guidelines to treat grade 1−2 (G1−G2) neuroendocrine tumors (NET). However, a precise therapeutic algorithm has not yet been established. This study aims at identifying and comparing the main therapeutic sequences in G1−G2 NET.
Methods
A retrospective observational Italian multicenter study was designed to collect data on therapeutic sequences in NET. Median progression-free survival (PFS) was compared between therapeutic sequences, as well as the number and grade of side effects and the rate of dose reduction/treatment discontinuation.
Results
Among 1182 patients with neuroendocrine neoplasia included in the ELIOS database, 131 G1–G2 gastroenteropancreatic, lung and unknown primary NET, unresectable or persistent/relapsing after surgery, treated with ≥2 systemic treatments, were included. Four main therapeutic sequences were identified in 99 patients: (A) somatostatin analogs (SSA) standard dose to SSA high dose (n = 36), (B) SSA to everolimus (n = 31), (C) SSA to chemotherapy (n = 17), (D) SSA to peptide receptor radionuclide therapy (PRRT) (n = 15). Median PFS of the second-line treatment was not reached in sequence A, 33 months in sequence B, 20 months in sequence C, 30 months in sequence D (p = 0.16). Both total number and severity of side effects were significantly higher in sequences B and C than A and D (p = 0.04), as well as the rate of dose reduction/discontinuation (p = 0.03).
Conclusions
SSA followed by SSA high dose, everolimus, chemotherapy or PRRT represent the main therapeutic sequences in G1−G2 NET. Median PFS was not significantly different between sequences. However, the sequences with SSA high dose or PRRT seem to be better tolerated than sequences with everolimus or chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine neoplasias are a heterogeneous group of tumors that originate from different tissues and organs, with more than half of the cases involving the digestive system [1]. Their incidence has dramatically increased in the last 40 years, being now estimated to be about 7 per 100,000 persons [2]. Most cases arise from pancreas, intestine and lung and are well-differentiated neuroendocrine tumors (NET), grade 1 (G1) and grade 2 (G2), which are characterized by indolent growth rate and long-time survival. However, more than 50% of the cases present with regional or distant metastases at the time of diagnosis [2]. Whereas the treatment of choice of localized NET is surgical resection, a wide spectrum of therapeutic options is available for patients with advanced, unresectable NET. The management of patients with metastatic or regionally advanced G1 or G2 gastroenteropancreatic (GEP) and lung NET is based on different therapeutic options such as somatostatin analogs (SSA), molecular targeting therapies (everolimus and sunitinib), chemotherapy and peptide receptor radionuclide therapy (PRRT) [3,4,5]. These can be used alone or as a multimodal approach. All these treatments can be considered in this setting according to guidelines [6,7,8,9]. However, a precise therapeutic algorithm defining the sequence of treatments in patients with unresectable G1−G2 NET has not yet been established.
In the present study, we aim to identify the most frequent therapeutic sequences in patients with G1−G2 NET, as well as to compare efficacy and toxicity between the identified sequences.
Patients and methods
Study design
This is a retrospective observational study. Data have been collected through an e-CRF and stored in a centralized computer database ad hoc created and active since 2011 in the context of the project “ELIOS” (Educational Learning Investigational Observational Study). A first observational study has been performed to evaluate the response to SSA according to Ki67 index in patients with NET [10]. In September 2016 the ELIOS group started a new observational study to evaluate and compare efficacy and tolerability of well-defined therapeutic sequences in patients with GEP, lung and unknown primary site NET. The current one is an Italian multicenter study where retrospective data of patients in follow-up in one of the centers of the ELIOS group from January 2005 to September 2017 were included. For this project, eight centers were involved in the ELIOS group, Federico II University of Naples, Cardarelli Hospital, National Cancer Institute of Naples Fondazione G. Pascale, Monaldi Hospital, University of Palermo, Istituto Oncologico del Mediterraneo, IRCCS Casa Sollievo della Sofferenza, Lecce Hospital.
Because no previous study explored the relationship between therapeutic sequences with PFS in patients with NET, it was not possible to define a relevant effect size and, consequently, a priori power analysis to determine the required sample size. Therefore, the sampling was based on the retrospective revision of a multicentric NET database, by taking into account those NET patients fulfilling the inclusion criteria reported below and receiving the most frequent therapeutic sequences among those used in the clinical practice until now.
Inclusion criteria were: age ≥ 18 yrs, histopathologically confirmed GEP, lung and unknown primary NET, well or moderately differentiated (G1 or G2 according to WHO 2010 for GEP NET or typical and atypical lung carcinoids according to WHO 2015 for thoracic NET) [11, 12], metastatic or locally advanced disease at diagnosis or persisting/relapsing after surgery, functioning or nonfunctioning, at least two types of antitumor treatments among SSA, targeted therapy, chemotherapy, PRRT, administered sequentially as first- and second-line therapy, first outpatient visit in the NET Center between 1 January 2005 and 30 September 2016, ≥1 yr follow-up, adequate liver and renal function.
The treatment sequences were established patient by patient by the multidisciplinary NET team, based on local availability and personal preferences. Treatment discontinuation occurred in patients with Response Evaluation Criteria in Solid Tumors (RECIST) progression or in those experiencing an unacceptable toxicity (grade 3 or 4). SSA at standard doses schedule, including octreotide LAR 30 mg or lanreotide autogel 120 mg every 28 days, and high doses schedule, including octreotide LAR 30 mg and lanreotide autogel 120 mg every 14−21 days, were considered as separate treatments in the analysis of sequences, according to the last ENETS guidelines where a higher than standard dose of SSA can be considered in NET patients with radiological progression [6]. Supportive concomitant therapies and external palliative radiotherapy were allowed.
Exclusion criteria were: age < 18 yrs, pregnant or breast-feeding patients, NET G3, poorly differentiated neuroendocrine carcinoma and mixed tumors, NET of primary sites other than GEP and lung.
The primary endpoints of the study were in G1−G2 unresectable treatment-naive NET: (a) to identify the most frequent therapeutic sequences (first- and second-line treatment) among those used in the clinical practice until now; (b) to compare the median progression-free survival (PFS) after first- and second-line treatment, the rate of total and serious adverse events as well as the rate of treatment discontinuation/dose reduction between the identified therapeutic sequences.
Patient characteristics
A total number of 1182 patients has been included in the ELIOS database at September 2017, involving subjects followed-up in one of the NET centers of the ELIOS group between January 2005 and September 2016. Of them, 420 patients with diagnosis of G1−G2 NET of pancreatic, gastrointestinal, lung or unknown primary were examined as potentially eligible for the study. Finally, 131 of 420 patients fulfilled all the inclusion criteria and were analyzed for the therapeutic sequences. A central pathology review was performed for all tumors included in the ELIOS database, according to the last WHO classifications of GEP and lung NET [11, 12].
Efficacy and safety assessment
Efficacy was assessed through the evaluation of median PFS after both first- and second-line treatment, defined as the time from the beginning of each line of treatment until tumor progression, treatment discontinuation, or until the last follow-up evaluation or death. Tumor progression was evaluated according to the RECIST v1.1. The radiologic assessment of tumor lesions was performed by contrast enhanced computerized tomography (CT) or magnetic resonance imaging, at baseline and every 3−6 months during the follow-up period. Toxicity was evaluated through the record of adverse events according to Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Also the rate of dose modification and treatment discontinuation after each sequence of treatment were considered.
Statistical analysis
Data were expressed as mean (±standard deviation) for continuous variables and as frequencies and percentages (%) for categorical data. To determine differences among sequences in terms of demographics and clinico-pathological characteristics, ANOVA, χ2 tests or Fisher exact were performed as appropriate. Kaplan−Meier analysis and log-rank test were used to compare PFS among sequences. Cox regression analyses were performed to provide hazard ratios (HRs). Finally, comparisons of treatment toxicity between the four sequences were performed using the χ2 test or Fisher exact test as appropriate. The statistical software R, version 3.2.5 [13] was used for all statistical analyses. The p value < 0.05 was taken as the level of significance.
Results
Patient parameters
Among the 131 patients fulfilling the inclusion criteria of the study, four most recurrent sequences were identified: (A) SSA standard dose to SSA high dose, (B) SSA to everolimus; (C) SSA to chemotherapy, (D) SSA to PRRT. SSA therapy represented the first line in all sequences. These four sequences involved 99 of 131 patients (75.6%) who formed the final dataset for the statistical analysis, as follows: sequence A 36 patients, sequence B 31 patients, sequence C 17 patients, sequence D 15 patients (Table 1). The remaining 32 patients, treated with different therapeutic sequences, were excluded (Table 2).
The final group of 99 patients included 57.6% males and 42.4% females, age at diagnosis 55.9 years on average (Table 3). According to primary site, there were 45.4% gastrointestinal NET (GI-NET), 32.3% pancreatic NET (P-NET) and 16.2% lung NET (L-NET), while 6.1% of tumors had unknown primary (UP). GI-NET were G1 in 48.9% and G2 in 51.1%. P-NET were G1 in 53.1% and G2 in 46.9%. Unknown primary tumors were G1 in 16.7% and G2 in 83.3%. Bronchial carcinoids were typical in 43.7% and atypical in 56.3%. All typical carcinoids were G1 and all atypical carcinoids were G2. As a whole 47.5% of the tumors were G1 and 52.5% were G2. Primary tumor was resected in 47.5%. Disease status before the first-line treatment was available in 80 patients, which were progressive in 56.2%, stable in 43.8% (Table 3).
Baseline characteristics such as age, sex, tumor stage, functional status, resection of primary tumor and disease status at baseline were similar between sequences, while tumor site and grading varied but not significantly (Table 4). Chemotherapy included capecitabine in nine patients, temozolomide in six patients and the combination in two patients. Concomitant supportive therapies for symptomatic bone metastases were performed in ten patients, equally distributed according to sequences (three in sequence A, four in B, two in C, one in D). Five of them were treated with external radiotherapy.
Efficacy
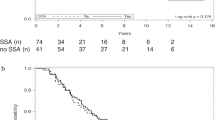
Every sequence had two lines of treatment and PFS was calculated for each line. As expected, there was no statistical difference in PFS of the first line between the four sequences, which consist in SSA in all of them (p = 0.77). Median PFS was 16.5 months for sequence A, 17 months for B, 20 months for C and 20 months for D (Fig. 1a).
Kaplan−Meier analysis: a Median PFS of the first-line treatment was 16.5 months for sequence A (SSA standard dose to SSA high dose), 17 months for B (SSA to everolimus), 20 months for C (SSA to chemotherapy) and 20 months for D (SSA to PRRT), without statistical difference in PFS between the four sequences (p = 0.77). b Median PFS of the second-line treatment was not reached in sequence A, whereas it was 33 months for B, 20 months for C and 30 months for D, without statistical difference in PFS between sequences (p = 0.16). PFS progression-free survival, SSA somatostatin analogs, PRRT peptide receptor radionuclide therapy
PFS was not significantly different between sequences (p = 0.16) for the second line of treatment as well (Fig. 1b). Median PFS was not reached in sequence A, whereas it was 33 months for B, 20 months for C and 30 months for D. HRs for PFS, for the two lines of treatment, are reported in Table 5.
When analyzing the second-line median PFS with respect to primary site, no significant difference was found (p = 0.89), while median PFS was longer in G1 than G2 NET (40 vs. 27 months, p = 0.04), as well as in stable tumors rather than in those progressive at baseline (57 vs. 24 months, p = 0.02).
An objective response rate was observed in 11 patients (11.1%), including 4 patients in sequence A (11.1%), 3 patients in sequence B (9.7%), 3 patients in sequence C (17.6%) and 1 patient in sequence D (6.7%), without significant difference (p = 0.81).
Toxicity
In 45 patients out of 99 (45%), no adverse events were recorded. The remaining 55% of cases, on the other hand, presented most frequently grade 1−3 adverse events. The different sequences were significantly associated with adverse events (p = 0.04). Table 6 shows the distribution of adverse events over the four sequences. A higher level of toxicity was found for sequence B (67.7%) and C (70.6%) than sequence A (44.4%) and D (33.3%) (p = 0.043). As for the severity of side effects, grade 3 or 4 adverse events were 5.6% in sequence A, 25.8% in sequence B, 29.4% in sequence C and 6.7% in sequence D (p = 0.030). Side effects lead to discontinuation of the treatment or reduction of the administered doses in significantly different rates over the four different sequences (p = 0.028). The rates of discontinuation/dose reduction were 2.8% for those treated with sequence A and 6.7% for those treated with sequence D. The other two sequences, instead, showed higher rates of discontinuation/dose reduction (16.1% for sequence B and 29.4% for C).
Discussion
The spectrum of treatments now available for NET includes SSA such as octreotide and lanreotide, targeted therapies such as everolimus and sunitinib, chemotherapy and finally PRRT with radiolabelled SSA [14,15,16,17,18,19,20]. Although no randomized trial has been published until now to study the higher than standard dose of SSA in NET, except the Netter study where octreotide LAR 60 mg per month represents the control arm, this schedule is also an option for progressive G1-G2 NET, as reported in the ENETS guidelines [6].
NET guidelines have been progressively improved. The last ENETS, NANETS, NCCN, ESMO guidelines all try to provide NET treatment algorithms based on stage and grade of NET rather than on primary site [6,7,8,9]. This is a first attempt to place the biology of the tumor at the center of decision-making and therapeutic choice, while waiting for an algorithm based on the genetic alterations of NET as for other tumor models. All the biological characteristics of NET available nowadays, such as neuroendocrine differentiation, tumor grade, somatostatin receptor status, functional status, help select or exclude certain therapies instead of others [21]. However, a therapeutic strategy, i.e. the definition of the sequence of treatments to be used over time, has never been established.
In the vast group of patients with GEP and lung, G1 and G2, loco-regionally advanced or metastatic, surgically unresectable NET, the clinical trials performed until now only concern efficacy and toxicity of a single line of treatment with SSA, everolimus, sunitinib, chemotherapy, PRRT, while the effects of a whole sequence are not known. This would be relevant to optimize the efficacy of NET therapy and to increase the patient tolerability to each drug administered as well as to the whole sequence of treatments. On the other hand, a high tolerability is also related to low rates of drug interruption and discontinuation and consequently is likely to induce a better effectiveness.
In this setting, whereas SSA are widely accepted as first-line treatment, because of their positive benefit/risk ratio, no data are available to establish the best choice as second-line treatment [22, 23]. This is the first study with the purpose of trying to answer this unmet need, by analyzing and comparing the main therapeutic sequences in a large retrospective series of G1−G2 NET. Four main therapeutic sequences were identified. As expected, all started with SSA as first-line treatment and continued with more aggressive approaches after progression. Some other sequences starting with chemotherapy, targeted therapy or PRRT were less represented in this population.
The first-line treatment with SSA appeared to be as homogeneously efficacious as expected, with a median PFS from 16.5 to 20 months between sequences. This result is in the middle between those of Promid and Clarinet trials, where the experimental arm had a time to progression of 14.3 in the former and a median PFS not reached in the latter [14, 15]. The difference of median PFS could be in part explained by baseline tumor progression rate, which was 56% in the current study, while only 4% in the Clarinet and not indicated, but likely much higher, in Promid study. In the Clarinet extension study, the subgroup of patients previously progressing on placebo had a median PFS of 19 months after therapy with lanreotide [24]. Interestingly, also the comparison between the second-line treatments of the four sequences revealed no significant difference in median PFS. The median PFS was not reached in sequence A containing SSA high dose, while it was 33 months in sequence B containing everolimus, 20 months in sequence C containing chemotherapy, 30 months in sequence D involving PRRT.
One of the strengths of this project is the study population that included a large number of G1−G2 patients, selected on the basis of well-defined inclusion and exclusion criteria. All patients were treatment naive, thus avoiding confounding factors due to previous treatments which can impact on both efficacy and cumulative toxicity. Among the weaknesses of the study, it has to be considered the retrospective design. However, because the four subgroups of patients were homogenous with respect to demographics and clinico-pathological characteristics, only univariate analysis was performed. Beyond this, there are no comparable reports available in literature and this represents a pilot study on therapeutic sequences in NET, useful to open the way for further prospective trials. Indeed, a highly informative phase 3 trial, the SEQTOR trial (ClinicalTrials.gov Identifier: NCT02246127), whose aim is to compare prospectively in pancreatic NET two different therapeutic sequences, everolimus followed by streptozocin/fluorouracil vs. streptozocin/fluorouracil followed by everolimus, is still ongoing.
Very few studies evaluated retrospectively efficacy and tolerability of antitumor agents in NET patients according to previous treatments. In all cases, study design was retrospective. In particular, everolimus resulted to induce similar PFS rates according to previous treatments, but to be poorly tolerated in NET patients previously treated with PRRT or chemotherapy and even more in those treated with the combination [25, 26]. Another study has retrospectively compared the sequence everolimus to sunitinib vs. the sequence sunitinib to everolimus in a series of 31 progressive metastatic G1−G2 pancreatic NET [27]. PFS was not significantly different between sequences, while toxicity was reported higher with sunitinib compared to everolimus both in terms of frequency of serious adverse events and rate of treatment discontinuation. However, none of these parameters reached the statistical significance. Interestingly, a tendency to a higher frequency of serious adverse events in sequential than in first-line treatments was observed both for everolimus and sunitinib, but also in this case there was no statistical significance [27]. As for Angelousi and co-workers, in the present study PFS was similar between sequences even if the comparison was between different classes of drugs such as SSA, everolimus, chemotherapy and PRRT. This finding is not surprising if we consider that G1−G2 NET are slowly growing tumors that can be stabilized as best response to each one of these treatments. The median PFS in this study was higher as compared to that from Angelousi but also to some phase-3 trials with SSA high dose, PRRT or everolimus [16, 18, 27]. One possible explanation is the longer follow-up time in the former compared to that reported in most of the other studies. Conversely, the Clarinet extension study, characterized by a long-time follow-up, reported a median PFS > 30 months in patients treated with lanreotide autogel 120 mg every 28 days [20, 24]. Another point is that most of the previous studies enrolled patients who had been previously heavily treated [16,17,18, 27]. In particular, in the retrospective study comparing everolimus and sunitinib, other treatments such as SSA, PRRT, chemotherapy and liver-directed therapy had been used in 71%, 58%, 39% and 23% of cases, respectively [27]. Radiant-2, -3 and -4 trials, comparing everolimus and placebo, enrolled patients previously treated with SSA (50−80%), chemotherapy (25−50%), radiation therapy (21−22%) [16, 17]. In Netter trial, comparing PRRT and SSA high dose in patients who progressed after SSA standard dose, almost half of the patients had received previous systemic treatments before SSA [18]. Anyway, the retrospective data of the current study need to be prospectively confirmed.
Whether efficacy does not allow discrimination between the four sequences, this is not the case with regard to toxicity. The better tolerability of sequences with SSA and PRRT and without targeted therapy and chemotherapy is in line with a recent national survey, where chemotherapy, everolimus and sunitinib had an odd ratio of poor perceived tolerance which was respectively 1.7, 3.4 and 5.9 compared to SSA [28]. Furthermore, for everolimus and sunitinib the commonest cause of discontinuation was toxicity rather than progression [28]. This is a crucial point that suggests to favor sequences with SSA as first treatment and SSA high doses or PPRT as sequential treatment. More aggressive options like chemotherapy and targeted therapy should be then considered in case of further progression.
In conclusion, this is the first study comparing different therapeutic sequences in patients with G1−G2 NET not previously treated. A similar efficacy is suggested for sequences starting with SSA and continuing with SSA high dose, everolimus, chemotherapy or PRRT, while those with high dose or radio-labeled SSA seem to be better tolerated. These findings represent a step forward in NET therapy by suggesting a therapeutic algorithm in G1−G2 GEP and lung NET, but require to be confirmed in prospective trials involving large study populations.
References
A. Faggiano, P. Ferolla, F. Grimaldi, D. Campana, M. Manzoni, M.V. Davì, A. Bianchi, R. Valcavi, E. Papini, D. Giuffrida, D. Ferone, G. Fanciulli, G. Arnaldi, G.M. Franchi, G. Francia, G. Fasola, L. Crinò, A. Pontecorvi, P. Tomassetti, A. Colao, Natural history of gastro-entero-pancreatic and thoracic neuroendocrine tumors. Data from a large prospective and retrospective Italian epidemiological study: the NET management study. J. Endocrinol. Invest. 35, 817–823 (2012)
A. Dasari, C. Shen, D. Halperin, B. Zhao, S. Zhou, Y. Xu, T. Shih, J.C. Yao, Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 3, 1335–1342 (2017)
M. Cives, J. Strosberg, Treatment strategies for metastatic neuroendocrine tumors of the gastrointestinal tract. Curr. Treat. Options Oncol. 18, 14 (2017)
N. Fazio, A. Ungaro, F. Spada, C.A. Cella, E. Pisa, M. Barberis, C. Grana, D. Zerini, E. Bertani, D. Ribero, L. Funicelli, G. Bonomo, D. Ravizza, J. Guarize, F. De Marinis, F. Petrella, E. Del Signore, G. Pelosi, L. Spaggiari, The role of multimodal treatment in patients with advanced lung neuroendocrine tumors. J. Thorac. Dis. 9, S1501–S1510 (2017)
A. Faggiano, F. Lo Calzo, G. Pizza, R. Modica, A. Colao, The safety of available treatments options for neuroendocrine tumors. Expert Opin. Drug. Saf. 16, 1149–1161 (2017)
M. Pavel, J. W. Valle, B. Eriksson, A. Rinke, M. Caplin, J. Chen, F. Costa, J. Falkerby, N. Fazio, V. Gorbounova, W. de Herder, M. Kulke, C. Lombard-Bohas, J. O’Connor, H. Sorbye, R. Garcia-Carbonero: Antibes Consensus Conference Participants; Antibes Consensus Conference participants. ENETS Consensus guidelines for the standards of care in neuroendocrine neoplasms: systemic therapy—biotherapy and novel targeted agents. Neuroendocrinology. 105, 266−280 (2017)
J.R. Strosberg, T.R. Halfdanarson, A.M. Bellizzi, J.A. Chan, J.S. Dillon, A.P. Heaney, P.L. Kunz, T.M. O’Dorisio, R. Salem, E. Segelov, J.R. Howe, R.F. Pommier, K. Brendtro, M.A. Bashir, S. Singh, M.C. Soulen, L. Tang, J.S. Zacks, J.C. Yao, E.K. Bergsland, The North American Neuroendocrine Tumor Society Consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas 46, 707–714 (2017)
M.H. Shah, W.S. Goldner, T.R. Halfdanarson, E. Bergsland, J.D. Berlin, D. Halperin et al. NCCN guidelines insights: neuroendocrine and adrenal tumors, version 2.2018. J. Natl. Compr. Canc. Netw. 16, 693–702 (2018)
K. Öberg, U. Knigge, D. Kwekkeboom, A. Perren; ESMO Guidelines Working Group, Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 23, 124–130 (2012)
A. Faggiano, A.C. Carratù, E. Guadagno, S. Tafuto, F. Tatangelo, F. Riccardi, C. Mocerino, G. Palmieri, V. Damiano, R. Siciliano, S. Leo, A. Mauro, L.F. Tozzi, C. Battista, G. De Rosa, A. Colao, Somatostatin analogues according to Ki67 index in neuroendocrine tumours: an observational retrospective-prospective analysis from real life. Oncotarget 7, 5538–5547 (2016)
F.T. Bosman, World Health Organization, International Agency for Research on Cancer: WHO Classification of Tumours of the Digestive System. 4th edn. (International Agency for Research on Cancer, Lyon, 2010)
W.D. Travis, E. Brambilla, A.P. Burke, A. Marx, A.G. Nicholson, WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart (International Agency for Research on Cancer, Lyon, 2015)
R Core Team, R: A Language and Environment for Statistical Computing, Version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria, 2018), http://www.R-project.org/
A. Rinke, H.H. Müller, C. Schade-Brittinger, K.J. Klose, P. Barth, M. Wied, C. Mayer, B. Aminossadati, U.F. Pape, M. Bläker, J. Harder, C. Arnold, T. Gress, R. Arnold; PROMID Study Group, Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J. Clin. Oncol. 27, 4656–4663 (2009)
M.E. Caplin, M. Pavel, J.B. Ćwikła, A.T. Phan, M. Raderer, E. Sedláčková, G. Cadiot, E.M. Wolin, J. Capdevila, L. Wall, G. Rindi, A. Langley, S. Martinez, J. Blumberg, P. Ruszniewski, CLARINET Investigators, Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 371, 224–233 (2014)
M.E. Pavel, J.D. Hainsworth, E. Baudin, M. Peeters, D. Hörsch, R.E. Winkler, J. Klimovsky, D. Lebwohl, V. Jehl, E.M. Wolin, K. Öberg, E. Van Cutsem, J.C. Yao, RADIANT-2 Study Group, Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 378, 2005–2012 (2011)
James C. Yao, Nicola Fazio, Simron Singh, Roberto Buzzoni, Carlo Carnaghi, Edward Wolin, Jiri Tomasek, Markus Raderer, Harald Lahner, Maurizio Voi, Lida Bubuteishvili Pacaud, Nicolas Rouyrre, Carolin Sachs, Juan W. Valle, Gianfranco Delle Fave, Eric Van Cutsem, Margot Tesselaar, Yasuhiro Shimada, Do-Youn Oh, Jonathan Strosberg, Matthew H. Kulke, Marianne E. Pavel, for the RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group, Everolimus for the treatment of advanced, nonfunctional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 387, 968–977 (2016)
J. Strosberg, G. El-Haddad, E. Wolin, A. Hendifar, J. Yao, B. Chasen, E. Mittra, P.L. Kunz, M.H. Kulke, H. Jacene, D. Bushnell, T.M. O’Dorisio, R.P. Baum, H.R. Kulkarni, M. Caplin, R. Lebtahi, T. Hobday, E. Delpassand, E. Van Cutsem, A. Benson, R. Srirajaskanthan, M. Pavel, J. Mora, J. Berlin, E. Grande, N. Reed, E. Seregni, K. Öberg, M. Lopera Sierra, P. Santoro, T. Thevenet, J.L. Erion, P. Ruszniewski, D. Kwekkeboom, E. Krenning, NETTER-1 Trial Investigators, Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 376, 125–135 (2017)
E. Raymond, L. Dahan, J.L. Raoul, Y.J. Bang, I. Borbath, C. Lombard-Bohas, J. Valle, P. Metrakos, D. Smith, A. Vinik, J.S. Chen, D. Hörsch, P. Hammel, B. Wiedenmann, E. Van Cutsem, S. Patyna, D.R. Lu, C. Blanckmeister, R. Chao, P. Ruszniewski, Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 364, 501–513 (2011)
M.E. Caplin, M. Pavel, J.B. Ćwikła, A.T. Phan, M. Raderer, E. Sedláčkova et al. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study. Endocr. Relat. Cancer 23, 191–199 (2016)
A.E. Hendifar, D. Dhall, J.R. Strosberg, The evolving treatment algorithm for advanced neuroendocrine neoplasms: diversity and commonalities across tumor types. Oncologist 24, 54–61 (2018)
I. Uri, S. Grozinsky-Glasberg, Current treatment strategies for patients with advanced gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Clin. Diabetes Endocrinol. 11, 16 (2018)
L. Lee, T. Ito, R.T. Jensen, Everolimus in the treatment of neuroendocrine tumors: efficacy, side-effects, resistance, and factors affecting its place in the treatment sequence. Expert Opin. Pharmacother. 19, 909–928 (2018)
J.B. Cwikla, E.M. Wolin, M. Pavel, A.T. Phan, M. Raderer, E. Sedláčková et al. Final analysis of time to subsequent disease progression/death in patients with metastatic enteropancreatic neuroendocrine tumours progressing under placebo and switched to lanreotide autogel/depot 120mg in the Clarinet open-label extension. Ann. Oncol. 28, v142–v157 (2017)
F. Panzuto, M. Rinzivillo, N. Fazio, F. de Braud, G. Luppi, M.C. Zatelli et al. Real-world study of everolimus in advanced progressive neuroendocrine tumors. Oncologist 19, 966–974 (2014)
K. Kamp, B. Gumz, R.A. Feelders, D.J. Kwekkeboom, G. Kaltsas, F.P. Costa, W.W. de Herder, Safety and efficacy of everolimus in gastrointestinal and pancreatic neuroendocrine tumors after (177)Lu-octreotate. Endocr. Relat. Cancer 20, 825–831 (2013)
A. Angelousi, K. Kamp, M. Kaltsatou, D. O’Toole, G. Kaltsas, W. de Herder, Sequential everolimus and sunitinib treatment in pancreatic metastatic well-differentiated neuroendocrine tumours resistant to prior treatments. Neuroendocrinology 105, 394–402 (2017)
A. Plante, E. Baudin, C. Do Cao, O. Hentic, O. Dubreuil, E. Terrebonne, V. Granger, D. Smith, C. Lombard-Bohas, T. Walter, Patient-reported tolerance in treatments approved in neuroendocrine tumors: a national survey from the French Group of Endocrine Tumors. Clin. Res Hepatol. Gastroenterol. 42, 153–159 (2018)
Acknowledgements
Thanks to all the participants to the ELIOS (Educational Learning Investigational Observational Study) Multicentre Study for their support.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was conducted in accordance with the 1964 Declaration of Helsinki and approved by the ethics committee of each institution. All patients gave written informed consent.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Faggiano, A., Di Maio, S., Mocerino, C. et al. Therapeutic sequences in patients with grade 1−2 neuroendocrine tumors (NET): an observational multicenter study from the ELIOS group. Endocrine 66, 417–424 (2019). https://doi.org/10.1007/s12020-019-01894-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-01894-0