Abstract
Purpose
To evaluate the applicability of ultrasound (US)-based Thyroid Imaging Reporting and Data System (TI-RADS) for evaluating medullary thyroid carcinoma (MTC).
Materials and methods
US images and medical records of patients with cytopathology-confirmed MTC between June 2003 and November 2016 were retrospectively reviewed. Four independent reviewers (two experienced and two inexperienced radiologists) evaluated 57 pre-operative US images of patients with MTC for shape, composition, echogenicity, margin, calcification of the MTC nodules, and categorized the nodules using TI-RADS classification. Weighted Kappa statistics was used to determine the inter-observer agreement of TI-RADS. Univariate and multivariate analyses were performed to assess US findings associated with lymph node metastasis.
Results
Ninety-five percent of nodules were classified as either high suspicion (68%) or intermediate suspicion (26%). The overall inter-rater agreement was good (Kappa 0.84, agreement 91.52%), and inexperienced reviewers also showed good agreements with the most experienced reviewer (weighted Kappa 0.73 and 0.81). According to the univariate analysis, TI-RADS category 5, shape, microcalcification, and extrathyroid extension were significantly associated with lymph node metastasis in MTC patients (p = 0.003, 0.008, 0.001, and 0.021, respectively). As per the multivariate analysis, the presence of microcalcification and the irregular shape of the nodule were significantly associated with metastatic lymph nodes in MTC patients (odds ratio, 26.6; 95% CI, 2.7–263.7, p = 0.005, odds ratio, 14.7; 95% CI, 1.3–170, p = 0.031, respectively).
Conclusion
TI-RADS is applicable for the evaluation of MTC nodules with good inter-observer agreement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thyroid ultrasound (US) is useful for the initial evaluation of thyroid nodules [1], as it provides a way to differentiate between benign and malignant nodules [2]. To date, several classification systems have been proposed in efforts to standardize the assessment of thyroid nodules using US [1, 3, 4]. The Thyroid Imaging Reporting and Data System (TI-RADS), based on a widely used Breast Imaging Reporting and Data System® (BI-RADS®) [5] for breast nodules, has been proposed by Korean Society of Thyroid Radiology to stratify thyroid nodules in accordance with the risk of malignancy [4]. Recently, TI-RADS has received much attention due to its simplicity and clinical feasibility [4, 6]. Thyroid nodules are classified into five categories based on solidity, echogenicity, and three suspicious US features (microcalcification, non-parallel orientation, and spiculated/microlobulated margin). Recommendations for fine-needle aspiration (FNA) are made in accordance with the category and size.
However, most classification systems, including TI-RADS, are primarily focused on papillary thyroid carcinoma (PTC), due to its high prevalence, which accounts for >80% of primary thyroid malignancies [7]. Conversely, US findings of medullary thyroid carcinoma (MTC), which accounts for 3–5% of thyroid cancers, have rarely been reported [8,9,10]. MTC is a neuroendocrine tumor that originates from the parafollicular C-cells, and is known to have a poorer prognosis than papillary and follicular thyroid carcinomas [11].
Nonetheless, US diagnosis of MTC remains challenging. US features for suspicious malignant nodules [12, 13] have been reported to be less frequent in MTC [9, 14]. Moreover, some previous studies reported that in one-third of MTC cases, MTCs may appear as benign nodules with no malignant features [15, 16]. The optimal surgical extent depends on the presence of lymph node metastasis at the time of the operation [17]. Early detection of MTC with thorough evaluation of the tumor plays an important role in the prognosis of patients [11, 18]. Most previous studies regarding MTC were focused on describing US findings of MTCs or comparing those with benign nodules or PTCs [9, 19, 20]. However, to the best of our knowledge, there is no study evaluating the efficacy of TI-RADS in assessing MTC nodules. Moreover, despite the high prevalence of the lymph node metastasis and its clinical importance in MTCs [7, 11, 21], there is a paucity of data regarding US findings associated with lymph node metastasis.
Therefore, the purpose of this study was to evaluate the applicability of TI-RADS in MTC patients and to assess US characteristics of MTC nodules associated with lymph nodes metastasis.
Material and methods
Patient selection
The local institutional review board approved the protocol of this study; informed consent was waived due to its retrospective nature. Using our hospital database, a search of medical records revealed that 60 patients were diagnosed with MTC between June 2003 and November 2016. Among them, pre-operative US images were available in 57 patients, and they were included in this study. The median age of patients was 63 years (range, 27–87 years); there were 23 men and 34 women. The final histopathologic diagnosis of MTC was confirmed at surgical resection (n = 50) or at biopsy either by FNA (n = 3) or core needle biopsy (CNB) (n = 4). The median maximum diameter of MTCs was 1.5 cm (range 0.3–4.9 cm). RET gene mutation analysis was performed in 22 patients, and RET gene mutation was identified in 3 (14%) patients. The pre-operative calcitonin level was available in 44 (77%) patients, which revealed a median value of 403 pg/ml (range 5–20,600). The baseline characteristics are summarized in Table 1. FNA or CNB revealed the following: two benign (Bethesda category 2) nodules (3%), seven atypia with undetermined significance (Bethesda category 3) nodules (12%), seven suspicious for MTC (Bethesda category 5) nodules (12%), and 41 MTC (Bethesda category 6) nodules (72%).
Procedures
All US examinations were performed using 7–12 MHz linear probe (iU22; Philips Medical Systems, Bothell, WA). Retrospective review of US images was conducted by two experienced reviewers (11 years and 4 years of experience in thyroid US) and two inexperienced reviewers (first-year residents). To familiarize the two inexperienced readers with TI-RADS, education (5 h) on thyroid US and TI-RADS was provided, with at least 300 cases of US examination and interpretation, prior to the image evaluation. Four reviewers independently reviewed US images of 57 MTC nodules and categorized the nodules using TI-RADS. All readers were blinded to the clinical information and pathology results. US images were reviewed on the same monitor and under the same light condition.
Nodules were characterized on US by shape, composition, echogenicity, margin, orientation, and presence of calcification in accordance with the standard methodology from previously published reports [4, 22]. Size was measured in three dimensions. Shape was classified as either round-to-ovoid or irregular. The orientation or direction of growth was categorized as either parallel (when the anteroposterior diameter of the nodule was shorter than its longitudinal or transverse diameter) or non-parallel (when the anteroposterior diameter of the nodule was longer than its longitudinal or transverse diameter). The composition was classified as solid, predominantly solid ( ≤ 50% cystic component), predominantly cystic ( > 50% cystic component), or cystic. Echogenicity was categorized as hypoechoic, isoechoic, or hyperechoic. Predominant echogenicity was used as the reference when the echogenicity of a solid component of the nodule was heterogeneous. Calcification was categorized as none, microcalcifications (punctuate echogenic foci of 1 mm or less), macrocalcification (echogenic foci larger than 1 mm with posterior shadowing) or rim calcification (peripheral eggshell calcification at the margin). Margins were classified as smooth or ill-defined or spiculated/microlobulated [23].
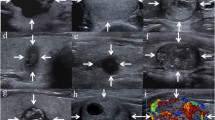
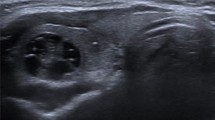
Reviewers graded the nodule in accordance with TI-RADS, which included solidity, echogenicity, and three suspicious US features (microcalcification, non-parallel orientation, spiculated/microlobulated margin) [4]. The categories were as follows: (1) “no nodule”, (2) “benign”—including spongiform nodule, partially cystic nodule with comet tail artifact, and pure cyst; (3) “low suspicion” (Fig. 1a)—partially cystic or isohyperechoic nodule without any of the three suspicious US features, (4) “intermediate suspicion” (Fig. 1b)—hypoechoic solid nodule without any of the three suspicious US features or partially cystic or isohyperechoic nodule with any one of the three suspicious US features, and (5) “high suspicion” (Fig. 1c)—solid hypoechoic nodule with any one of the three suspicious US features [4]. The presence of suspicious lymph nodes was rated as either present or absent. Suspicious lymph nodes were assessed with respect to cystic change, hyperechogenicity, microcalcification, and vascularity in the peripheral portion of the lymph node (Fig. 2). Extrathyroidal extension was recorded as positive when the nodule was abutting the thyroid capsule or there was a discontinuity of the capsule (Fig. 1c).
Transverse US images of medullary thyroid carcinoma nodules categorized by TI-RADS. a A partially cystic nodule without any suspicious feature, graded as TI-RADS 3 (low suspicion). b A hypoechoic solid nodule without any suspicious feature, graded as TI-RADS 4 (intermediate suspicion). c A hypooechoic solid nodule with spiculated margin and microcalcifications (white arrows), graded as TI-RADS 5 (high suspicion). Note the presence of extrathyroid extension to strap muscle (arrowheads) and metastatic lymphadenopathy (black arrows)
The diagnosis of MTC was initially made using either FNA or CNB. The result of FNA was interpreted according to the Bethesda System for Reporting Thyroid Cytopathology, and that of CNB was based on the pathology reporting system. After making the diagnosis, seven patients were lost to follow-up and three patients underwent total thyroidectomy without lymph node dissection. A total of 47 patients underwent total thyroidectomy with neck dissection. Patients were grouped into two categories with respect to the presence of metastatic lymph node on surgical specimen.
Statistical analysis
The inter-observer percentages regarding TI-RADS classification were calculated by dividing the number of cases of complete agreement by the total number of cases. The weighted kappa coefficient statistics were used to determine the degree of agreement after taking into account the agreement expected by chance. The kappa statistics were rated in the following manner: k = 0–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81 or greater, excellent agreement [24].
US analysis data from the most experienced reviewer was used for comparative statics. Chi-square test or independent t-test was used to determine US findings significantly associated with lymph node metastasis. After univariate analysis, variables with a p-value of <0.05 were included in the multivariate logistic regression analysis with the enter method to determine independent features associated with lymph node metastasis. All statistical analyses were performed using R statistical software v3.1.0 and SPSS 20.0 (SPSS, Chicago, IL). A p-value of <0.05 was considered statistically significant.
Results
Ninety-five percent of nodules (54/57) were classified as either high suspicion (TI-RADS 5; 68%) or intermediate suspicion (TI-RADS 4; 26%); only three nodules were classified as low suspicion (TI-RADS 3). Ninety-five percent of nodules (54/57) were indicated to further FNA based on TI-RADS (size > 5 mm in TI-RADS 5, > 10 mm in TI-RADS 4, > 15 mm in TI-RADS 3 and presence of suspicious lymph node). The average kappa value was 0.84, and the mean percent of agreement between the four readers was 91.52%. The inter-observer agreement of each pair of readers is summarized in Table 2. All kappa values were at good level. Two inexperienced readers were discrepant in 6/57 and 9/57 of cases, compared with the most experienced reader. Most discrepant cases were rated as TI-RADS 5 by the most experienced reader, while they were rated as TI-RADS 4 by inexperienced readers.
The US findings of MTCs are summarized in Table 3. Most MTCs were presented as solid nodules (88%), whereas 12% were presented with partially solid component; 95% were hypoechoic; 61% showed a round-to-oval shape, while 39% showed irregular shape; 77% were parallel, whereas 23% were non-parallel; 44% were smooth, 31% were ill-defined and 25% spiculated; 40% had microcalcification, whereas 9% had macrocalcification; 26% had suspicious lymph node on US; and 33% had sonographic evidence of extrathyroidal extension. Lymph nodes suspected for metastasis from MTCs showed loss of fatty hilum (94%), cystic internal content (19%), microcalcification (69%), hyperechogenicity (50%), and hypervascularity (86%).
The result of univariate analysis regarding US imaging factors associated lymph node metastasis is summarized in Table 4. The grade of TI-RADS was statistically significantly higher in lymph node-positive MTCs than in lymph node-negative MTCs (TI-RADS 5 18/20 [90%] in lymph node-positive group vs. 13/27 [48%] in the lymph node-negative group; p = 0.003). The size of the nodule was larger in the lymph node-positive group than in the lymph node-negative group (Median (cm); 2.1 vs. 1.5), but without statistical significance (p < 0.26). The irregular shape was seen more commonly in the lymph node-positive group (12/20 [60%]) than in a lymph node-negative group (6/27 [22%]; p = 0.008). The presence of microcalcification was observed more frequently in the lymph node-positive group (13/20 [65%]) than in the lymph node-negative group (5/27 [18%]; p = 0.001). Another feature was extrathyroid extension, which was found more commonly in the lymph node-positive group (11/20 [55%]) than in the lymph node-negative group (6/27 [22%]; p = 0.021). Multivariate logistic regression analysis by the enter method showed that the presence of microcalcification and irregular shape of the nodule were significant variables associated with metastatic lymph nodes in MTC patients (odds ratio, 26.6; 95% CI, 2.7–263.7, p = 0.005, odds ratio, 14.7; 95% CI, 1.3–170, p = 0.031, respectively)
Discussion
The results of our study demonstrate that TI-RADS effectively stratified the risk of malignancy in our cohort comprised of MTCs, providing a basis for further management (95% of MTC nodules were indicated for fine-needle aspiration) with good inter-observer agreement. Common US findings of MTC in this study were solid hypoechoic nodules with a round-to-oval shape, parallel orientation, ill-defined or spiculated margin, and microcalcification. Metastatic lymph nodes in MTC showed hyperechogenicity, loss of fatty hilum, and hypervascularity. MTC nodules with irregular shape and microcalcification were significantly associated with lymph node metastasis.
To our knowledge, our study included the largest number of patients in demonstrating the applicability of TI-RADS in MTC. To date, there is only one previous study applying the 2015 American thyroid association sonographic patterns [10] in 30 MTC nodules. Our results were consistent with this previous study, which showed that >80% of MTCs (24 out of 30) were classified as “intermediate” to “high” suspicion.
In this present study, 26% of MTCs were classified as intermediate suspicion. MTC nodules with intermediate suspicion on US might lead to delayed diagnosis and treatment [15, 25]. “Non-malignant looking” nodules were regular in shape and solid on sonographic images, equivalent to intermediate suspicion nodules. Previous studies found that MTC nodules more frequently fall into the intermediate sonographic category, compared with PTCs, as suspicious sonographic findings are less prevalent in MTCs [1]. Therefore, intermediate sonographic features should be assessed promptly in cases with MTC suspicion. Serum calcitonin measurement is recommended in aiding the diagnosis of MTC [26].
Interestingly, MTCs in our study population tended to have more malignant features than benign features, except for the round-to-oval shape. Our results differed from previous studies [9, 14] in that the frequency of MTCs with taller-than-wide shape (13 to 19%) was much lower than that of PTCs (36.4 to 42.1%). In addition, 60% of MTCs had ill-defined or spiculated margins. Recently, Zhou et al. [27] reported that small MTCs more frequently showed spiculated margins, while MTCs with a diameter of larger than 1 cm showed a smooth margin, with overall 34% of nodules showing spiculated margins. We speculate that the resolution of US may have influenced the results.
In the current study, 40% of MTCs had microcalcification and 9% had macrocalcification. Our study results are grossly in line with a recent meta-analysis by Valderrabano et al. [10], in which they reported the overall prevalence of microcalficiation and macrocalcification of MTCs to be 31.7% and 26%, respectively. Multiple “bright echogenic spots” represent pathologic findings of focal deposits of calcium in the background of amyloid [14]. The amyloid deposition is the pathologic hallmark of MTC, distinguishing MTC from other thyroid tumors [8], including faint homogenous calcifications found in different thyroid tumors [28].
Calcified lymph nodes can be noted in metastasis from thyroid tumors [29]. Two previous studies have reported “bright echogenic spots” in metastatic lymph nodes from MTC [28, 30]. This supports the result of our study, where frequent microcalcification was noted in suspicious metastatic cervical lymph nodes. In our study, loss of fatty hilum (94%), cystic component (19%), and hypervascularity (86%) were also observed in metastatic lymph nodes of MTC patients. Although there is no literature supporting our results, these features are not different from findings seen in metastatic lymph nodes from papillary thyroid cancer. Moreover, it has been established that an increased peripheral vascularity of metastatic MTC lesion is shared with other types of neuroendocrine tumors [11]. Pombo et al. [29] found that hypervascularity in metastatic lymph nodes of MTCs were presented as high attenuation on CT. However, it is noteworthy that our study used high-resolution US images to evaluate increased vascularity on Doppler US images in metastatic lymph nodes of MTCs.
To the best of our knowledge, this is the first report describing the association between US characteristics of MTC and lymph node metastasis in those who underwent surgical resection for MTC. We showed a reliable correlation between US characteristics and surgical pathology results. US features of irregular shape and presence of microcalcification were two independent factors associated with lymph node metastasis in MTC patients. Park et al. [31] recently reported that US features classified by TI-RADS are associated with lymph node metastasis in PTC patients. In cases where MTC nodules show irregular shape and presence of microcalcification with lymph node metastasis, there needs to be a thorough evaluation of lymph nodes. Based on the result that malignant features of MTC and TI-RADS are applicable for MTC patients, we conjecture that the TI-RADS score could potentially be used as a marker for lymph node metastasis in MTC patients. However, further prospective studies with larger population is necessary to elucidate this conclusion.
There are several limitations to consider when interpreting the findings of this study. First, given the retrospective nature of this study, there might be inherent selection bias. Second, due to the small number of study population from a single center, generalizability of this data may be limited. A more comprehensive prospective study from a multicenter may be necessary. Finally, we could not investigate the relationship between nodule characteristics and prognosis. Although lymph node metastasis in MTC is related to poorer survival, to our knowledge, a correlation between imaging findings in MTC with patients’ prognosis has not been reported to date. Future follow-up studies are warranted with respect to the relationship of US findings with lymph node metastasis and with prognosis to provide more in-depth information on whether US findings could potentially be used as an imaging prognostic marker for MTC.
In conclusion, TI-RADS is applicable in evaluation of MTC nodules with good inter-observer agreement. Application of TI-RADS criteria and having the knowledge of US image findings associated with lymph node metastasis might be helpful to properly manage patients with suspected or known MTC.
References
B.R. Haugen, E.K. Alexander, K.C. Bible, G.M. Doherty, S.J. Mandel, Y.E. Nikiforov, F. Pacini, G.W. Randolph, A.M. Sawka, M. Schlumberger, K.G. Schuff, S.I. Sherman, J.A. Sosa, D.L. Steward, R.M. Tuttle, L. Wartofsky, 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid.: Off. J. Am. Thyroid. Assoc. 26(1), 1–133 (2016). https://doi.org/10.1089/thy.2015.0020
S.I. Sherman, Thyroid carcinoma. Lancet (Lond., Engl.) 361(9356), 501–511 (2003)
H.J. Moon, E.K. Kim, J.H. Yoon, J.Y. Kwak, Malignancy risk stratification in thyroid nodules with nondiagnostic results at cytologic examination: combination of thyroid imaging reporting and data system and the Bethesda System. Radiology 274(1), 287–295 (2015). https://doi.org/10.1148/radiol.14140359
D.G. Na, J.H. Baek, J.Y. Sung, J.H. Kim, J.K. Kim, Y.J. Choi, H. Seo, Thyroid Imaging Reporting and Data System risk stratification of thyroid nodules: Categorization based on solidity and echogenicity. Thyroid.: Off. J. Am. Thyroid. Assoc. 26(4), 562–572 (2016). https://doi.org/10.1089/thy.2015.0460
E.B. Mendelson, M. Böhm-Vélez, W.A. Berg, et al. ACR BI-RADS® Ultrasound. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology (2013).
E.J. Ha, W.J. Moon, D.G. Na, Y.H. Lee, N. Choi, S.J. Kim, J.K. Kim, A. Multicenter Prospective, Validation study for the Korean Thyroid Imaging Reporting and Data System in patients with thyroid nodules. Korean J. Radiol. 17(5), 811–821 (2016). https://doi.org/10.3348/kjr.2016.17.5.811
P. Trimboli, L. Giovanella, A. Crescenzi, F. Romanelli, S. Valabrega, G. Spriano, N. Cremonini, R. Guglielmi, E. Papini, Medullary thyroid cancer diagnosis: an appraisal. Head Neck. 36(8), 1216–1223 (2014). https://doi.org/10.1002/hed.23449
B. Gorman, J.W. Charboneau, E.M. James, C.C. Reading, L.E. Wold, C.S. Grant, H. Gharib, I.D. Hay, Medullary thyroid carcinoma: role of high-resolution US. Radiology 162(1 Pt 1), 147–150 (1987). https://doi.org/10.1148/radiology.162.1.3538147
S. Lee, J.H. Shin, B.K. Han, E.Y. Ko, Medullary thyroid carcinoma: comparison with papillary thyroid carcinoma and application of current sonographic criteria. AJR Am. J. Roentgenol. 194(4), 1090–1094 (2010). https://doi.org/10.2214/ajr.09.3276
P. Valderrabano, D.L. Klippenstein, J.B. Tourtelot, Z. Ma, Z.J. Thompson, H.S. Lilienfeld, B. McIver, New American Thyroid Association Sonographic Patterns for thyroid nodules perform well in medullary thyroid carcinoma: institutional experience, systematic review, and meta-Analysis. Thyroid.: Off. J. Am. Thyroid. Assoc. 26(8), 1093–1100 (2016). https://doi.org/10.1089/thy.2016.0196
D. Ganeshan, E. Paulson, C. Duran, M.E. Cabanillas, N.L. Busaidy, C. Charnsangavej, Current update on medullary thyroid carcinoma. AJR Am. J. Roentgenol. 201(6), W867–876 (2013). https://doi.org/10.2214/AJR.12.10370
H. Gharib, E. Papini, R. Paschke, D.S. Duick, R. Valcavi, L. Hegedus, P. Vitti, American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical Guidelines for clinical practice for the diagnosis and management of thyroid nodules. J. Endocrinol. Invest. 33(5 Suppl), 1–50 (2010)
J.P. Brito, M.R. Gionfriddo, A. Al Nofal, K.R. Boehmer, A.L. Leppin, C. Reading, M. Callstrom, T.A. Elraiyah, L.J. Prokop, M.N. Stan, M.H. Murad, J.C. Morris, V.M. Montori, The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 99(4), 1253–1263 (2014). https://doi.org/10.1210/jc.2013-2928
S.H. Kim, B.S. Kim, S.L. Jung, J.W. Lee, P.S. Yang, B.J. Kang, H.W. Lim, J.Y. Kim, I.Y. Whang, H.S. Kwon, C.K. Jung, Ultrasonographic findings of medullary thyroid carcinoma: a comparison with papillary thyroid carcinoma. Korean J. Radiol. 10(2), 101–105 (2009). https://doi.org/10.3348/kjr.2009.10.2.101
P. Trimboli, L. Giovanella, S. Valabrega, M. Andrioli, R. Baldelli, N. Cremonini, F. Rossi, L. Guidobaldi, A. Barnabei, F. Rota, A. Paoloni, L. Rizza, G. Fattorini, M. Latini, C. Ventura, P. Falasca, F. Orlandi, A. Crescenzi, F. D’Ambrosio, V. Cantisani, F. Romanelli, R. Negro, E. Saggiorato, M. Appetecchia, Ultrasound features of medullary thyroid carcinoma correlate with cancer aggressiveness: a retrospective multicenter study. J. Exp. Clin. Cancer Res.: CR 33, 87 (2014). https://doi.org/10.1186/s13046-014-0087-4
C. Kim, J.H. Baek, E. Ha, J.H. Lee, Y.J. Choi, D.E. Song, J.K. Kim, K.W. Chung, W.B. Kim, Y.K. Shong, Ultrasonography features of medullary thyroid cancer as predictors of its biological behavior. Acta Radiol. (Stockh., Swed.: 1987) 58(4), 414–422 (2017). https://doi.org/10.1177/0284185116656491
J.E. Ahn, J.H. Lee, J.S. Yi, Y.K. Shong, S.J. Hong, D.H. Lee, C.G. Choi, S.J. Kim, Diagnostic accuracy of CT and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J. Surg. 32(7), 1552 (2008)
S. Roman, R. Lin, J.A. Sosa, Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 107(9), 2134–2142 (2006). https://doi.org/10.1002/cncr.22244
K.E. Cho, H.M. Gweon, A.Y. Park, M.R. Yoo, J. Kim, J.H. Youk, Y.M. Park, E.J. Son, Ultrasonographic features of medullary thyroid carcinoma: Do they correlate with pre and postoperative calcitonin levels? Asian Pac. J. Cancer Prev.: APJCP 17(7), 3357–3362 (2016)
P. Trimboli, N. Nasrollah, S. Amendola, F. Rossi, G. Ramacciato, F. Romanelli, P. Aurello, A. Crescenzi, O. Laurenti, E. Condorelli, C. Ventura, S. Valabrega, Should we use ultrasound features associated with papillary thyroid cancer in diagnosing medullary thyroid cancer? Endocr. J. 59(6), 503–508 (2012)
C. Scollo, E. Baudin, J.P. Travagli, B. Caillou, N. Bellon, S. Leboulleux, M. Schlumberger, Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J. Clin. Endocrinol. Metab. 88(5), 2070–2075 (2003). https://doi.org/10.1210/jc.2002-021713
J.H. Yoon, H.S. Lee, E.K. Kim, H.J. Moon, J.Y. Kwak, Malignancy risk stratification of thyroid nodules: Comparison between the Thyroid Imaging Reporting and Data System and the 2014 American Thyroid Association Management Guidelines. Radiology 278(3), 917–924 (2016). https://doi.org/10.1148/radiol.2015150056
J.H. Shin, J.H. Baek, J. Chung, E.J. Ha, J.-h Kim, Y.H. Lee, H.K. Lim, W.-J. Moon, D.G. Na, J.S. Park, Y.J. Choi, S.Y. Hahn, S.J. Jeon, S.L. Jung, D.W. Kim, E.-K. Kim, J.Y. Kwak, C.Y. Lee, H.J. Lee, J.H. Lee, J.H. Lee, K.H. Lee, S.-W. Park, J.Y. Sung, Ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 17(3), 370–395 (2016)
J.R. Landis, G.G. Koch, The measurement of observer agreement for categorical data. Biometrics 33(1), 159–174 (1977).
M. Fukushima, Y. Ito, M. Hirokawa, A. Miya, K. Kobayashi, H. Akasu, K. Shimizu, A. Miyauchi, Excellent prognosis of patients with nonhereditary medullary thyroid carcinoma with ultrasonographic findings of follicular tumor or benign nodule. World J. Surg. 33(5), 963–968 (2009). https://doi.org/10.1007/s00268-009-9939-z
C. Daumerie, D. Maiter, D. Gruson, Serum calcitonin estimation in medullary thyroid cancer: basal or stimulated levels? Thyroid Res. 6(Suppl 1), S4 (2013). https://doi.org/10.1186/1756-6614-6-S1-S4
L. Zhou, B. Chen, M. Zhao, H. Zhang, B. Liang, Sonographic features of medullary thyroid carcinomas according to tumor size: comparison with papillary thyroid carcinomas. J. Ultrasound Med.: Off. J. Am. Inst. Ultrasound Med. 34(6), 1003–1009 (2015). https://doi.org/10.7863/ultra.34.6.1003
T. McCook, C. Putman, J. Dale, S. Wells, Medullary carcinoma of the thyroid: radiographic features of a unique tumor. Am. J. Roentgenol. 139(1), 149–155 (1982)
F. Pombo, E. Rodriguez, J. Cao, C. Martinez-Isla, Cervical lymph node metastases of medullary thyroid carcinoma: CT findings. Eur. Radiol. 7(1), 99–101 (1997)
B. Saller, L. Moeller, R. Gorges, O.E. Janssen, K. Mann, Role of conventional ultrasound and color Doppler sonography in the diagnosis of medullary thyroid carcinoma. Exp. Clin. Endocrinol. Diabetes.: Off. J., Ger. Soc. Endocrinol. Ger. Diabetes. Assoc. 110(8), 403–407 (2002). https://doi.org/10.1055/s-2002-36546
V.Y. Park, E.K. Kim, H.J. Moon, J.H. Yoon, J.Y. Kwak, The thyroid imaging reporting and data system on US, but not the BRAFV600E mutation in fine-needle aspirates, is associated with lateral lymph node metastasis in PTC. Medicine 95(29), e4292 (2016). https://doi.org/10.1097/md.0000000000004292
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
This retrospective study was approved by the institutional review board of Seoul National University Bundang Hospital and informed consent was waived.
Rights and permissions
About this article
Cite this article
Yun, G., Kim, Y.K., Choi, S.I. et al. Medullary thyroid carcinoma: Application of Thyroid Imaging Reporting and Data System (TI-RADS) Classification. Endocrine 61, 285–292 (2018). https://doi.org/10.1007/s12020-018-1594-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1594-4