Abstract
Purpose
To evaluate the effectiveness and safety of microwave ablation (MWA), including cooled MWA (cMWA) and uncooled MWA (uMWA), for the treatment of benign thyroid nodules (BTNs).
Methods
The databases of MEDLINE, EMBASE and Cochrane library were searched up to 3 Jun, 2018. In this meta-analysis, data of volume reduction rates (VRRs) at the 3-, 6- and 12-month follow-up, and complications are obtained to evaluate the effectiveness and safety of cMWA and uMWA for the treatment of BTNs.
Results
Nine studies involving 1461 patients with 1845 BTNs were included. The pooled VRR at the 3-month follow-up after MWA therapy reached 54.3% (95% CI: 45.3–63.3%, I2 = 97.6%), 73.5% (95% CI: 66.7–80.3%, I2 = 94.9%) at the 6-month follow-up, and 88.6% (95% CI: 84.9–92.4%, I2 = 92.7%) at the 12-month follow-up. The pooled proportions of overall, major and minor complications were 52.4% (95% CI: 29.8–74.9%; I2 = 99.5%), 4.8% (95% CI: 2.7–7.0%; I2 = 55.9%) and 48.3% (95% CI: 31.2–65.4%; I2 = 99.7%). Both cMWA and uMWA achieved similar pooled VRR at the 3-month follow-up (58.4 vs 45.3%, P = 0.07) and pooled proportion of major complications (4.9 vs 5.0%, P = 0.49), while uMWA had higher pooled proportions of overall and minor complications than cMWA (97.8 vs 29.7%, P < 0.01; 97.8 vs 21.0%, P < 0.01), with more patients suffering pain and skin burn after uMWA (100 vs 5.5%, P < 0.01; 47.2 vs 0.2%, P < 0.01).
Conclusion
MWA is an effective treatment modality for BTNs. When considering the patient’s comfort, cMWA would be a more preferable procedure with less complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid nodule is one of the most common diseases in clinical practice and has been increasingly detected in the adult population by ultrasonography (US) [1]. Considering most of the thyroid nodules are benign, treatment is mainly concerned on patients with subjective symptoms or cosmetic problems, which are related to nodular volume [2]. Although surgery is the well-established therapeutic option for benign thyroid nodules (BTNs), its risk of complications and effect on quality of life remain concerns [3, 4].
Recently, various minimally invasive non-surgical therapeutic modalities, such as ethanol ablation (EA), radiofrequency ablation (RFA), laser ablation (LA) and microwave ablation (MWA) have been proposed to treat BTNs [5,6,7,8,9]. Although RFA and LA have been recommended for symptomatic BTNs because of the larger number of clinical applications [2], MWA, taking experience from its use in other organs like liver [10], is the more recent percutaneous technique developed [9]. Several studies have demonstrated that MWA also showed good results in sufficient necrosis and subsequent nodule shrinkage in BTNs with excellent volume reduction ratios (VRRs) of 82.5–90.0% at 12-month follow-up and a low major complication rate of 6.6% [11,12,13]. There have been also reviews which concluded that MWA is a safe and effective technique for the treatment of BTNs [9, 14]. However, there were limitations on small sample of patients in previous initial studies, or studies in previous reviews. Besides, there have been two kinds of MWA methods reported in treating BTNs, the internally water-cooled system of MWA applicator (cMWA, most applied in China) and the real-time temperature control without cooling system of MWA applicator (uMWA, most applied in Germany). Though cMWA is more used in multiple organ sites [15], few studies or reviews concern about the possibly different influences between cMWA and uMWA in thyroid. Therefore, it is necessary to collect the latest data regarding the use of MWA, including cMWA and uMWA, to evaluate its effectiveness and safety for treating BTNs and help clinicians to make a better choice of MWA application.
To the best of our knowledge, it is the first meta-analysis study to evaluate both two MWA methods for the treatment of BTNs. We evaluated the volume reduction and complications of MWA and compared the two options for BTNs.
Materials and methods
Literature search strategy
A computerized search of MEDLINE, EMBASE and Cochrane library was performed to identify relevant original literature on the efficacy and safety of cMWA and uMWA for treatment of benign thyroid nodules. The following search terms were used: thyroid nodule AND (microwave ablation OR MWA). Our search was limited to studies published in English till 3 Jun, 2018. To identify other suitable articles, the bibliographies of the articles were screened.
Study selection
Studies where MWA was used as the treatment for benign thyroid nodules were included. They were required to meet all of the following criteria: (1) the study participation was human; (2) the study demonstrated the clinical value of MWA for benign thyroid nodules; (3) the study reported the result of the volume reduction at the 3-, 6- or 12-month follow-up, or complications. Studies with overlapping patients and data, case reports and series with a sample size of fewer than eight patients, review articles, editorials, letters, comments, and conference proceedings were excluded.
Data extraction and quality assessment
Two reviewers independently extracted the following information from the selected studies: (1) study characteristics: authors, nation, year of publication, sample size, and study design; (2) demographic and clinical characteristics of the patients: mean age, sex, MWA techniques (e.g., use of transisthmic approach, moving shot technique, or hydrodissection; mean ablation time; mean power; gauge of antenna), and nodule characteristics (size and composition); (3) volume reduction ratio (VRR); and (4) major and minor complications. Disagreements were resolved by consensus with a third reviewer.
If the study provided medians and interquartile ranges instead of means and standard deviations (SDs), we imputed the means and SDs as described by Hozo et al [16]. VRR was defined as VRR (%) = [(initial volume − final volume) × 100] / initial volume. Major and minor complications were as defined by the Society of Interventional Radiology [17, 18] and a recent classification [19]. A major complication was defined as one that, if left untreated, might threaten the patient’s life, lead to substantial morbidity or disability, or result in a lengthened hospital stay, including a transient or permanent voice change, rupture of a treated nodule, hypothyroidism, brachial plexus injury, Horner’s syndrome, shoulder weakness, and severe Graves’ ophthalmopathy. All other complications, such as hematoma, vomiting, skin burns, and pain, were considered minor.
The quality of the included studies was also independently assessed by two reviewers using the MINORS evaluation tool [20]. Disagreements were also resolved by consensus with the third reviewer.
Data synthesis
We used RevMan Manger 5.3 and STATA 12.0 to perform the statistical analyses. The pooled VRR and proportions of complications after MWA were used as the main indices for this meta-analysis. For all analysis, P < 0.05 was considered statistically significant. Meta-analytic pooling was conducted by the inverse variance method for calculating weights.
Heterogeneity among studies was assessed by using chi-square testing and I² statistics (0–40%, may not be important; 30–60%, may represent moderate heterogeneity; 50–90%, may represent substantial heterogeneity; 75–100%, may represent considerable heterogeneity) [21]. Publication bias was visually assessed by funnel plots, and statistical significance was evaluated by Egger’s test [22].
We summarized the pooled VRR and pooled proportions of complications using a fixed-effect model in the case of no or non-significant heterogeneity, otherwise, in the case of significant heterogeneity, a random-effects model was used.
Results
Literature search
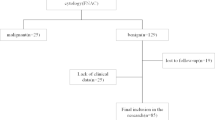
The study selection process is described in Fig. 1. The literature search of the MEDLINE, EMBASE and Cochrane library generated 81 initial articles, of which 30 were screened for eligibility after removing duplicates. Of the remaining 51 articles, we excluded 9 review articles, 11 abstracts/comments/letters, and 10 articles that were not in the field of interest. The full texts of the remaining 21 articles were retrieved. An additional search of the bibliographies of these articles identified no further eligible studies. Of these 21 articles, five were further excluded after reviewing the full text without reporting the volume reduction or complications, and seven with partially overlapping patient cohorts [23,24,25,26,27,28,29]. Finally, nine eligible studies, which included total sample sizes of 1461 patients and 1845 thyroid nodules, were included in our meta-analysis [11,12,13, 30,31,32,33,34,35]. cMWA was used to treat 1784 thyroid nodules in 1408 patients (96.4%), and uMWA was used to treat 61 thyroid nodules in 53 patients (3.6%).
Characteristics of the included studies
Characteristics and quality of the nine included studies included in the meta-analysis were summarized in Table 1. Of the nine studies, five were retrospective [11, 12, 31, 33, 34], and four studies were prospective [13, 30, 32, 35]. In terms of the demographic characteristics of the patients in the included studies, the mean ages of these cases ranged between 42.0 and 66.0 years. Three studies were comparative (cMWA vs uMWA [32], and cMWA vs RFA [13], cMWA vs surgery [35]), and six studies were single-arm studies, four with cMWA only [11, 12, 30, 33] and two with uMWA only [31, 34]. The mean initial nodule volume was 15.0 mL (range, 2.1–102.1 mL) for all the studies, but the mean initial nodule volume of cMWA (11.3 mL; range, 2.1–99.0 mL) was lower than that of uMWA (47.8 mL; range, 19.8–102.1 mL; P < 0.01). There were five studies reported to use a moving shot technique [11,12,13, 31, 35], five studies to use a hydrodissection approach [11,12,13, 33, 35], and three studies to use a transisthmic approach [31, 32, 34].
Pooled VRR of MWA treatment
Using the random-effect model, MWA showed statistically significant reduction in nodule volume with a pooled VRR of 54.3% (95% CI: 45.3–63.3%, I2 = 97.6%) at the 3-month follow-up, 73.5% (95% CI: 66.7–80.3%, I2 = 94.9%) at the 6-month follow-up, and 88.6% (95% CI: 84.9–92.4%, I2 = 92.7%) at the 12-month follow-up. There was no significant publication bias noted for overall VRR at the 6-month and 12-month follow-up (P = 0.14, P = 0.72), while a significant publication bias was noted for that at the 3-month follow-up (P < 0.01). Besides, significant heterogeneities were noted for all the overall VRR.
The subgroup analysis for VRR is presented in Fig. 2 and Table 3. Considering that there is only one literature [31] reporting the VRR of uMWA at the 6-month follow-up and none reporting at the 12-month follow-up, only the VRR at the 3-month follow-up between both MWA methods was analyzed. The VRR at the 3-month follow-up was 58.4% (95% CI: 49.3–67.4%, I2 = 97.0%) for cMWA, and 45.3% (95%CI: 29.5–61.1%, I2 = 92.7%) for uMWA. There was no significant difference between cMWA and uMWA in the VRR (P = 0.07). There was no significant publication bias noted for uMWA group (P = 0.97) either, while a significant publication bias was noted for cMWA group (P = 0.03). Besides, significant heterogeneities were noted for both cMWA and uMWA.
Pooled proportions of overall complications
The overall complications of the included studies were summarized in Table 2. 262 complications of MWA were reported among 1845 thyroid nodules in 1461 patients. The overall complication rate was 52.4% (95% CI: 29.8–74.9%; I2 = 99.5%). There was no significant publication bias noted for overall MWA complications (P = 0.08), while significant heterogeneity was noted.
The subgroup analysis for over complication is presented in Fig. 3 and Table 3. The incidence of overall complications was higher in uMWA group (97.8%, 95% CI: 94.0–100%; I2 = 0) than in cMWA group (29.7%, 95% CI: 18.8–40.5%; I2 = 97.4%; P < 0.01). There was no significant publication bias noted for neither cMWA group (P = 0.40) nor uMWA group (P = 0.11). Besides, significant heterogeneity was noted for cMWA, while there was no heterogeneity noted for uMWA.
Pooled proportions of major complications
The major complications of MWA were also summarized in Table 2. 72 complications were reported. The major complication rate of MWA was 4.8% (95% CI: 2.7–7.0%; I2 = 55.9%). There was no significant publication bias noted for MWA major complications (P = 0.42), while heterogeneity was noted.
The subgroup analysis for major complication is also presented in Fig. 4 and Table 3. The rates of major complications were 4.9% (95% CI: 2.4–7.4%; I2 = 69.9%) for cMWA, and 5.0% (95% CI: 0–10.8%, I2 = 0) for uMWA, without significant difference between both groups (P = 0.49). Significant publication bias was noted for neither cMWA group (P = 0.58) nor uMWA group (P = 0.07). Besides, heterogeneity was noted for cMWA, while there was no heterogeneity noted for uMWA.
The major complications include voice change, thyroid dysfunction, nodule rupture, and Horner’s syndrome. The most common complaint was transient voice change, with a rate of 4.0% (58/1461). All these patients received cMWA and recovered within 1 day to 3 months.
Pooled proportions of minor complications
The minor complications of MWA were also summarized in Table 2. 190 complications were reported. The minor complication rate of MWA was 48.3% (95% CI: 31.2–65.4%; I2 = 99.7%). There was no significant publication bias noted for MWA minor complications (P = 0.09), while heterogeneity was noted.
The subgroup analysis for minor complication is also presented in Fig. 5 and Table 3. The incidence of minor complications was higher in uMWA group (97.8%, 95% CI: 94.0–100%; I2 = 0%) than in cMWA group (21.0%, 95% CI: 13.2–28.8%; I2 = 98.0%; P < 0.01). Significant publication bias was noted for neither cMWA group (P = 0.45) nor uMWA group (P = 0.11). Besides, heterogeneity was noted for cMWA, while there was no heterogeneity noted for uMWA.
The minor complications include pain, skin burn, hemorrhage/hematoma, hyperthyroidism, and vomiting. The most common complaints of MWA were pain, with a rate of 8.9% (130/1461); skin burn, with a rate of 1.9% (28/1461); and hemorrhage/hematoma, with a rate of 2.0% (29/1461). We identified all patients with pain (53/53, 100%) after uMWA procedure while 77 cMWA patients suffered pain (77/1408, 5.5%, P < 0.01); 25 patients with skin burn (25/53, 47.2%) after uMWA procedure while only three cMWA patients suffered skin burn (3/1408, 0.2%, P < 0.01), and two patients with hemorrhage/hematoma (2/53, 3.8%) after uMWA procedure while 27 cMWA patients suffered hemorrhage/hematoma (27/1408, 1.9%, P = 0.28).
Discussion
The present meta-analysis demonstrates that MWA, including both the options, shows a significant reduction in nodule volume with a pooled VRR of 54.3% (95% CI: 45.3–63.3%) at the 3-month follow-up, 73.5% (95% CI: 66.7–80.3%) at the 6-month follow-up, and 88.6% (95% CI: 84.9–92.4%) at the 12-month follow-up; and a pooled proportion of major complication of 4.8% (95% CI: 2.7–7.0%). It also shows that both cMWA and uMWA have similar pooled VRR at the 3-month follow-up (58.4 vs 45.3%, P = 0.07) and pooled proportion of major complication (4.9 vs 5.0%, P = 0.49), while the pooled proportion of overall complication and minor complication of uMWA was significantly higher than that of cMWA (97.8 vs 29.7%, P < 0.01; 97.8 vs 21.0%, P < 0.01). Considering these results, MWA is an effective and relatively safer thermal ablation technique for treating BTNs, and cMWA reveals superior safety to uMWA for less overall and minor complications. Based on the above, cMWA should be a more acceptable MWA option for the treatment of BTNs.
In principle, microwaves produce thermal energy by stimulating water molecules in the ablated tissue to oscillate during ablation [36]. It heats tissue to cytotoxic levels through which cellular death is caused, afterwards the created coagulative necrosis is degraded by the patients’ own immune system [37]. This explains the main mechanism of MWA leading to the significant volume reduction in BTNs. This volume reduction caused by MWA seems to be less effective in the long-term follow-up when comparing to RFA [13], but it may be caused by the influence of the un-matched enrolled patients, since a study achieved similar VRR of RFA and MWA at 1–12 month follow-up when well-matched the enrolled patients of both RFA and MWA groups [25]. In a meta-analysis of RFA [38], a pooled VRR of 76.1% (95% CI: 70.1–82.1%) was achieved at the 6-month follow-up, which is similar as MWA with a pooled VRR of 73.5% (95% CI: 66.7–80.3%) in our meta-analysis. Some researchers [13] also proposed that carbonization, more easily caused by MWA due to higher central temperature in tissue >150 °C [39, 40] (<110 °C by RFA [41]), which is difficult to dissolve so that inevitably causes less VRR, may be another possible explanation. However, this speculation probably lacks direct evidence, as there are many factors, such as initial nodule volume [42], internal nodule component and energy delivery [43], are considered to be correlated with final VRR. Further controlled and randomized prospective clinical studies focusing on effectiveness comparison should be needed if we want to draw a conclusion about which thermal ablation technique is better.
Basically, MWA is safe for the treatment of BTNs, with an incidence of major complication at a low level of 4.8% (95% CI: 2.7–7.0%). Injury of the nerve located adjacent to the thyroid gland is the most common major complication of MWA, including injury of the laryngeal recurrent nerve (showing voice change) and the sympathetic nerve (showing Horner’s syndrome) during the ablation. Voice change after ablation is the most common complaints [13]. We identified 58 patients (4.0%, 58/1461) with voice change but completely recovered within 1 day to 3 months. It might be caused by both thermal injury and hemorrhage [44], or other factors such as lidocaine injection, inflammation and fibrosis around the nerve [44, 45]. Many prevention measures are proposed. A most commonly accepted viewpoint is that several methods, such as moving shot technique, hydrodissection technique and transisthmic approach may be useful to advoid nerve injury, in addition to sufficient knowledge of neck anatomy, accurate preoperative US evaluation of target BTNs and the adjacent structures, US monitoring, necessary short-term pauses and communication with patients [13, 41, 46, 47], but it lacks powerful evidence from comparison studies. Mader et al. [32] also found that hemorrhage might be less seen when increase heat among the uncooled antenna shaft (uMWA) through which vessels around are destroyed, but whether the increase heat would aggravate thermal injury remains unknown. A recent research [48] on RFA for BTNs proposed a radial-movement technique without the transisthmic approach which would help reduce the risk of hemorrhage by reducing insertion numbers and shorten the skin-to-nodule pathway, though a similar rate of voice change (4.3%) was reported. Further controlled and randomized prospective clinical studies focusing on possible prevention measures might give reliable answers.
Regarding the devices, the generators used for uMWA are capable of producing 24–36 W at a frequency of 902–928 MHz [31, 32, 34] while the systems used for cMWA are capable of producing 20–60 W at a frequency of 2450 MHz and include an internal water-cooling system [11,12,13, 30, 32, 33, 35]. Several studies believed that these two systems of MWA might be related to the effectiveness of ablation. Sun et al. [49] showed that microwaves with a lower frequency of 915 MHz create significantly larger ablation zones and furthermore have a deeper penetration depth than microwaves with a higher frequency of 2450 MHz, while Mader et al. [32] indicated that the cooling system of an ablation device might have a larger influence on the volume reduction than the frequency the system is running with. However, our meta-analysis demonstrated a similar VRR after both MWA procedures (58.4 vs 45.3%, P = 0.07), which may suggest similar efficacy of cMWA and uMWA at the 3-month follow-up. One explanation for the seemingly similar efficacy of both modalities may be relatively small volume of thyroid gland and the use of moving-shot technique in BTNs [25]. Although the tissue surrounding the uncooled antenna will be heated up more quickly, thyroid gland is relative small and BTNs also make the safety margin unnecessary. Besides, moving the antenna during the procedure maximizes ablation zone to effectively reduce the nodule volume and allow the nodule to be treated safely. However, it should be also noted that the initial nodule volume of uMWA groups was larger than that of cMWA (47.8 mL vs 11.3 mL, P < 0.01), which may lead to a lack of knowledge on whether the efficacy of uMWA is underestimated, as an RFA study showed better VRR presented in smaller nodules less than 12 mL [42]. Besides, it is unclarified whether the two modalities differ in long-term efficacy and overall superiority such as energy delivered per milliliter of thyroid tissue and treatment time. A fair comparison would need to be based on a prospective randomized study additionally taking overall cost and quality of life issues into consideration in long-term follow-up studies.
Eventhough both the options of MWA have similar volume reduction for BTNs, uMWA has a higher incidence of overall complications than cMWA (97.8 vs 29.7%, P < 0.01). Since both MWA have a similar incidence of major complications (4.9 vs 5.0%, P = 0.49), the higher rate of overall complications is mainly due to a higher incidence of minor complications (97.8 vs 21.0%, P < 0.01), which is consisted by more patients with pain and skin burn. We identified all patients with pain (53/53, 100%) after uMWA procedure while 77 cMWA patients suffered pain (77/1408, 5.5%, P < 0.01), and 25 patients with skin burn (25/53, 47.2%) after uMWA procedure while only three cMWA patients suffered skin burn (3/1408, 0.2%, P < 0.01) in our meta-analysis. The higher temperature during uMWA, which was measured in liver models up to 90 °C at the non-internally cooled antennas, comparing with less than 20 °C during internally cooled antennas shaft [50], may help partly explain the higher incidence, as both complications are associated with thermal injury. Although it is believed that the increasing heat in the uMWA helps prevent hemorrhage through destroying surrounding vessels [32], our meta-analysis did not show significant difference in the incidence of hemorrhage/hematoma between both MWA procedures (3.8 vs 2.0%, P = 0.29). In that case, to achieve an increase of patient’s safety and comfort during MWA procedure, cMWA would be a more acceptable option for the treatment of BTNs with relatively lower incidence of pain and skin burn. However, there are also other factors such as parenchymal edema (associated with pain) and a moving antenna close to the skin (associated with skin burn) [47]. Operators must pay more attention during MWA procedure, and whether prevention measures are necessary needs more direct evidence.
This review has some limitations of note. First, it included relatively few studies, of which most were retrospective studies and focused on cMWA with relatively larger sample size, which can lead to significant heterogeneties. Though we used subgroup analysis, the heterogeneities regarding the pooled VRR and complications of cMWA and uMWA could not be overcome well. It has been postulated in this regard that most heterogenety is due to technical differences between the subjects, institutions or operators. Second, only short-term VRRs (at the 3-month follow-up) of both MWA methods were compared, since there is only one study [32] reporting the VRR of uMWA at the 6-month follow-up and none reporting at the 12-month follow-up, which is insufficient to prove the effectiveness of uMWA at the long-term follow-up. Third, the pooled proportion of overall (52.4%, 95% CI: 29.8–74.9%) and minor complications (48.3%, 95% CI: 31.2–65.4%) in this meta-analysis were very high, e.g., the pooled proportion of minor complications of cMWA (21.0%, 95% CI: 13.2–28.8%) was much higher than that reported by a multicenter research [13] showing 2.5%. A possible explanation is that pain, which is reported by most studies with rates varying from 2 to 60% while undergoing thermal ablation, is considered as a complication instead of a side effect according to a recent review [19]. Fourth, gray literature, which may have caused a publication bias, was excluded, such as letters, conference abstracts, and unpublished data. However, it was difficult to extract accurate data for the meta-analysis.
In conclusion, this systemic review with meta-analysis demonstrates that MWA is an effective treatment modality for BTNs. When considering the patient’s safety and comfort, cMWA would be a more preferable procedure with less complications.
References
H. Gharib, E. Papini, R. Paschke, D.S. Duick, R. Valcavi, L. Hegedus, P. Vitti, American association of clinical endocrinologists, associazione medici endocrinologi, and European thyroid association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. J. Endocrinol. Invest. 33, 1–50 (2010)
H. Gharib, L. Hegedus, C.M. Pacella, J.H. Baek, E. Papini, Clinical review: Nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J. Clin. Endocrinol. Metab. 98, 3949–3957 (2013)
J.J. Sancho, R. Prieto, J.P. Duenas, C. Ribera, J. Ripolles, A. Larrad, A. Sitges-Serra, A randomized trial of hemithyroidectomy versus Dunhill for the surgical management of asymmetrical multinodular goiter. Ann. Surg. 256, 846–842 (2012)
L. Rosato, N. Avenia, P. Bernante, M. De Palma, G. Gulino, P.G. Nasi, M.R. Pelizzo, L. Pezzullo, Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J. Surg. 28, 271–276 (2004)
R. Cesareo, A. Palermo, V. Pasqualini, R. Cianni, G. Gaspa, S. Manfrini, C.M. Pacella, Radiofrequency ablation for the management of thyroid nodules: A critical appraisal of the literature. Clin. Endocrinol. 87, 639–648 (2017)
E. Papini, R. Gugliemi, C.M. Pacella, Laser, radiofrequency, and ethanol ablation for the management of thyroid nodules. Curr. Opin. Endocrinol. Diabetes Obes. 23, 400–406 (2016)
M.K. Shahrzad, Laser thermal ablation of thyroid benign nodules. J. Lasers Med. Sci. 6, 151–156 (2015)
A.P. Mainini, C. Monaco, L.C. Pescatori, C. De Angelis, F. Sardanelli, L.M. Sconfienza, G. Mauri, Image-guided thermal ablation of benign thyroid nodules. J. Ultrasound 20, 11–22 (2017)
Y.L. Yang, C.Z. Chen, X.H. Zhang, Microwave ablation of benign thyroid nodules. Future Oncol. 10, 1007–1014 (2014)
P. Liang, Y. Wang, X. Yu, B. Dong, Malignant liver tumors: Treatment with percutaneous microwave ablation—complications among cohort of 1136 patients. Radiology 251, 933–940 (2009)
W. Wu, X. Gong, Q. Zhou, X. Chen, X. Chen, B. Shi, US-guided percutaneous microwave ablation for the treatment of benign thyroid nodules. Endocr. J. 64, 1079–1085 (2017)
Y.J. Liu, L.X. Qian, D. Liu, J.F. Zhao, Ultrasound-guided microwave ablation in the treatment of benign thyroid nodules in 435 patients. Exp. Biol. Med. 242, 1515–1523 (2017)
Z. Cheng, Y. Che, S. Yu, S. Wang, D. Teng, H. Xu, J. Li, D. Sun, Z. Han, P. Liang, US-guided percutaneous radiofrequency versus microwave ablation for benign thyroid nodules: A prospective multicenter study. Sci. Rep. 7, 9554 (2017)
F. Morelli, A. Sacrini, G. Pompili, A. Borelli, S. Panella, A. Masu, L. De Pasquale, R. Giacchero, G. Carrafiello, Microwave ablation for thyroid nodules: A new string to the bow for percutaneous treatments? Gland Surg. 5, 553–558 (2016)
J. Yu, P. Liang, Status and advancement of microwave ablation in China. Int. J. Hyperthermia 33, 278–287 (2017)
S.P. Hozo, B. Djulbegovic, I. Hozo, Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 5, 13 (2005)
D.R. Burke, C.A. Lewis, J.F. Cardella, S.J. Citron, A.T. Drooz, Z.J. Haskal, J.W. Husted, T.C. McCowan, A. Van Moore, S.B. Oglevie, D. Sacks, J.B. Spies, R.B. Towbin, C.W. Bakal, Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J. Vasc. Interv. Radiol. 14, S243–246 (2003)
C.A. Lewis, T.E. Allen, D.R. Burke, J.F. Cardella, S.J. Citron, P.E. Cole, A.T. Drooz, E.A. Drucker, Z.J. Haskal, L.G. Martin, A. Van Moore, C.D. Neithamer, S.B. Oglevie, K.S. Rholl, A.C. Roberts, D. Sacks, O. Sanchez, A. Venbrux, C.W. Bakal, Quality improvement guidelines for central venous access. The standards of practice committee of the society of cardiovascular and interventional radiology. J. Vasc. Interv. Radiol. 8, 475–479 (1997)
I. J. Nixon, P. Angelos, A. R. Shaha, A. Rinaldo, M. D. Williams, A. Ferlito, Image-guided chemical and thermal ablations for thyroid disease: Review of efficacy and complications. Head Neck (2018)
O. Dent, Methodological index for non-randomized studies. ANZ J. Surg. 73, 675–676 (2003)
J. Higgins, S. Green, Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Updated March 2011. Accessed 15 Aug 2015
M. Egger, G. Davey Smith, M. Schneider, C. Minder, Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997)
Y. Korkusuz, K. Kohlhase, D. Groner, C. Erbelding, W. Luboldt, C. Happel, S. Ahmad, T.J. Vogl, F. Gruenwald, Microwave ablation of symptomatic benign thyroid nodules: Energy requirement per ml volume reduction. RoFo 188, 1054–1060 (2016)
B. Wang, Z. Y. Han, J. Yu, Z. Cheng, F. Liu, X. L. Yu, C. Chen, J. Liu, P. Liang, Factors related to recurrence of the benign non-functioning thyroid nodules after percutaneous microwave ablation. Int. J. Hyperthermia 33, 459–464 (2017)
W.W. Yue, S.R. Wang, F. Lu, L.P. Sun, L.H. Guo, Y.L. Zhang, X.L. Li, H.X. Xu, Radiofrequency ablation vs. microwave ablation for patients with benign thyroid nodules: A propensity score matching study. Endocrine 55, 485–495 (2017)
H. Korkusuz, C. Happel, K. Heck, H. Ackermann, F. Grunwald, Percutaneous thermal microwave ablation of thyroid nodules. Preparation, feasibility, efficiency. Nuklearmedizin 53, 123–130 (2014)
Y. Wei, L. Qian, J. B. Liu, Y. Liu, Sonographic measurement of thyroid nodule changes after microwave ablation: relationship between multiple parameters. Int. J. Hyperthermia 34, 660–668 (2018)
W. Wu, X. Gong, Q. Zhou, X. Chen, X. Chen, Ultrasound-guided percutaneous microwave ablation for solid benign thyroid nodules: Comparison of MWA versus control group. Int. J. Endocrinol. 2017, 9724090 (2017)
Y. Korkusuz, D. Groner, N. Raczynski, O. Relin, Y. Kingeter, F. Grunwald, C. Happel, Thermal ablation of thyroid nodules: Are radiofrequency ablation, microwave ablation and high intensity focused ultrasound equally safe and effective methods? Eur. Radiol. 28, 929–935 (2018)
B. Feng, P. Liang, Z. Cheng, X. Yu, J. Yu, Z. Han, F. Liu, Ultrasound-guided percutaneous microwave ablation of benign thyroid nodules: experimental and clinical studies. Eur. J. Endocrinol. 166, 1031–1037 (2012)
K. Heck, C. Happel, F. Grunwald, H. Korkusuz, Percutaneous microwave ablation of thyroid nodules: Effects on thyroid function and antibodies. Int. J. Hyperth. 31, 560–567 (2015)
O.M. Mader, N.F. Tanha, A. Mader, C. Happel, Y. Korkusuz, F. Grunwald, Comparative study evaluating the efficiency of cooled and uncooled single-treatment MWA in thyroid nodules after a 3-month follow up. Eur. J. Radiol. Open 4, 4–8 (2017)
W. Yue, S. Wang, B. Wang, Q. Xu, S. Yu, Z. Yonglin, X. Wang, Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients. Eur. J. Radiol. 82, e11–16 (2013)
H. Korkusuz, F. Nimsdorf, C. Happel, H. Ackermann, F. Grunwald, Percutaneous microwave ablation of benign thyroid nodules. Functional imaging in comparison to nodular volume reduction at a 3-month follow-up. Nuklearmedizin 54, 13–19 (2015)
X. Zhi, N. Zhao, Y. Liu, J. B. Liu, C. Teng, L. Qian, Microwave ablation compared to thyroidectomy to treat benign thyroid nodules. Int. J. Hyperthermia 34, 644–652 (2018)
C.L. Brace, Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: What are the differences? Curr. Probl. Diagn. Radiol. 38, 135–143 (2009)
Y. Korkusuz, O.M. Mader, W. Kromen, C. Happel, S. Ahmad, D. Groner, M. Koca, A. Mader, F. Grunwald, H. Korkusuz, Cooled microwave ablation of thyroid nodules: Initial experience. Eur. J. Radiol. 85, 2127–2132 (2016)
E.J. Ha, J.H. Baek, K.W. Kim, J. Pyo, J.H. Lee, S.H. Baek, H. Dossing, L. Hegedus, Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and bayesian network meta-analysis. J. Clin. Endocrinol. Metab. 100, 1903–1911 (2015)
M. Ahmed, C.L. Brace, F.T. Lee Jr., S.N. Goldberg, Principles of and advances in percutaneous ablation. Radiology 258, 351–369 (2011)
E.M. Knavel, C.L. Brace, Tumor ablation: Common modalities and general practices. Tech. Vasc. Interv. Radiol. 16, 192–200 (2013)
J.H. Shin, J.H. Baek, E.J. Ha, J.H. Lee, Radiofrequency ablation of thyroid nodules: basic principles and clinical application. Int. J. Endocrinol. 2012, 919650 (2012)
R. Cesareo, V. Pasqualini, C. Simeoni, M. Sacchi, E. Saralli, G. Campagna, R. Cianni, Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J. Clin. Endocrinol. Metab. 100, 460–466 (2015)
S.L. Jung, J.H. Baek, J.H. Lee, Y.K. Shong, J.Y. Sung, K.S. Kim, D. Lee, J.H. Kim, S.M. Baek, J.S. Sim, D.G. Na, Efficacy and safety of radiofrequency ablation for benign thyroid nodules: A prospective multicenter study. Korean J. Radiol. 19, 167–174 (2018)
J.H. Baek, J.H. Lee, J.Y. Sung, J.I. Bae, K.T. Kim, J. Sim, S.M. Baek, Y.S. Kim, J.H. Shin, J.S. Park, D.W. Kim, J.H. Kim, E.K. Kim, S.L. Jung, D.G. Na, Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology 262, 335–342 (2012)
C. Kim, J.H. Lee, Y.J. Choi, W.B. Kim, T.Y. Sung, J.H. Baek, Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur. Radiol. 27, 3128–3137 (2017)
W.K. Jeong, J.H. Baek, H. Rhim, Y.S. Kim, M.S. Kwak, H.J. Jeong, D. Lee, Radiofrequency ablation of benign thyroid nodules: Safety and imaging follow-up in 236 patients. Eur. Radiol. 18, 1244–1250 (2008)
J.F. Wang, T. Wu, K.P. Hu, W. Xu, B.W. Zheng, G. Tong, Z.C. Yao, B. Liu, J. Ren, Complications followingradiofrequency ablation of benign thyroid nodules: A systematic review. Chin. Med. J. (Engl.) 130, 1361–1370 (2017)
R. Cervelli, S. Mazzeo, L. De Napoli, A. Boccuzzi, B. Pontillo-Contillo, G. Materazzi, P. Miccoli, R. Cioni, D. Caramella, Radiofrequency ablation in the treatment of benign thyroid nodules: An efficient and safe alternative to surgery. J. Vasc. Interv. Radiol. 28, 1400–1408 (2017)
Y. Sun, Y. Wang, X. Ni, Y. Gao, Q. Shao, L. Liu, P. Liang, Comparison of ablation zone between 915- and 2,450-MHz cooled-shaft microwave antenna: Results in in vivo porcine livers. AJR Am. J. Roentgenol. 192, 511–514 (2009)
N. He, W. Wang, Z. Ji, C. Li, B. Huang, Microwave ablation: an experimental comparative study on internally cooled antenna versus non-internally cooled antenna in liver models. Acad. Radiol. 17, 894–899 (2010)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zheng, Bw., Wang, Jf., Ju, Jx. et al. Efficacy and safety of cooled and uncooled microwave ablation for the treatment of benign thyroid nodules: a systematic review and meta-analysis. Endocrine 62, 307–317 (2018). https://doi.org/10.1007/s12020-018-1693-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1693-2