Abstract
Purpose
We investigated the impact of different cut-offs on the prevalence of 25-hydroxyvitamin D [25-(OH)D] deficiency.
Methods
We used baseline data of 4149 participants (45–75 years, 50% women) of the population-based Heinz Nixdorf Recall study. Serum 25-(OH)D was measured with the Roche Cobas assay. Quartiles (p25, p50, and p75) were calculated. Data were stratified by months, sex, and age. According to the recommendations of ‘Dachverband Osteologie’, Endocrine Society and National Institute of Health we used 25-(OH)D thresholds of 12, 20, and 30 ng/ml to estimate vitamin D deficiency.
Results
Overall the median of 25-(OH)D was 19.8 ng/ml (p25 = 14.4 ng/ml, p75 = 26.6 ng/ml), with highest concentrations in July (p50 = 23.8 ng/ml, p25 = 18.2 ng/ml, and p75 = 31.2 ng/ml) and lowest in March (p50 = 15.8 ng/ml, p25 = 11.5 ng/ml, and p75 = 20.6 ng/ml). Prevalence of vitamin D deficiency rose from 16, 51 up to 83% using the cut-offs of <12, <20 ng/ml, and <30 ng/ml, respectively. With respect to seasonal variance, prevalence of vitamin D deficiency rose to 92% in February/March using the cut-off <30 ng/ml (<12: 28%, <20 ng/ml: 71%) whereas in June/July prevalence of vitamin D deficiency decreased to 71% (<12: 6%, <20 ng/ml: 30%). The chance to attest the diagnosis of vitamin D deficiency for cut-off 12 ng/ml in March is 6.4-fold higher than in June, for cut-off 20 ng/ml, 5.5-fold higher and for cut-off 30 ng/ml, 3.1-fold higher.
Conclusions
Guidelines to define vitamin D deficiency revealed extremely different prevalence rates ranging between 6 and 92%. Accounting for collection time and antecedent sun exposure are important to reduce bias in research studies and improve decision-making in clinical care. Vitamin D thresholds have to be rethought.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D deficiency is assumed to be very prevalent. In Europe and the U.S., the prevalence of vitamin D deficiency varies between 14 and 100% [1, 2]. Vitamin D is important for many metabolic pathways and different health outcomes, and the physiology of vitamin D is very complex. Most of the body’s vitamin D (80–90%) is produced endogenously, if sun exposure is adequate, and only 10–20% is absorbed by ingestion [3]. Endogenous vitamin D3 or cholecalciferol production involves cutaneous synthesis in response to ultraviolet B rays of sunlight. The time needed to produce adequate vitamin D3 in the skin depends on the strength of the ultraviolet B rays, length of time spent in the sun, and the amount of pigment in the skin [4]. Thus, serum concentrations of vitamin D vary substantially during the seasons, reaching a peak 30–60 days after maximal sunlight exposure in the summer months and being lowest at the end of winter [5, 6].
Vitamin D3 of the skin is hydroxylized in the liver into 25-hydroxyvitamin D [25-(OH)D] and subsequently in the kidney by the 1α-hydroxylase into its active metabolite 1,25-dihydroxyvitamin D. Beside season, multiple factors like skin pigmentation, ethnicity, age, sex, diseases, and medication influence vitamin D concentrations [3].
Following diseases are reported to be associated with vitamin D deficiency: osteoporosis and fractures [7, 8], rickets in children and osteomalacia in adults, cardiovascular disease [9], metabolic disease [10], hypertension [11], falls among the elderly [12], cancer [13], multiple sclerosis [14], diabetes mellitus type 1 [15], cognition alteration [16], chronic pain [17], inflammation [18] and all-cause mortality [19]. The role of vitamin D in bone metabolism and muscular function is accepted, but there is a lively debate on the multifold other vitamin D implications [20, 21].
There is no internationally agreed definition for sufficient and consequently deficient vitamin D status. This is a general issue for analyses under investigational and methodological aspects such as analytics, and is influenced by the fact that cut-offs vary depending on the health outcomes under investigation. Currently, the definition of vitamin D deficiency is a laboratory analytical, based on the assay. There is agreement that serum concentrations of 25(OH)D increase in proportion to cutaneous synthesis and dietary intake of vitamin D, and currently represent the best indicator of vitamin D status [5].
The following societies proceeded to define a cut-off for 25(OH)D as a marker for vitamin D deficiency especially in the issue bone health. According to recommendations of the ‘Dachverband Osteologie’ [22] and the National Institutes of Health [23], thresholds for 25(OH)D of 20 ng/ml are considered to be adequate for health, whereas the Endocrine Society [24] recommends values ≥30 ng/ml (Table 1). In addition, the National Institutes of Health [23] differentiates between values <12 ng/ml, which are supposed to be a risk factor for rickets in children and osteomalacia in adults, and values between 12 and 20 ng/ml, which are considered to be inadequate for bone and overall health. Those differences in recommendations show that we need more evidence to define vitamin D deficiency. Recommendations do not account for seasonal aspects, but vitamin D is highly influenced by season. Seasonal variations, together with variable cut-offs, may lead to highly biased conclusions in epidemiological cohorts.
In this study, we analyzed the vitamin D status of the participants of the Heinz Nixdorf Recall Study who are representative for the middle-aged German population, considering sex, age and seasonal differences of blood sampling. Additionally, we investigated the impact of different cut-offs on the prevalence of vitamin D deficiency, taking into account seasonal differences to provide a basis for accurate or less biased definition of vitamin D thresholds.
Methods
Study population
The study has been described in detail elsewhere [25]. Briefly, the Heinz Nixdorf Recall study is a large population-based cohort study, started in 2000. Our study design was cross-sectional and we analyzed data collected at baseline between 2000 and 2003. All participants provided written informed consent, gave permission for future measurements and the study was approved by the institutional ethic committee. The study was certified and recertified according to DIN EN ISO 9001:2000/2008. Participants were investigated in the Heinz Nixdorf study center located in Essen, which was established for this study. Participants were men and women aged 45–75 years from the general population living in three large adjacent cities (Bochum, Essen, Muelheim/Ruhr) in Germany. They were recruited from a random sample derived from mandatory citizen registries. Response rate was 56% and in total 4814 participants (50% women) were enrolled [26].
Measurements
Computer-assisted face-to-face interviews, clinical examinations and comprehensive laboratory tests were conducted according to standard protocols. Serum aliquots were stored at −80 °C. Accurate temperature was controlled by an in-house master display and long-term storage does not affect serum 25-(OH)D level [27]. Total serum 25-(OH)D was measured in 2007 on thawed serum samples with the Roche Cobas assay. This assay employed polyclonal antibodies that bind specifically to human 25(OH)D. According to the product insert the intra-assay variation was <6.5%, the intermediate precision to describe the precision of a repeated value determination of a sample with a deliberate change of one analytic parameter was <11.5%, the functional sensitivity was 4.01 ng/ml and the detection limit 3.00 ng/ml. In comparison with the reference method (LC-MS/MS) the accuracy was: y = 1.09*x−0.510; pearson r, 0.894. The control of the instrument was performed according to the product insert (quality control of the manufacturer). 25(OH)D values of 20–32 ng/ml are minimal and values ≥30 ng/ml are favored (product insert). Participants were asked about all kind of medication intake at baseline including vitamin substitution. For information about the possible sun exposure of our participants, we used resources of the German Weather Service who supplied us with data about the daily sun radiation in J/cm² in Bochum (Germany, latitude 51°49′ North) from 11December 2000 to 13 August 2003.
Statistical analyses
Results are based on the analysis of data of 4149 participants with 25-(OH)D readings at baseline (50.4% women, mean age: 59.7 ± 7.8 years). Medians (50th percentile [p50]) and quartiles (25th percentile [p25], 75th percentile [p75]) of 25-(OH)D were calculated, stratified by sex, age group (45–54, 55–64, and 65–75 years) and investigation month. Monthly variability of 25-(OH)D according to the mean sun radiation of the investigation month was calculated. We estimated the impact of sun radiation on 25-(OH)D in a model including dummy variables for month and year. According to the recommendations of the German ‘Dachverband Osteologie’ [22], Endocrine Society [24] and National Institutes of Health [23], prevalence of 25-(OH)D deficiency was calculated according to the cut-offs <12, <20, and <30 ng/ml stratified by investigation months. Odds ratios to attest the diagnosis of vitamin D deficiency in one month compared to June were estimated. Analyses were performed using SAS software, version 9.2.
Results
The sample consisted of 4149 participants with 25-(OH)D readings (50.4% women, mean age: 59.7 ± 7.8 years). Of those 65 (1.6%) participants reported oral multivitamin intake and 46 (1.1%) participants reported oral vitamin D intake. Table 2 depicts the medians and quartiles of 25-(OH)D stratified by sex, age group, and monthly mean power of global sun radiation. The median 25-(OH)D-concentration was 19.8 ng/ml (p25: 14.4, p75: 26.6 ng/ml). The median 25-(OH)D in women was lower than in men (p50: 18.9 ng/ml, respect to 20.9 ng/ml). Older women showed the lowest 25-(OH)D serum concentration (45–54/55–64/65–75 years: 19.7/20.1/16.7 ng/ml), whereas no age effects could be observed in men (20.4/21.6/20.2 ng/ml). The highest 25-(OH)D concentrations were obtained in the sunny season (e.g., July, p50: 23.8, p25: 18.2, p75: 31.2 ng/ml) and lowest medians in winter (e.g., March, p50: 15.8, p25: 11.5, p75: 20.6 ng/ml).
There was a latency of 1–2 months after high sun exposure and the curves of p25, p50, and p75 are nearly parallel (Fig. 1). Stratified by the year of investigation, there is a high variability of the monthly median 25-(OH)D in the different years in spring (Fig. 1). There was a difference of 9.2 ng/ml in median 25-(OH)D values in April between 2001 and 2003. The peak-trough difference in 25-(OH)D concentration during the year in men was greater than in women. The maximal range of monthly medians in men was 10.2 vs. 8 ng/ml in women. The estimated impact of sun radiation on 25-(OH)D was 12.6%. The effect estimates for the dummy variables (included for month and year of the examination) showed some variation from year to year and a strong seasonal component along the course of the sun radiation.
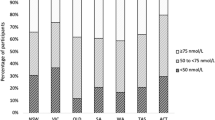
Table 3 shows the prevalence of vitamin D deficiency according to the cited different threshold recommendations. The prevalence was as low as 16% using the threshold <12 ng/ml, increased to 51% (<20 ng/ml) and even 83% (<30 ng/ml). The highest prevalence rates of vitamin D deficiency were observed in February/March (28, 71, and 92%) and lowest in June/July (6, 30, 71%). The chance to attest the diagnosis of vitamin D deficiency for cut-off 12 ng/ml in March is 6.4-fold higher than in June, for cut-off 20 ng/ml 5.5-fold higher and for cut-off 30 ng/ml 3.1-fold higher, with a prevalence of vitamin D deficiency of 92% in February and March (Table 3).
Discussion
Our study shows that the influence of sun radiation on the prevalence of vitamin D deficiency according to different 25-(OH)D thresholds is very high, which is not considered in the current recommendations of the ‘Dachverband Osteologie’ [22], Endocrine Society [24] and National Institutes of Health [23]. Additionally, we observed a small influence of sex and age on 25-(OH)D concentration.
According to those international guidelines, 16% [25-(OH)D < 12 ng/ml], 51% [25-(OH)D < 20 ng/ml] or 83% [25-(OH)D < 30 ng/ml] of our cohort, which is representative for the middle-aged German population, had vitamin D deficiency. The variation of 25-(OH)D concentration by investigation month was very high, because global sun radiation from October to March in general is not strong enough in Germany to produce sufficient vitamin D in the skin [3]. We measured the lowest 25-(OH)D median values at the end of winter (February/March) and the highest at midsummer (June/July). In March, the prevalence for 25-OHD < 20 ng/ml was more than double as high as in July (70 vs. 30%). For the threshold <30 ng/ml in March, almost everybody (92%) in our study population was categorized as vitamin D deficient, and in July still 71%. According to those guidelines, the chance to attest the diagnosis of vitamin D deficiency for the cut-off 12 ng/ml in March is 6.4-fold higher than in June, for the cut-off 20 ng/ml 5.5-fold higher and for the cut-off 30 ng/ml 3.1-fold higher. Our prevalence of vitamin D deficiency in men and women is in line with the literature in Europe and the U.S., which reported a prevalence of 14–100% using the same cut-offs [1, 2]. A study from Sweden (latitude 57°41′ North) reported 25-(OH)D levels during January to March below the thresholds of 20 and 30 ng/ml of 58 and 88%, respectively, and during July to September of 11 and 50% [28]. The still very high but lower prevalence of vitamin D deficiency than in our study might be due to supplement of dairy products with vitamin D in Sweden and a younger study population with a higher percentage of men. Guidelines producing a prevalence of deficiency of more than 50% and also different chances of deficiency according to the time of blood sampling are impractical. Therefore, current thresholds should be challenged.
Other studies showed the dependence of 25-(OH)D values on sun exposure as well [6, 29–31]. A study in the United States found that serum 25-(OH)D concentrations varied in a sinusoidal manner [32]. A study from Switzerland (latitude 46° North) generated centile curves of 25-(OH)D in a sinusoidal manner of the general adult population to help interpreting patients vitamin D status independently of measurement time, taking into account age, gender and body mass index [33]. The intra-individual analyses of a longitudinal study from Sweden showed a mean increase in 25-(OH)D by 3.2 ng/ml per month between April and August [28]. A larger peak-trough difference in 25-(OH)D concentration during the year in men (9.4 ng/ml) than in women (6.8 ng/ml) was reported [32], which is in accordance with our results (men: 10.2 ng/ml, women: 8 ng/ml). Our observation that the 25-(OH)D curves of p25, p50, and p75 were nearly parallel (Fig. 1) indicates a relatively stable trend within a year. In our model 12.6% of the underlying 25-(OH)D variation was explained by sun radiation. We also see relevant differences between the years of investigation. The difference of median 25-(OH)D values in April of 2001 compared to 2003 is 9.2 ng/ml. This huge difference was explained by the fact that the sun radiation in February, March, and April was much more intense in 2003 than in 2001 (Fig. 1). Single 25-(OH)D measurements mislead the diagnosis of vitamin D status. Using a centile curve for temperate zones, particularly for measurements in winter or summer months would lead to greater precision. The interpretation of measurements in spring or autumn month remains difficult, because the annual increase and decrease of 25-(OH)D in this periods depend on variable weather and individual factors.
We also observed an age effect on 25-(OH)D concentrations in women but not in men, although it was much lower than the seasonal effect. In the literature, the effect of age on 25-(OH)D is controversial. Most investigators found significantly lower mean values in the elderly [31, 34]. A lower efficiency of cutaneous synthesis of vitamin D in older people was suggested [35, 36]. On the other hand, some investigators suggested that mean plasma 25-(OH)D concentrations in healthy old people living at home might be not lower than in younger people [37–39]. Further studies to explain the 25-(OH)D decrease by age, especially in older women, and whether they are physiological or pathological still have to be performed.
We observed a sex effect on 25-(OH)D values and, similarly to the age effect, it was much lower than the seasonal effect. The 25-(OH)D medians of women were lower than those of men, especially in women older than 65 years. Gender differences were also found in young healthy Italian persons [40]. This difference might have physiological reasons. It could be linked to androgen-related differences in vitamin D-binding protein concentrations, or to either the precursor production by the skin or its 25-hydroxylation by the liver [40]. Further studies are necessary to explain the differences in gender, which might be important for more precise definition of sufficient or insufficient 25-(OH)D status.
As mentioned before, vitamin D deficiency is associated with many diseases. However, the evidence for many of those associations is inconsistent [19, 41, 42]. Large interventional studies to be sure of a positive effect of vitamin D supplementation regarding different outcomes are needed. The ‘normal’ vitamin D concentration in blood should be defined firstly and then the need of supplementation should be discussed.
The diagnosis of vitamin D insufficiency based on the current thresholds leads unavoidably to interventions, like supplementation with vitamin D or monitoring. On the other hand, a pronounced vitamin D deficiency might be trivialized if vitamin D deficiency can be diagnosed in almost everybody. According to current recommendations, the majority of our population would need vitamin D substitution.
Evidence for negative effects after general long-term substitution is missing. Most trials of higher doses of vitamin D are not adequately designed to assess any harm after long-term. The National Institutes of Health reported that symptoms of toxicity are unlikely at daily intakes below 10,000 IU/day [23]. The Women’s Health Initiative reported a 17% increased risk of kidney stones in postmenopausal women with vitamin D and calcium supplementation [43]. Substances that directly activate the vitamin D receptor are not recommended for patients with chronic kidney disease because they were associated with increased risk of hypercalcemia and hyperphosphatemia [44, 45]. Meaningful long-term studies to investigate positive as well as negative effects of vitamin D substitution are much-needed.
Furthermore, a number of different vitamin D assays are used worldwide, resulting in hardly comparable readings [46, 47]. Examinations by the Vitamin D External Quality Assessment Scheme (DEQAS) [48] revealed wide variation of 3.2–6.4 ng/ml in the mean serum 25-(OH)D level by each specific methodology for the same sample [47]. Differences up to 10 ng/ml in mean 25-(OH)D level are reported in another test population measured by different assays [49]. The interassay differences between the mean of methodologies varied between 1 and 6.4 ng/ml [47]. The accuracy of various 25-(OH)D assays including our Roche Cobas assay differs widely from the National Institute for Standards and Technology standard and is ±10% or even higher [47]. Reliable and validated standard analytical procedures are needed to adequately determine 25-(OH)D concentrations. Additionally, better vitamin D analysis might prove or disprove associations and presumptions about vitamin D and health.
In clinical practice, results of vitamin D assays should be viewed highly critically, as it has to be done with most laboratory parameters. Currently, the definition of vitamin D deficiency is laboratory analytical, based on the assay. It is very likely that low 25-OHD is not equal to vitamin D deficiency. Other anamnestic parameters have to be considered. For example, a person who prefers to stay indoors, women with burka, infants or geriatric patients are likely to have vitamin D deficiency. In clinical practice vitamin D should be judged in conjunction with other labor parameters, like creatinine, alcaline phosphatase and parathormone.
Serum 25-(OH)D is strongly associated with parameters related to sun exposure, but only weakly with intake of vitamin D supplements [28]. This is why we did not exclude from analysis those participants who reported oral vitamin D substitution.
We identified several strengths and limitations of our study. Strengths are the large randomly-selected community sample and the high quality of data collection and data handling, which was confirmed by external certification of the Heinz Nixdorf Recall study. Limitation of our study is the high intra-assay variation of the Roche Cobas of <6%, but very better-performing vitamin D assays are currently not available. Another limitation is that participants of our study were all Caucasians, so we could not investigate ethnic aspects. Overall, our study provides essential data to represent the middle aged German population.
Vitamin D status is widely and controversially debated in the context of morbidity and mortality, although causality has not really been determined. Recommended guidelines to define vitamin D deficiency revealed extremely different numbers of subjects with vitamin D deficiency in our study population. According to those guidelines, up to 92% of a German metropolitan population aged 45–75 years has insufficient vitamin D values. Guidelines which produce a deficiency prevalence of more than 50% are impractical. Therefore, current thresholds should be challenged. New guidelines for vitamin D which consider sun exposure are needed. This is especially important with respect to recommended supplementation of vitamin D. Our data could be used for a first approximation of new guidelines for the elderly German population. Further accounting for blood samples collection time and antecedent sun exposure are important to reduce bias in research studies, as well as in clinical care, to improve decision-making. In temperate zones interpretation of vitamin D measured in winter or summer month is more precise than in spring or autumn because of variable course of annual increase and decrease as a function of weather and individual factors. Vitamin D thresholds have to be rethought to diagnose vitamin D deficiency and to define people who really benefit from vitamin D substitution.
References
M.C. Chapuy, P. Preziosi, M. Maamer et al., Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos. Int. 7, 439–443 (1997)
M.F. Holick, High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 81, 353–373 (2006)
Bundesinstitut für Risikobewertung: http://www.bfr.bund.de/de/ausgewaehlte_fragen_und_antworten_zu_vitamin_d-131898.html (2012). Accessed 22 Oct 2016
T. Kulie, A. Groff, J. Redmer, J. Hounshell, S. Schrager, Vitamin D: an evidence-based review. J. Am. Board Fam. Med. 22, 698–706 (2009)
J.S. Adams, M. Hewison, Update in vitamin D. J. Clin. Endocrinol. Metab. 95, 471–478 (2010)
S.S. Sherman, B.W. Hollis, J.D. Tobin, Vitamin D status and related parameters in a healthy population: the effects of age, sex, and season. J. Clin. Endocrinol. Metab. 71, 405–413 (1990)
H.A. Bischoff-Ferrari, D.P. Kiel, B. Dawson-Hughes et al., Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J. Bone Miner. Res. 24, 935–942 (2009)
J.A. Cauley, A.Z. Lacroix, L. Wu et al., Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann. Intern. Med. 149, 242–250 (2008)
H. Dobnig, S. Pilz, H. Scharnagl et al., Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch. Intern. Med. 168, 1340–1349 (2008)
D.M. Lee, M.K. Rutter, T.W. O’Neill et al., Vitamin D, parathyroid hormone and the metabolic syndrome in middle-aged and older European men. Eur. J. Endocrinol. 161, 947–954 (2009)
S.K. Kunutsor, T.A. Apekey, M. Steur, Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur. J. Epidemiol. 28, 205–221 (2013)
R.L. Prince, N. Austin, A. Devine, I.M. Dick, D. Bruce, K. Zhu, Effects of ergocalciferol added to calcium on the risk of falls in elderly high-risk women. Arch. Intern. Med. 168, 103–108 (2008)
H. van der Rhee, J.W. Coebergh, E. de Vries, Is prevention of cancer by sun exposure more than just the effect of vitamin D? A systematic review of epidemiological studies. Eur. J. Cancer 49, 1422–1436 (2013)
B. Pozuelo-Moyano, J. Benito-Leon, A.J. Mitchell, J. Hernandez-Gallego, A. Systematic, Review of randomized, double-blind, placebo-controlled trials examining the clinical efficacy of vitamin D in multiple sclerosis. Neuroepidemiology 40, 147–153 (2012)
C. Mathieu, C. Gysemans, A. Giulietti, R. Bouillon, Vitamin D and diabetes. Diabetologia 48, 1247–1257 (2005)
C. Balion, L.E. Griffith, L. Strifler et al., Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. 79, 1397–1405 (2012)
S. Straube, S. Derry, R.A. Moore, H.J. McQuay, Vitamin D for the treatment of chronic painful conditions in adults. Cochrane. Database. Syst. Rev. 1, CD007771 (2010)
X. Guillot, L. Semerano, N. Saidenberg-Kermanac’h, G. Falgarone, M.C. Boissier, Vitamin D and inflammation. Joint Bone Spine 77, 552–557 (2010)
G. Bjelakovic, L. Gluud, D. Nikolova et al., Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst. Rev. 7, CD007470 (2011)
K.C. Norris, S.F. Williams, Race/ethnicity, serum 25-hydroxyvitamin D, and heart disease. JAMA 310, 153–155 (2013)
M.C. Robertson, L.D. Gillespie, Fall prevention in community-dwelling older adults. JAMA 309, 1406–1407 (2013)
DVO-LEITLINIE: http://www.dv-osteologie.org/dvo_leitlinien/osteoporose-leitlinie-2014 (2014). Accessed 22 Oct 2016
National Institutes of Health: http://ods.od.nih.gov/factsheets/Vitamin-D-HealthProfessional/#h5 (2014). Accessed 22 Oct 2016
Endocrine society: http://www.hormone.org/patient-guides/2011/vitamin-D-deficiency (2014). Accessed 22 Oct 2016
A. Schmermund, S. Möhlenkamp, A. Stang et al., Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study. Risk factors, evaluation of coronary calcium and lifestyle. Am. Heart J. 144, 212–218 (2002)
A. Stang, S. Moebus, N. Dragano et al., Baseline recruitment and analyses of nonresponse of the Heinz Nixdorf recall study: identifiability of phone numbers as the major determinant of response. Eur. J. Epidemiol. 20, 489–496 (2005)
C. Agborsangaya, A.T. Toriola, K. Grankvist et al., The effects of storage time and sampling season on the stability of serum 25-hydroxy vitamin D and androstenedione. Nutr. Cancer 62, 51–57 (2010)
E. Klingberg, G. Oleröd, J. Konar et al., Seasonal variations in serum 25-hydroxy vitamin D levels in a Swedish cohort. Endocrine 49, 800–808 (2015)
M.J. Bolland, A.B. Grey, R.W. Ames et al., The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency. Am. J. Clin. Nutr. 86, 959–964 (2007)
R. Jorde, M. Sneve, M. Hutchinson, N. Emaus, Y. Figenschau, G. Grimnes, Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am. J. Epidemiol. 171, 903–908 (2010)
T.C. Stamp, J.M. Round, Seasonal changes in human plasma levels of 25-hydroxyvitamin D. Nature 247, 563–565 (1974)
A.B. Shoben, B. Kestenbaum, G. Levin et al., Seasonal variation in 25-hydroxyvitamin D concentrations in the cardiovascular health study. Am. J. Epidemiol. 174, 1363–1372 (2011)
P. Vuistiner, V. Rousson, H. Henry, A. Population-Based, Model to consider the effect of seasonal variation on serum 25(OH)D and Vitamin D status. Biomed. Res. Int 2015, 168189 (2015)
E. Lester, R.K. Skinner, M.R. Wills, Seasonal variation in serum-25-hydroxyvitamin-D in the elderly in Britain. Lancet 1, 979–980 (1977)
M.F. Holick, L.Y. Matsuoka, J. Wortsman, Age, vitamin D, and solar ultraviolet. Lancet 2, 1104–1105 (1989)
A.G. Need, H.A. Morris, M. Horowitz, C. Nordin, Effects of skin thickness, age, body fat, and sunlight on serum 25-hydroxyvitamin D. Am. J. Clin. Nutr. 58, 882–885 (1993)
D. Corless, S.P. Gupta, D.A. Sattar, S. Switala, B.J. Boucher, Vitamin D status of residents of an old people’s home and long-stay patients. Gerontology 25, 350–355 (1979)
K. Guggenheim, M. Kravitz, R. Tal, N.A. Kaufmann, Biochemical parameters of vitamin D nutriture in old people in Jerusalem. Nutr. Metab. 23, 172–178 (1979)
G. Toss, S. Almqvist, L. Larsson, H. Zetterqvist, Vitamin D deficiency in welfare institutions for the aged. Acta Med. Scand 208, 87–89 (1980)
V. Carnevale, S. Modoni, M. Pileri et al., Longitudinal evaluation of vitamin D status in healthy subjects from southern Italy: seasonal and gender differences. Osteoporos. Int. 12, 1026–1030 (2001)
M.J. Bolland, A. Grey, G.D. Gamble, I.R. Reid, The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol 2, 307–320 (2014)
A. Cranney, T. Horsley, S. O’Donnell, H.A. Weiler et al., Effectiveness and safety of vitamin D in Relation to bone health. Evidence report/technology assessment No. 158 (Prepared by the University of Ottawa evidence-based practice center (UO-EPC) under contract no. 290-02-0021. AHRQ Publication No. 07-E013. Rockville, MD: Agency for Healthcare Research and Quality (2007)
R.D. Jackson, A.Z. LaCroix, M. Gass et al., Calcium plus vitamin D supplementation and the risk of fractures. N. Engl. J. Med. 354, 669–683 (2006)
S.D. Anker, S. von Haehling, Vitamin D in chronic kidney disease: more questions than answers. JAMA 307, 722–723 (2012)
S.C. Palmer, D.O. McGregor, P. Macaskill et al., Meta-analysis: vitamin D compounds in chronic kidney disease. Ann. Intern. Med. 147, 840–853 (2007)
G.D. Carter, R. Carter, J. Jones, J. Berry, How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin. Chem. 50, 2195–2197 (2004)
Gel-H. Fuleihan, R. Bouillon, B. Clarke et al., Serum 25-Hydroxyvitamin D levels: variability, knowledge gaps, and the concept of a desirable range. J. Bone Miner. Res. 30, 1119–33 (2015)
Vitamin D External Quality Assessment Scheme (DEQAS) http://www.deqas.org/. Accessed 12 Dec 2016
G. Snellman, H. Melhus, R. Gedeborg et al., Determining vitamin D status: a comparison between commercially available assays. PLoS. One 5(7), e11555 (2010)
Acknowledgments
We are indebted to all the study participants and to the dedicated personnel of both the study center of the HNR study and the EBT-scanner facilities Prof. D. Grönemeyer, Bochum and Dr. R. Seibel, Mülheim as well as to the investigative group, in particular to U. Roggenbuck, U. Slomiany, E.M. Beck, A. Öffner, S. Münkel, M. Bauer, S. Schrader, R. Peter, and H. Hirche. We acknowledge the support of the SarstedtAG&Co. (Nümbrecht, Germany) concerning laboratory equipment. We thank Prof. K. Lauterbach (Adjunct Prof., Harvard School of Public Health, Boston, USA) for his valuable contributions in an earlier phase of the study. We thank the Heinz Nixdorf Foundation [Chairman: Martin Nixdorf; Past Chairman: Dr Jur Gerhard Schmidt (deceased)], for their generous support of this study. This study is also supported by the German Federal Ministry of Education and Research (BMBF) that transferred the monitoring of the study Deutsches Zentrum für Luft- und Raumfahrt (DLR), Bonn, Germany. An international advisory board and quality control as well as event committee were established, but had no role concerning the study design, data collection, analysis, interpretation, or writing the report. The Deutsche Forschungsgemeinschaft (DFG) supported the study (DFG project: ER 155/6-1 and ER 155/6-2). This study is also supported by the Kulturstiftung Essen.
Author Contributions
S.H.S.: analysis and interpretation of data, drafting the manuscript. K.H.J. and R.E.: study concept and design. H.L., K.H.J., R.E., D.F., S.M.: assisted with drafting the manuscript for intellectual content. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The study was approved by the institutional ethics committee.
Informed consent
All participants provided written informed consent.
Rights and permissions
About this article
Cite this article
Schramm, S., Lahner, H., Jöckel, KH. et al. Impact of season and different vitamin D thresholds on prevalence of vitamin D deficiency in epidemiological cohorts—a note of caution. Endocrine 56, 658–666 (2017). https://doi.org/10.1007/s12020-017-1292-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1292-7