Abstract
Objectives
To investigate the safety and efficacy of once-weekly glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide as monotherapy or add-on to other antihyperglycaemic agents (AHAs) in patients with type 2 diabetes mellitus (T2DM).
Methods
PubMed, Embase, Cochrane library and ClinicalTrials.gov were searched from the inception to January 18, 2018. Randomised controlled trials (RCTs) comparing semaglutide with placebo or other AHAs in T2DM patients were included in our meta-analysis. Risk ratio (RR) and mean difference (MD) with 95% confidence intervals (CI) were used to evaluate the outcomes.
Results
A total of 11 studies with 9519 patients were included in our meta-analysis. The results revealed that compared with placebo or other AHAs, semaglutide had further reduced the level of haemoglobin A1c (HbA1c) [MD 1.03%, 95% CI (0.85%, 1.22%), p < 0.00001], self-measured plasma glucose (SMPG) [MD 1.19 mmol/L, 95% CI (0.84 mmol/L, 1.53 mmol/L), p < 0.00001], fasting plasma glucose (FPG) [MD 1.33 mmol/L, 95% CI (0.97 mmol/L, 1.69 mmol/L), p < 0.00001] and weight [MD 3.61 kg, 95% CI (3.05 kg, 4.17 kg), p < 0.00001] and significantly increased participants who achieved HbA1c < 7.0% [RR 2.26, 95% CI (1.89, 2.70), p < 0.00001] in T2DM patients. Semaglutide had a significant increase in the incidence of adverse events (AEs) [RR 1.06, 95% CI (1.02, 1.11), p < 0.0001] and an analogous incidence in serious adverse events (SAEs) [RR 0.94, 95% CI (0.86, 1.02), p = 0.11] and hypoglycaemic events (severe or blood glucose (BG)-confirmed symptomatic) [RR 0.93, 95% CI (0.74, 1.16), p = 0.50] compared with the control group.
Conclusions
This article revealed that semaglutide had a favourable efficacy and safety in treating T2DM patients. It maybe a superior choice for T2DM patients who have obesity or a poor adherence to daily AHAs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of T2DM is rising in many countries, affecting an estimated 114.4 million (10.9%) adults in China and 425 million (8.8%) adults worldwide [1, 2]. Although several T2DM treatments are available [3], many patients with type 2 diabetes do not achieve recommended blood glucose concentrations [4], therefore, at risk of developing several chronic complications of diabetes, including cardiovascular disease [5, 6]. Additionally, avoidance of both hypoglycaemia and weight gain is recommended as important therapeutic considerations when selecting treatments and individualising treatment goals [7, 8].
GLP-1 receptor agonists are a novel series of AHAs. GLP-1 receptor agonists decrease blood glucose of T2DM patients by stimulating insulin secretion and inhibiting the release of glucagon in a glucose-dependent manner, targeting the pathophysiological factors underlying the islet cell dysfunction associated with type 2 diabetes [9]. Importantly, GLP-1 receptor agonists have been shown to a low risk of hypoglycaemia and reduce body weight as a consequence of reduced appetite and energy intake [10,11,12].
Short-acting GLP-1 receptor agonists that require administration once or twice per day were the first generation in this class. However, a substantial proportion of people with T2DM do not take their medication as prescribed [13, 14]. Recent efforts have been made to develop GLP-1 receptor agonists that require less frequent dosing, which could improve patient adherence and reduce treatment burden. Several GLP-1 receptor agonists were approved that are dosed once a week, and the results of meta- analysis showed that these agonists varied in safety and efficacy [15].
Semaglutide is a once-weekly GLP-1 analogue which was approved by the US Food and Drug Administration for the treatment of type 2 diabetes. It has 94% structural homology to natural GLP-1, and is similar to liraglutide [16]. The GLP-1 moiety of semaglutide is modified by the addition of a fatty diacid chain and two amino acid substitutions, and important structural modifications make semaglutide less susceptible to degradation by the enzyme DPP-4, and thus more enzymatically stable [16, 17]. Recently, many studies [17,18,19] which had tested the efficacy and safety of semaglutide as monotherapy or add-on to other AHAs in the treatment of T2DM were published. Nevertheless, there still lacks a comprehensive evaluation of the available evidence to support the use of semaglutide in clinical practice.
We conducted a systematic review and meta-analysis to test the efficacy and safety of semaglutide in patients with T2DM.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for conducting a high-quality meta-analysis [20, 21] and the Cochrane handbook guidelines [22]. This meta-analysis was registered in PTOSPERO (CRD42018090285).
Data source and searching
We systemically searched Pubmed, Embase and Cochrane library for eligible studies from the inception to January 18, 2018. We used the combination of the following medical subject heading (MeSH) and free-text terms: diabetes, diabetes mellitus, type 2 diabetes mellitus, DM, T2DM, semaglutide, ozempic, NN9936, NN9935 and NN9934. To find out newly developed clinical trials, we searched the ClinicalTrials.gov. Finally, we carried out an additionally manual search of the references of included trials, former meta-analyses and diabetes-related journals to identify other newly published and unpublished studies. The detailed search strategy was clearly described in Supplemental Table 1.
Study selection
Two researchers independently selected eligible studies which were included in the meta-analysis. If there existed a disagreement, they would solve by consulting another researcher. Inclusion criteria were listed as the followings: (1) RCTs; (2) semaglutide versus placebo or any other AHAs; (3) treatment duration ≥12 weeks; (4) T2DM patients; (5) at least one of the following outcomes was reported in a trial: reduction in HbA1c, reduction in SMPG, reduction in FPG, number of participants achieving HbA1c <7.0%, weight loss, AEs, SAEs and hypoglycaemic events (severe or BG-confirmed symptomatic) and (6) patients were ≥18 years. Studies were excluded if they are (1) non-RCTs; (2) published in the form of abstracts, short communications, or brief reports; (3) trials tested in animals or healthy human subjects; (4) not report information of interest and (5) trials whose treatment duration was shorter than 12 weeks. If several papers had been published about one trial, the paper which contains more adequate information was included in our meta-analysis.
Data extraction
We extracted the information of included studies in three aspects: the baseline characteristics of included trials and participants, the basic outcomes and the quality of included studies. Two independent researchers extracted the required information, if there existed a disagreement, they would reach a consensus by discussing with a third researcher. We collected the following information in each trial: first author, publication year, National Clinical Trial (NCT) number, medications in treatment and control group, sample size, average age, gender ratio, baseline HbA1c, diabetes duration, body weight, treatment duration and safety and efficacy outcomes (reduction in HbA1c, reduction in SMPG, reduction in FPG, number of participants achieving HbA1c <7.0%, weight loss, AEs, SAEs and hypoglycaemic events (severe or BG-confirmed (<3.1 mmol/L) symptomatic)). The authors of these included studies were not contacted for additional information.
Quality assessment
We assessed the risk of bias in the included studies with the Cochrane Collaboration’s tool [20]. The risk of bias was described and assessed in seven specific domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. The results of these domains were graded as a ‘low’ risk of bias, a ‘high’ risk of bias or an ‘unclear’ risk of bias.
Statistical analysis
RR and 95% CI were applied to dichotomous outcomes, whereas MD and 95% CI were applied to continuous outcomes. Two-tailed, p < 0.05 was considered statistically significant. Statistical heterogeneity was assessed by χ2 test, p < 0.10 and I2 > 50% was considered to be significant heterogeneity. Pooled analyses were performed using a random-effect model. Subgroup analysis was performed according to different dosages of semaglutide and different treatment methods in the control group. Sensitivity analysis was made to test the robustness of a primary outcome. All analyses were conducted with Revman5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, Denmark) and Stata11.0 (Stata Corp, College Station, TX, USA).
Results
Search results
The study identification and selection process were summarised in Fig. 1. Of 366 records identified by initial electronic search, 11 studies [17,18,19, 23,24,25,26,27,28,29,30] involving 9519 T2DM patients met our inclusion criteria for narrative synthesis. No additional study was identified by manual search. The characteristics of the included trials and patients were described in Table 1. All trials have a NCT number and therefore were registered in ClinicalTrials.gov. All studies were published between 2016 and 2018. The sample size ranged from 75 to 3297. Among 11 trials, 5 trials [17, 23, 25, 28, 29] compared the efficacy and safety of semaglutide with placebo and 6 trials [18, 19, 24, 26, 27, 30] compared the efficacy and safety of semaglutide with other AHAs. In the included studies, the diabetes duration ranged from 3.62 to 14.3 years and the treatment duration ranged from 12 to 104 weeks.
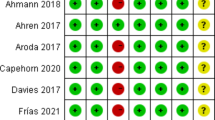
Risk of bias in included studies, and quality of evidence
The bias assessment of all 11 trials was detailed in Fig. 2. A total of 10 studies [17,18,19, 23,24,25,26,27, 29, 30] explicitly described the random sequence generation, mainly by interactive voice response system or web response system. In total, nine studies [17,18,19, 24,25,26,27, 29, 30] used unpredicted methods to generate a random sequence which stated a low risk of allocation concealment process, whereas one study [28] lacked random sequence generation method and one study [23] that performed allocation in an unblinded method was regarded as having an unclear risk and a high risk in allocation concealment, respectively. In total, five trials [17, 18, 23, 28, 29] indicated that they adopted a double-blind design, while six trials [19, 24,25,26,27, 30] employed an open design. All 11 trials [17,18,19, 23,24,25,26,27,28,29,30] had a blinded design in outcome assessment. There was no trial that described neither the number of withdrawal or loss to follow-up and the reason for these aspects, therefore all trials were regarded as having a low risk in this domain. All included studies were considered to have a low risk of bias in selective reporting, according to the review of their protocols in ClinicalTrials.gov. All trials were considered to have an unclear risk of bias in the domain of other bias.
Efficacy outcomes
All outcomes were reported in total and subgroup analysis. We made subgroup analysis of both efficacy and safety outcomes according to predefined groups.
Reduction in HbA1c is the primary outcome in this meta-analysis. In total, ten trials [17,18,19, 24,25,26,27,28,29,30] reported data on reduction in HbA1c (Fig. 3). The pooled evidence showed that compared with placebo or other AHAs, semaglutide had further reduced the level of HbA1c in T2DM patients [MD 1.03%, 95% CI (0.85%, 1.22%), p < 0.00001].
Compared with the control group, semaglutide was associated with a significantly stronger reduction in SMPG [MD 1.19 mmol/L, 95% CI (0.84 mmol/L, 1.53 mmol/L), p < 0.00001], FPG [MD 1.33 mmol/L, 95% CI (0.97 mmol/L, 1.69 mmol/L), p < 0.00001] and weight [MD 3.61 kg, 95% CI (3.05 kg, 4.17 kg), p < 0.00001] (Supplemental Figures 1–3). As shown in Supplemental Figure 4, there were significantly more participants who achieved HbA1c < 7.0% in the semaglutide group than in the control group [RR 2.26, 95% CI (1.89, 2.70), p < 0.00001].
Safety outcomes
The safety endpoints were AEs, SAEs and hypoglycaemic events (severe or BG-confirmed symptomatic). As shown in Supplemental Figure 5, semaglutide had slightly increased the incidence of AEs compared with the control group [RR 1.06, 95% CI (1.02, 1.11), p < 0.0001]. The pooled evidence indicated that compared with the control group, semaglutide had an analogous safety background in terms of SAEs [RR 0.94, 95% CI (0.86, 1.02), p = 0.11] and hypoglycaemic events (severe or BG-confirmed symptomatic) [RR 0.93, 95% CI (0.74, 1.16), p = 0.50] (Supplemental Figure 6–7).
Subgroup analysis and sensitivity analysis
By dividing the treatment group into 0.5-mg semaglutide group and 1.0-mg semaglutide group, we made subgroup analysis of all eight outcomes. The results of subgroup analysis were described in Table 2.
Meta-analysis revealed that HbA1c significantly decreased by 0.89% [95% CI (0.64%, 1.15%), p < 0.00001] with 0.5-mg semaglutide and 1.03% [95% CI (0.89%, 1.40%), p < 0.00001] with 1.0-mg semaglutide. SMPG significantly decreased by 1.04 mmol/L [95% CI (0.49 mmol/L, 1.59 mmol/L), p < 0.00001] with 0.5-mg semaglutide and 1.31 mmol/L [95% CI (0.86 mmol/L, 1.76 mmol/L), p < 0.00001] with 1.0-mg semaglutide. FPG significantly decreased by 1.05 mmol/L [95% CI (0.51 mmol/L, 1.59 mmol/L), p < 0.00001] with 0.5-mg semaglutide and 1.55 mmol/L [95% CI (1.08 mmol/L, 2.01 mmol/L), p < 0.00001] with 1.0-mg semaglutide. The number of participants achieving HbA1c < 7.0% significantly increased with 0.5-mg semaglutide [RR 2.14, 95% CI (1.66, 2.75), p < 0.00001] and 1.0-mg semaglutide [RR 2.37, 95% CI (1.81, 3.11), p < 0.00001]. Body weight significantly decreased by 2.67 kg [95% CI (2.00 kg, 3.35 kg), p < 0.00001] with 0.5-mg semaglutide and 4.31 kg [95% CI (3.74 kg, 4.88 kg), p < 0.00001] with 1.0-mg semaglutide. AEs were slightly increased with 0.5-mg semaglutide [RR 1.09, 95% CI (1.02, 1.17), p = 0.0008] and 1.0-mg semaglutide [RR 1.05, 95% CI (1.00, 1.11), p = 0.004]. Both doses of semaglutide have a similar incidence rate to the control group in SAEs and hypoglycaemia (severe or BG-confirmed symptomatic).
As shown in Table 2, we also carried out subgroup analysis by dividing the control group into placebo-controlled group and active-controlled group. Compared with placebo, semaglutide significantly increased the reduction in HbA1c [MD 1.33%, 95% CI (1.02%, 1.64%), p < 0.00001], SMPG [MD 1.88 mmol/L, 95% CI (1.62 mmol/L, 2.14 mmol/L), p < 0.00001], FPG [MD 1.95 mmol/L, 95% CI (1.41 mmol/L, 2.48 mmol/L), p < 0.00001] and weight [MD 3.74 kg, 95% CI (3.03 kg, 4.46 kg), p < 0.00001]. There were significantly more patients achieving HbA1c < 7% in semaglutide arm than in placebo arm [RR 4.01, 95% CI (2.84, 5.66), p < 0.00001]. Compared with placebo, semaglutide was associated with a significant increase in AEs [RR 1.05, 95% CI (0.99, 1.12), p = 0.01] and a significant decrease in SAEs [RR 0.91, 95% CI (0.83, 0.99), p = 0.03]. No significant difference was found in hypoglycaemia (severe or BG-confirmed symptomatic) [RR 1.07, 95% CI (0.93, 1.25), p = 0.30] between semaglutide and placebo. The main AHAs used in the active-controlled group were sitagliptin, exenatide ER, insulin glargine and dulaglutide. Compared with these AHAs, semaglutide had significantly reduced the level of HbA1c [MD 0.85%, 95% CI (0.64%, 1.06%), p < 0.00001], SMPG [MD 0.90 mmol/L, 95% CI (0.55 mmol/L, 1.25 mmol/L), p < 0.00001], FPG [MD 1.08 mmol/L, 95% CI (0.67 mmol/L, 1.49 mmol/L), p < 0.00001] and weight [MD 3.51 kg, 95% CI (2.69 kg, 4.33 kg), p < 0.00001]. More patients achieved HbA1c < 7% in semaglutide arm than in other AHA arms [RR 1.83, 95% CI (1.56, 2.15), p < 0.00001]. Semaglutide significantly increased the risk of AEs [RR 1.07, 95% CI (1.01, 1.12), p = 0.01] and decreased the risk of hypoglycaemia (severe or BG-confirmed symptomatic) [RR 0.69, 95% CI (0.49, 0.97), p = 0.03] compared with these AHAs. No significant difference was found in SAEs [RR 1.08, 95% CI (0.89, 1.31), p = 0.44] between semaglutide and other AHAs.
Sensitive analysis was conducted with Stata software. As shown in Supplemental Figure 8, we found similar overall results for the primary outcome after excluding each individual study.
Discussion
The results of this meta-analysis showed that 0.5-mg and 1.0-mg semaglutide given once per week were superior to placebo or other AHAs in improving glycaemic control and weight loss in patients with T2DM. In the subgroup analysis of placebo-controlled group and active-controlled group, semaglutide had significantly improved the glycaemic control and weight loss compared with either placebo or other AHAs. Semaglutide had a similar safety background to placebo and other AHAs in terms of AEs, SAEs and hypoglycaemia (severe or BG-confirmed symptomatic). A sensitivity analysis on primary outcome generated similar results, which indicated that the results of the present meta-analysis were generalisable.
ADA recommends that the optimal HbA1c target is 7% for most nonpregnant adults with T2DM and HbA1c should be maintained at 7% at every stage of their disease [31, 32]. Our meta-analysis showed that 74% patients in the semaglutide group had achieved HbA1c < 7%, the proportion is more than two times of T2DM patients in the control group. The results achieved with semaglutide are of clinical relevance because improvements in HbA1c have been shown to reduce the risk of both diabetes-related complications and mortality [33]. With a glucose-dependent mechanism of action, GLP-1 receptor agonists have numerically fewer episodes of hypoglycaemia that occurred [34, 35]. Semaglutide has similar and significantly lower risk of hypoglycaemia (severe or BG-confirmed symptomatic) compared with placebo and other AHAs, respectively, which is complied with other GLP-1 receptor agonists. The low risk of hypoglycaemia and less medication frequency of semaglutide may contribute to improve adherence for T2DM patients.
Cardiovascular disease is the leading cause of death and complications in patients with T2DM [36]. To date, empagliflozin and liraglutide have already been shown to improve cardiovascular outcomes in patients with type 2 diabetes who were at high risk for cardiovascular events [37, 38]. SUSTAIN6 [29] was designed to assess cardiovascular safety of semaglutide in patients with T2DM. Despite an increase in pulse rate, 0.5- and 1.0-mg semaglutide led to a significant reduction in cardiovascular risk with fewer occurrences of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke compared with placebo. Nonetheless, long-term and large-scale RCTs are needed to test whether the findings of SUSTAIN6 are applicable to T2DM patients with no or fewer cardiovascular risk.
In this meta-analysis, both doses of semaglutide led to significant weight loss, compared with either placebo or other AHAs. Semaglutide is also associated with significant weight loss in subjects with obesity [39, 40]. Obesity is associated with an increase in the risk of cardiovascular complications and other comorbidities, as well as a reduction in quality of life [41,42,43]. The results from studies also suggest that weight gain can lead to frustration, reduced motivation, and decreased adherence to treatment [44, 45]. In addition, sustained weight loss in patients with T2DM contributed to improve glycaemic control and reduce the need for glucose-lowering medications [46].
Although acute pancreatitis has been reported after treatment with GLP-1 receptor agonists, a causal link has not been shown [47, 48], and the occurrence of pancreatitis in the studies included in this meta- analysis was low. Only 18 patients in the semaglutide group and 15 patients in the control group were reported to have pancreatitis throughout the course of clinical trials. There was an unexpected higher rate of retinopathy complications in the semaglutide group. There are studies which have reported that rapid glucose lowering is associated with worsening of retinopathy in patients with type 1 diabetes [49, 50]. Therefore, this finding might be related to the fast reduction in glucose concentrations, rather than a direct effect of semaglutide treatment.
To our knowledge, there are five [51,52,53,54,55] recently published meta-analyses that mentioned the efficacy and safety of semaglutide in T2DM patients. They also recommended semaglutide for T2DM patients which is consistent with our results. In the meta-analyses conducted by Witkowski et al. [51, 52] and Sharma et al. [53], they only made an indirect comparison by network meta-analysis and compared semaglutide with a class of antidiabetic agents such as GLP-1 receptor agonists and sodium–glucose cotransporter 2 inhibitors. Compared with the other two meta-analyses made by Shi et al. [54] and Andreadis et al. [55], we think we have several advantages: First, we focused on the safety and efficacy of semaglutide injection which was approved by US Food and Drug Administration. Second, we have assessed the quality of included trials in seven specific domains which was recommended by Cochrane Collaboration. Third, we have made subgroup analysis according to different dosages of semaglutide in the experimental group and different treatment therapies in the control group. Finally, sensitivity analysis was made to test the robustness of obtained outcomes.
We noted several limitations in this study. First, the power of our analysis may be restricted because of the limited study numbers and population sizes. Second, there was significant heterogeneity in some outcomes. Our research is a study-level meta-analysis, studies varied in relation to the study population, combined treatment method and treatment duration. All of these confounding factors may contribute to heterogeneity in some outcomes. Finally, only published data were included, which may lead to a reporting bias by overestimating the effect of semaglutide. All these aspects reinforce the need to perform more large, well-designed trials involving the safety and efficacy of semaglutide in patients with T2DM.
Conclusions
In conclusion, semaglutide had a favourable efficacy and safety as monotherapy or add-on to other AHAs in the treatment of T2DM patients. It maybe a superior choice for T2DM patients with obesity or T2DM patients who have a poor adherence to daily AHAs. Semaglutide is generally well tolerated and has obviously better efficacy than either placebo or other AHAs in treating T2DM.
References
IDF, IDF diabetes atlas, 8th edn (2017). http://www.diabetesatlas.org/. Accessed 15 July 2018
WHO, World Health Organization Global Report on Diabetes (2016). http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf?ua=1. Accessed 15 July 2018
S.E. Inzucchi, R.M. Bergenstal, J.B. Buse, M. Diamant, E. Ferrannini, M. Nauck, A.L. Peters, A. Tsapas, R. Wender, D.R. Matthews, Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38(1), 140–149 (2015)
K.J. Lipska, X. Yao, J. Herrin, R.G. McCoy, J.S. Ross, M.A. Steinman, S.E. Inzucchi, T.M. Gill, H.M. Krumholz, N.D. Shah, Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care 40(4), 468–475 (2017)
The ADVANCE Collaborative Group, Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358(24), 2560–2572 (2008)
R.R. Holman, S.K. Paul, M.A. Bethel, D.R. Matthews, H.A. Neil, 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359(15), 1577–1589 (2008)
O. Hamdy, S. Ashrafzadeh, A. Mottalib, Weight management in patients with type 2 diabetes: a multidisciplinary real-world approach. Curr. Diab. Rep. 18(9), 66 (2018)
H.W. Rodbard, L. Blonde, S.S. Braithwaite, E.M. Brett, R.H. Cobin, Y. Handelsman, R. Hellman, P.S. Jellinger, L.G. Jovanovic, P. Levy, J.I. Mechanick, F. Zangeneh, American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr. Pract. 13(Suppl 1), 1–68 (2007)
S. Madsbad, Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes. Metab. 18(4), 317–332 (2016)
A.R. Meloni, M.B. DeYoung, C. Lowe, D.G. Parkes, GLP-1 receptor activated insulin secretion from pancreatic beta-cells: mechanism and glucose dependence. Diabetes Obes. Metab. 15(1), 15–27 (2013)
J.E. Potts, L.J. Gray, E.M. Brady, K. Khunti, M.J. Davies, D.H. Bodicoat, The effect of glucagon-like peptide 1 receptor agonists on weight loss in type 2 diabetes: a systematic review and mixed treatment comparison meta-analysis. PLoS ONE 10(6), e0126769 (2015)
J. van Can, B. Sloth, C.B. Jensen, A. Flint, E.E. Blaak, W.H. Saris, Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int. J. Obes. 38(6), 784–793 (2014)
A. McGovern, W. Hinton, S. Calderara, N. Munro, M. Whyte, S. de Lusignan, A class comparison of medication persistence in people with type 2 diabetes: a retrospective observational study. Diabetes Ther. 9(1), 229–242 (2018)
K. Iglay, S.E. Cartier, V.M. Rosen, V. Zarotsky, S.N. Rajpathak, L. Radican, K. Tunceli, Meta-analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Curr. Med. Res. Opin. 31(7), 1283–1296 (2015)
F. Zaccardi, Z.Z. Htike, D.R. Webb, K. Khunti, M.J. Davies, Benefits and harms of once-weekly glucagon-like peptide-1 receptor agonist treatments: a systematic review and network meta-analysis. Ann. Intern. Med. 164(2), 102–113 (2016)
J. Lau, P. Bloch, L. Schaffer, I. Pettersson, J. Spetzler, J. Kofoed, K. Madsen, L.B. Knudsen, J. McGuire, D.B. Steensgaard, H.M. Strauss, D.X. Gram, S.M. Knudsen, F.S. Nielsen, P. Thygesen, S. Reedtz-Runge, T. Kruse, Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J. Med. Chem. 58(18), 7370–7380 (2015)
C. Sorli, S.I. Harashima, G.M. Tsoukas, J. Unger, J.D. Karsbøl, T. Hansen, S.C. Bain, Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 5(4), 251–260 (2017)
B. Ahrén, L. Masmiquel, H. Kumar, M. Sargin, J.D. Karsbøl, S.H. Jacobsen, F. Chow, Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 5(5), 341–354 (2017)
R.E. Pratley, V.R. Aroda, I. Lingvay, J. Lüdemann, C. Andreassen, A. Navarria, A. Viljoen, SUSTAIN 7 investigators, Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet. Diabetes Endocrinol. 6(4), 275–286 (2018)
D. Moher, A. Liberati, J. Tetzlaff, D.G. Altman, PRISMA Group, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535 (2009)
A. Liberati, D.G. Altman, J. Tetzlaff, C. Mulrow, P.C. Gøtzsche, J.P. Ioannidis, M. Clarke, P.J. Devereaux, J. Kleijnen, D. Moher, The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339, b2700 (2009)
J. P. T. Higgins, S. Green (eds.), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. http://handbook.cochrane.org (2011). Accessed 27 July 2018
C. Kapitza, K. Dahl, J.B. Jacobsen, M.B. Axelsen, A. Flint, Effects of semaglutide on beta cell function and glycaemic control in participants with type 2 diabetes: a randomised, double-blind, placebo-controlled trial. Diabetologia 60(8), 1390–1399 (2017)
K. Kaku, Y. Yamada, H. Watada, A. Abiko, T. Nishida, J. Zacho, A. Kiyosue, Safety and efficacy of once-weekly semaglutide vs additional oral antidiabetic drugs in Japanese people with inadequately controlled type 2 diabetes: a randomized trial. Diabetes Obes. Metab. 20(5), 1202–1212 (2018)
M. Davies, T.R. Pieber, M.L. Hartoft-Nielsen, O.K.H. Hansen, S. Jabbour, J. Rosenstock, Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA 318(15), 1460–1470 (2017)
A.J. Ahmann, M. Capehorn, G. Charpentier, F. Dotta, E. Henkel, I. Lingvay, A.G. Holst, M.P. Annett, V.R. Aroda, Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care 41(2), 258–266 (2018)
V.R. Aroda, S.C. Bain, B. Cariou, M. Piletič, L. Rose, M. Axelsen, E. Rowe, J.H. DeVries, Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 5(5), 355–366 (2017)
H.W. Rodbard, I. Lingvay, J. Reed, R. de la Rosa, L. Rose, D. Sugimoto, E. Araki, P.L. Chu, N. Wijayasinghe, P. Norwood, Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J. Clin. Endocrinol. Metab. 103(6), 2291–2301 (2018)
S.P. Marso, S.C. Bain, A. Consoli, F.G. Eliaschewitz, E. Jódar, L.A. Leiter, I. Lingvay, J. Rosenstock, J. Seufert, M.L. Warren, V. Woo, O. Hansen, A.G. Holst, J. Pettersson, T. Vilsbøll, SUSTAIN-6 investigators, semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375(19), 1834–1844 (2016)
Y. Seino, Y. Terauchi, T. Osonoi, D. Yabe, N. Abe, T. Nishida, J. Zacho, S. Kaneko, Safety and efficacy of semaglutide once weekly vs sitagliptin once daily, both as monotherapy in Japanese people with type 2 diabetes. Diabetes Obes. Metab. 20(2), 378–388 (2018)
D.M. Nathan, J.B. Buse, M.B. Davidson, E. Ferrannini, R.R. Holman, R. Sherwin, B. Zinman, American Diabetes Association, European Association for Study of Diabetes, Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32(1), 193–203 (2009)
American Diabetes Association, Standards of medical care in diabetes–2010. Diabetes Care 33(Suppl 1), S11–S61 (2010)
I.M. Stratton, A.I. Adler, H.A. Neil, D.R. Matthews, S.E. Manley, C.A. Cull, D. Hadden, R.C. Turner, R.R. Holman, Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321(7258), 405–412 (2000)
D. Russell-Jones, A. Vaag, O. Schmitz, B.K. Sethi, N. Lalic, S. Antic, M. Zdravkovic, G.M. Ravn, R. Simó, Liraglutide effect and action in diabetes 5 (LEAD-5) met + SU Study Group, liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomised controlled trial. Diabetologia 52(10), 2046–2055 (2009)
M. Diamant, L. Van Gaal, B. Guerci, S. Stranks, J. Han, J. Malloy, M.K. Boardman, M.E. Trautmann, Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trial. Lancet Diabetes Endocrinol 2(6), 464–473 (2014)
A.S. Matheus, L.R. Tannus, R.A. Cobas, C.C. Palma, C.A. Negrato, M.B. Gomes, Impact of diabetes on cardiovascular disease: an update. Int. J. Hypertens. 2013, 653789 (2013)
B. Zinman, C. Wanner, J.M. Lachin, D. Fitchett, E. Bluhmki, S. Hantel, M. Mattheus, T. Devins, O.E. Johansen, H.J. Woerle, U.C. Broedl, S.E. Inzucchi, EMPA-REG OUTCOME Investigators, empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373(22), 2117–2128 (2015)
S.P. Marso, G.H. Daniels, K. Brown-Frandsen, P. Kristensen, J.F. Mann, M.A. Nauck, S.E. Nissen, S. Pocock, N.R. Poulter, L.S. Ravn, W.M. Steinberg, M. Stockner, B. Zinman, R.M. Bergenstal, J.B. Buse, LEADER Steering Committee, LEADER Trial Investigators, Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375(4), 311–322 (2016)
J.B. Hjerpsted, A. Flint, A. Brooks, M.B. Axelsen, T. Kvist, J. Blundell, Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity. Diabetes Obes. Metab. 20(3), 610–619 (2018)
J. Blundell, G. Finlayson, M. Axelsen, A. Flint, C. Gibbons, T. Kvist, J.B. Hjerpsted, Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 19(9), 1242–1251 (2017)
P. Poirier, T.D. Giles, G.A. Bray, Y. Hong, J.S. Stern, F.X. Pi-Sunyer, R.H. Eckel, American Heart Association, Obesity Committee of the Council on Nutrition, Physical activity, and metabolism, obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113(6), 898–918 (2006)
R.T. Jung, Obesity as a disease. Br. Med. Bull. 53(2), 307–321 (1997)
T.S. Han, M.A. Tijhuis, M.E. Lean, J.C. Seidell, Quality of life in relation to overweight and body fat distribution. Am. J. Public Health 88(12), 1814–1820 (1998)
F.X. Pi-Sunyer, The impact of weight gain on motivation, compliance, and metabolic control in patients with type 2 diabetes mellitus. Postgrad. Med. 121(5), 94–107 (2009)
A. Farmer, A.L. Kinmonth, S. Sutton, Measuring beliefs about taking hypoglycaemic medication among people with type 2 diabetes. Diabet. Med. 23(3), 265–270 (2006)
J.P. Wilding, The importance of weight management in type 2 diabetes mellitus. Int. J. Clin. Pract. 68(6), 682–691 (2014)
T. Chalmer, T.P. Almdal, T. Vilsbøll, F.K. Knop, Adverse drug reactions associated with the use of liraglutide in patients with type 2 diabetes-focus on pancreatitis and pancreas cancer. Expert. Opin. Drug. Saf. 14(1), 171–180 (2015)
H. Wang, Y. Liu, Q. Tian, J. Yang, R. Lu, S. Zhan, J. Haukka, T. Hong, Incretin-based therapies and risk of pancreatic cancer in patients with type 2 diabetes: a meta-analysis of randomised controlled trials. Diabetes Obes. Metab. 20(4), 910–920 (2018)
The Diabetes Control and Complications Trial Research Group, Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch. Ophthalmol. 116(7), 874–886 (1998)
K. Dahl-Jørgensen, O. Brinchmann-Hansen, K.F. Hanssen, L. Sandvik, O. Aagenaes, Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: the Oslo study. Br. Med. J. 290(6471), 811–815 (1985)
M. Witkowski, L. Wilkinson, N. Webb, A. Weids, D. Glah, H. Vrazic, A systematic literature review and network meta-analysis comparing once-weekly semaglutide with other GLP-1 receptor agonists in patients with type 2 diabetes previously receiving 1-2 oral anti-diabetic drugs. Diabetes Ther. 9(3), 1149–1167 (2018)
M. Witkowski, L. Wilkinson, N. Webb, A. Weids, D. Glah, H. Vrazic, A systematic literature review and network meta-analysis comparing once-weekly semaglutide with other GLP-1 receptor agonists in patients with type 2 diabetes previously receiving basal insulin. Diabetes Ther. 9(3), 1233–1251 (2018)
R. Sharma, L. Wilkinson, H. Vrazic, E. Popoff, S. Lopes, S. Kanters, E. Druyts, Comparative efficacy of once-weekly semaglutide and SGLT-2 inhibitors in type 2 diabetic patients inadequately controlled with metformin monotherapy: a systematic literature review and network meta-analysis. Curr. Med. Res. Opin. 29, 1–9 (2018)
F.H. Shi, H. Li, M. Cui, Z.L. Zhang, Z.C. Gu, X.Y. Liu, Efficacy and safety of once-weekly semaglutide for the treatment of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 9, 576 (2018)
P. Andreadis, T. Karagiannis, K. Malandris, I. Avgerinos, A. Liakos, A. Manolopoulos, E. Bekiari, D. R. Matthews, A. Tsapas, Semaglutide for type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes. Metab. (2018). https://doi.org/10.1111/dom.13361. [Epub ahead of print]
Author contributions
G.L. and X.L. made contributions to have the idea for this study and design this study. G.L., X.W. and S.Q. contributed towards literature search, data extraction, data analysis, drafting and critical revision of the manuscript. X.L., Y.Z. and Y.L. made contributions to data interpretation, assess the quality of the studies, drafting and critical revision of the manuscript. All authors had reviewed the manuscript, approved the final draft and decided to submit it for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, X., Qie, S., Wang, X. et al. The safety and efficacy of once-weekly glucagon-like peptide-1 receptor agonist semaglutide in patients with type 2 diabetes mellitus: a systemic review and meta-analysis. Endocrine 62, 535–545 (2018). https://doi.org/10.1007/s12020-018-1708-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1708-z