Abstract
Purpose
This study aimed to determine whether resveratrol treatment alleviates endoplasmic reticulum stress and changes the expression of adipokines in adipose tissues and cells.
Methods
8-week-old male C57BL/6 mice were fed a high-calorie diet (HCD group) or high-calorie diet supplemented with resveratrol (high-calorie diet + resveratrol group) for 3 months. Insulin resistance, serum lipids and proinflammatory indices, the size and inflammatory cell infiltration in subcutaneous and visceral adipose tissues were analyzed. The gene expressions of endoplasmic reticulum stress, adipokines, and inflammatory cytokines were determined. The induced mature 3T3-L1 cells were pretreated with resveratrol and then palmitic acid, and the gene expressions of endoplasmic reticulum stress, adipokines, and inflammatory cytokines were determined.
Results
Subcutaneous and visceral adipose tissues in the high-calorie diet-fed mice exhibited adipocyte hypertrophy, inflammatory activation, and endoplasmic reticulum stress. Resveratrol alleviated high-calorie diet-induced insulin resistance and endoplasmic reticulum stress, increased expression of SIRT1, and reversed expression of adipokines in varying degrees in both subcutaneous and visceral adipose tissues. The effects of resveratrol on palmitic acid-treated adipocytes were similar to those shown in the tissues.
Conclusions
Resveratrol treatment obviously reversed adipocyte hypertrophy and insulin resistance by attenuating endoplasmic reticulum stress and inflammation, thus increasing the expression of SIRT1 and inverting the expression of adipokines in vivo and in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of obesity has reached epidemic proportions worldwide and is usually associated with insulin resistance (IR), which often leads to a number of interrelated metabolic derangements, collectively characterized as metabolic syndrome (MetS). Central to the development of MetS are adipose tissues, which have led to the idea that the manipulation of the biology of adipocytes might be a useful therapeutic strategy in MetS [1].
White adipose tissue mainly consists of subcutaneous adipose tissue (SAT) and internal adipose tissue (IAT). SAT makes up about 80% of the total fat mass, while IAT constitutes the other 20% and is mainly composed of visceral adipose tissue (VAT). Dysfunctional adipose tissue is characterized by adipocyte hypertrophy and eventually hyperplasia, macrophage infiltration, impaired insulin signaling, and ultimately IR.
Adipose tissue can secrete a multitude of soluble proteins, collectively called adipokines [2, 3]. Among them, more than 50 adipokines are pumped out by adipocytes, and participate in crosstalk with the rest of the body [4]. The result is the release of a host of inflammatory adipokines and excessive amounts of free fatty acids (FFAs), which promote ectopic fat deposition in muscle, liver, and pancreatic β cells; this is typically known as “lipotoxicity” [5]. Proinflammatory adipokines are mainly composed of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and chemokine monocyte chemoattractant protein-1 (MCP-1), which can play a central role in the development of obesity-induced IR in adipose tissue [6].
Endoplasmic reticulum (ER) stress causes adipocyte dysfunction and chronic inflammation [7, 8]. Within adipocytes, elevated lipid storage, lipogenesis, and adipokine synthesis may act as stress signals for the ER. Notably, ER stress is most prominent in adipose tissue and contributes to its dysfunction, however, many important questions remain to be answered, including the mechanism of ER stress in the inflammatory response and in the expression of adipokines in adipocytes. Therefore, it is necessary to study the relationship between ER stress and the expression of adipokines.
Resveratrol (RES) has attracted a great deal of attention because of its antioxidant and anti-inflammatory effects [9] that can reduce protein misfolding or ER stress [10]. There have been reports about RES participating in ER stress in HepG2 cells, human nasopharyngeal carcinoma cells, and retinal vascular degeneration [11–14], but the mitigatory effects of RES on ER stress in adipocytes are still unknown. We hypothesize that RES treatment can improve lipid metabolism and dysregulated adipokines by alleviating ER stress induced by high-calorie diet (HCD) and circulating FFAs in adipocytes.
Mitochondria take a central place in consumption of lipid, directly upstream of lipolysis. Mitochondrial ATPase family AAA-Domain containing protein 3 (ATAD3) is an important regulator of mitochondrial biogenesis and lipogenesis. RES attenuates mitochondrial oxidative stress and serves as a potent antioxidant. The antilipogenic effect of RES is further modulated by expression levels of mitochondrial ATAD3 [15].
We adopted a self-developed HCD to feed C57BL/6 mice, simultaneously accompanied by an RES treatment for three consecutive months. Saturated fatty acid palmitate (16:0) is one of the main lipids in the HCD and we found that the changes of palmitic acid (PA) was the most remarkable in the serum lipids, so it was chosen to treat mature adipose 3T3-L1 cells to induce ER stress and verify the mitigative effects of RES on ER stress in adipocytes. As far as we know, this is the first study in the literature that discusses the influence of RES on ER stress in adipose tissues and cells.
Materials and methods
Animals and RES treatment
Male C57BL/6 wild-type mice (8 weeks of age, 21.52 ± 2.03 g, n = 32) were purchased from the Experimental Animal Center of Anhui Medical University (Anhui, China) and acclimated for 1 week. The mice were randomly divided into four groups: a standard chow diet group without (SCD) and with RES treatment group (SCD + RES), a high-calorie group without (HCD) and with RES treatment group (HCD + RES). The HCD and HCD + RES mice were fed HCD (consisting of 20% lard, 20% sucrose, 10% custard powder, 1% cholesterol, and 49% basic forage) for 3 months. The SCD + RES and HCD + RES mice were given 400 mg/kg/d of RES [16] via gavage in addition to the SCD or HCD diet. All procedures were performed in accordance with the principles approved by the Animal Ethics Committee of Anhui Medical University.
Glucose tolerance test and insulin tolerance test
Glucose disposal and insulin sensitivity was determined by the glucose tolerance test (GTT) and insulin tolerance test (ITT). For the GTT assays, after an overnight fast, all mice were given glucose (2 g/kg BW) by intraperitoneal injection, then blood samples were obtained from the tail veins at 0, 30, 60, 90, and 120 min. Blood glucose was measured with a glucometer (OneTouch Ultra, Johnson & Johnson Medical, China), and the data were plotted as blood glucose concentration over time. ITT assays were performed using an intraperitoneal injection of neutral insulin (1 U/kg BW) after a 4–6-h fast. Blood glucose was measured at 0, 30, 45, 60, 90, and 120 min, and the data were plotted as blood glucose concentration over time according to the literature [17].
Analysis of serum biochemical indices and tissue sampling
After a 12-h fast, the mice were anesthetized via CO2 inhalation. Blood samples were then collected from the orbital sinus and the serum was separated for the biochemical analyses, including glucose, triglyceride (TG), total cholesterol (TC), high density lipoprotein-cholesterol (HDL-C), and low density lipoprotein-cholesterol (LDL-C), by colorimetric enzymatic assays in an automatic analyzer (Olympus AU640, Japan). Serum TNF-α, IL-6, and MCP-1 levels were measured by ELISA assays (R&D Systems, Billerica, USA). Serum FFA and PA levels were measured by gas chromatography according to the method of Han et al. [18]. Abdominal SAT and VAT (perirenal as a representative VAT) were rapidly taken and weighed, freshly frozen in liquid nitrogen, and stored at −80 °C until the time of biochemical analysis. The rest parts of tissues were fixed for histology and immunohistochemistry respectively.
Histology and immunohistochemistry
The SAT and VAT were fixed in 10% (v/v) phosphate-buffered formalin, dehydrated, and embedded in paraffin. Thin tissue slides (4 μm) were deparaffinized and stained with hematoxylin and eosin for routine pathological examination and analyzed for adipocyte sizes using Image Pro-Plus software. The SAT and VAT slides were also used to incubate overnight at 4 °C with rabbit anti-mouse leptin (Boster, China), TNF-α (Boster), and GRP78 (Abcam), respectively, which was followed by signal amplification with the Boster ABC kit (Boster). The reaction was developed by the addition of ABC chromogen substrate. Microphotographs were taken and quantified.
3T3-L1 culture, differentiation, and RES treatment
Mouse 3T3-L1 preadipocytes (American Type Culture Collection, Manassas, USA) were proliferated in 6-well plates in DMEM containing 10% NCS. After the cells had maintained confluency for 2 days, they were incubated in induction medium I (day 0) containing 1 μM dexamethasone, 0.5 mM 1-methyl-3-isobutyl-xanthine, and 5 μg/ml insulin in DMEM with 10% FBS for 2 days. Then the cells were placed in induction medium II (day 2), containing 5 μg/ml insulin in DMEM with 10% FBS, for 2 more days. Thereafter, the cells were cultured in DMEM with 10% FBS for the remainder of the differentiation process, which was as normal control (NC) group. Before the cell experiments, PA was coupled to fatty acid-free BSA at a 2:1 ratio (PA:albumin) and added to the cell culture at a concentration of 0.6 mM, which was as PA group, according to a previous study [19]. Before the addition of PA, 200 μM RES was used to pretreat the differentiated mature 3T3-L1 cells and as PA + RES group.
Realtime RT-PCR
Total RNAs were prepared from SAT, VAT, and the differentiated mature 3T3-L1 cells by Trizol reagent (Takara, Japan). Total RNA (1 μg) was reverse transcribed with OligodT primers and Moloney murine leukemia virus reverse transcriptase (Fermentas Life Sciences, CA, USA). The genes engaged in lipid metabolism, such as peroxisomal proliferator-activated receptor alpha (PPARα), peroxisomal proliferator-activated receptor gama (PPARγ) and sterol regulatory element binding protein-1c (SREBP1c), inflammatory cytokines such as TNFα and IL1β, adipokines such as leptin, adiponectin, and resistin, ER stress upstream markers activated transcription factor 6 (ATF6), ER-resident PKR-like eIF2α kinase (PERK), and inositol-requiring enzyme 1α (IRE1α), downstream effectors such as glucose regulated protein 78 (GRP78) and CCAAT/enhancer binding protein (C/EBP) homologous protein (CHOP), and the RES-activated gene silent information regulator 1 (SIRT1) (primer sequences were shown in Table 1) were measured by realtime RT-PCR in a StepOnePlus Real-Time PCR System (Applied Biosystems, CA, USA) using the iTaqTM Universal SYBR® Green Supermix (Bio-Rad, USA) and the ΔΔCT threshold cycle method. Gene expression levels were normalized to those of GAPDH and presented relative to the gene expressions in the SCD group or NC group.
Western blot analysis
Proteins were prepared by lysing SAT, VAT, and the differentiated mature 3T3-L1 cells in an ice-cold lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 1 mM phenylmethanesulphonyl fluoride, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 50 μg/ml aprotinin and 50 μg/ml leupeptin) for 30 min and centrifuged at 8000X g for 15 min at 4 °C to obtain the supernatant. Western blot analysis for β-actin (Abcam), IRE1α (Bioss, China), IL1β (Bioss), GRP78, CHOP (Bioss) and SIRT1 (Santa Cruz) were performanced using specific antibodies. Total protein concentrations were measured with the Bradford dye binding assay to correct for any differences in protein loading. The intensities of the bands were quantified using Gel-Pro Analyser 3.1 software (Informax).
Statistical analysis
Unless otherwise noted, all values are expressed as mean ± SD and analyzed by one-way ANOVA followed by the unpaired, two-tailed student’s T-test using SPSS16.0 software. Statistical significance was set at P < 0.05.
Results
Effects of RES on body and fat mass of HCD-fed mice
All male mice started the experiment with similar body masses. There were no overt differences in the food intakes between the three groups (P > 0.05). After 3 months, the HCD-fed mice had a significant body mass and SAT and VAT mass gain compared with the SCD-fed and SCD + RES mice, while body weight and fat mass decreased in the HCD + RES group, indicating that RES treatment might decrease lipogenesis and reduce body fat (Table 2).
Effect of RES on IR of HCD-fed mice
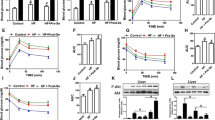
GTT and ITT experiments were performed to explore the role of RES in obesity-induced insulin resistance. The HCD-fed mice were significantly more glucose intolerant after the 3-month HCD challenge; they became glucose intolerant and had greater area under the curve, which meant reduced glucose disposal. However, administration of RES reduced glucose intolerance 30 and 60 min after intraperitoneal injection of glucose in the HCD-fed mice (Figs.1a, c). Consistent with the effects of RES on GTT, the reduction in glucose levels during ITT was greater in the HCD + RES group, indicated that RES treatment robustly improved glucose disposal and increased insulin sensitivity (Figs. 1b, d).
Resveratrol improved the insulin sensitivity and blood inflammation markers of the HCD-fed mice. 1a-d, Blood samples were obtained from tail veins, measured with a glucometer, and the data were plotted as blood glucose concentration over time. a glucose tolerance test; b insulin tolerance test; c and d the calculation of the area under the curve (AUC) for each of the experiments. 1e-g, Resveratrol decreased inflammation in the HCD-fed mice. Plasma TNF-α, IL-6, and MCP-1 were measured using an ELISA kit. * P < 0.05 vs. SCD group, # P < 0.05 vs. HCD group. Data are expressed as mean ± SD (n = 6)
Effect of RES on blood lipid and inflammation of HCD-fed mice
The levels of serum TC, TG, LDL-C, FFA, and PA in the HCD-fed mice significantly increased and the HDL-C levels markedly decreased as compared to the SCD-fed and SCD + RES mice (P < 0.05) (Table 3); RES treatment obviously inverted serum lipid levels. Because the HCD-fed mice showed increased plasma FFA levels, especially PA levels, meanwhile serum TC levels had no significant differences between the HCD and HCD + RES groups, we selected PA as a representative HCD ingredient in order to study its effects in differentiated mouse 3T3-L1 cells. TNF-α, IL-6, and MCP-1 are the main obesity-induced proinflammatory cytokines and chemokine—they were all markedly elevated (P < 0.05), which suggests inflammatory activation in the HCD-fed mice. Meanwhile, all three inflammatory factor levels were attenuated by RES treatment, indicating that RES had an anti-inflammatory effect (Figs. 1e–g).
Effect of RES on adipocyte size and gene expression in SAT and VAT
Adipocyte size has been shown to be an independent predictor of glucose intolerance [16]. Therefore, in order to detect the influence of RES on adipocyte size, we performed HE staining experiment. The adipocytes in the SAT and VAT of the HCD-fed mice were hypertrophic, and their diameters were almost two times the size of the SCD-fed and SCD + RES mice. The average sizes of adipocytes in the SAT and VAT of the HCD + RES mice were markedly smaller (Fig. 2a). Figure 2e provides statistical information about the sizes of adipocytes in all four groups, which were consistent with Fig. 2a.
Resveratrol influenced the cell morphology and gene expression in both SAT and VAT (×200). a HE staining of SAT and VAT; b Immunohistochemical analysis of leptin; c Immunohistochemical analysis of TNF-α; d Immunohistochemical analysis of GRP78; e Quantification of adipocyte size in SAT and VAT; f Quantification of protein expressions of leptin, g Quantification of protein expressions of TNFα, h Quantification of protein expressions of GRP78. Data are expressed as mean ± SD (n = 6). * P < 0.05 vs. SCD group, # P < 0.05 vs. HCD group. IOD represent Integral Optical Density
Next, we confirmed the effect of RES on gene expression using immunohistochemistry for leptin, TNF-α, and GRP78 in both SAT and VAT. Leptin, TNF-α, and GRP78 in the HCD-fed mice were significantly increased and mainly distributed in the cytoplasm as compared to the SCD-fed and SCD + RES mice. In contrast, leptin, TNF-α, and GRP78 were markedly decreased in the HCD + RES mice (Figs. 2b–d). The semi-quantification results indicated that the gene expression, except for the GRP78 expression in VAT, showed significant differences among the three groups (Figs. 2f–h), which meant that the lipid-lowering effects of RES are possibly related to its influence on the protein expression of adipokines, inflammatory factors, and ER stress. Therefore, we would continue to confirm the effects of RES on more adipokines or genes participating in inflammation and ER stress.
Because GTT, ITT, inflammatory factors, histological and immunohistochemical analysis of the adipose tissue showed no significant differences between SCD and SCD + RES group, no other parameters were measured for the SCD + RES group, and the SCD group alone served as the standard-diet control for the remaining experiments.
Effects of RES on ER stress, lipid metabolism, and adipokines in SAT and VAT
We further tested the effects of RES on the mRNA expression of adipokines, ER stress upstream markers ATF6, PERK and IRE1α, and downstream effectors such as GRP78 and CHOP mRNA in both SAT and VAT. The results showed that the ER stress related genes ATF6, PERK, IRE1α, GRP78, and CHOP were all increased in the SAT of the HCD-fed mice, while PERK mRNA expression was almost undetectable in the VAT, which indicates that the ER stress in SAT and VAT was activated by HCD feeding, and that RES treatment obviously decreased gene expression, thus alleviating ER stress (Figs. 3a, b).
Effects of resveratrol on mRNA and protein expression of ER stress, SIRT1, lipid metabolism, and adipokines in SAT and VAT. Gene expression were analyzed by realtime RT-PCR or Western blot. a IRE1α, PERK, and ATF6; b CHOP and GRP78; c leptin, adiponectin, and resistin; d TNF-α, SIRT1, and IL-1β; e SREBP1c, PPARα and PPARγ; f Western blot of IRE1α, IL1β, GRP78, CHOP and SIRT1, 1–3 represent protein expression in SAT, 1 SCD, 2 HCD, 3 HCD + RES, 4–6 represent protein expression in VAT, 4 SCD, 5 HCD, 6 HCD + RES; g and h Quantification of related protein expressions. Data are presented as mean ± SD. * P < 0.05 vs. SCD group, # P < 0.05 vs. HCD group
We also detected the influence of RES on the expression of SIRT1, inflammatory factors, lipid metabolism, and adipokines. As expected, the HCD feeding decreased the mRNA expression of SIRT1, while increasing the expressions of leptin and resistin, TNF-α and IL-1β, and SREBP1c (Figs. 3c–e). mRNA expression of adiponectin and PPARα showed no significant difference when compared with the SCD-fed mice. RES treatment obviously inverted the mRNA expression of the above indices and significantly increased the mRNA expression of SIRT1, adiponectin, and PPARα. There were also no differences in the expression of all these genes between SAT and VAT, except for PERK and IL-1β. Expression of PPARγ was increased in HCD group and consistent with increased size of adipocytes in SAT and VAT; RES treatment decreased expression of PPARγ significantly, which was consistent with decreased size of adipocytes (Fig. 3e). Changes of protein expression of IRE1α, GRP78, CHOP, SIRT1, and IL1β were similar with their mRNA expression in SAT and VAT (Figs. 3f–h).
Effects of RES on PA-treated mature 3T3-L1 cells
In order to confirm whether the changes of gene expression in adipose tissues also existed in adipocytes, we further tested the effects of RES on mRNA expression in ER stress in mature 3T3-L1 cells. The results showed that the ER stress-related genes ATF6, PERK, IRE1α, GRP78, and CHOP all increased in the 3T3-L1 cells, indicating that the ER stress in these cells was activated by PA. RES pretreatment obviously alleviated ER stress (Figs. 4a, b).
Effects of resveratrol on mRNA and protein expression of ER stress, SIRT1, lipid metabolism, and adipokines in mature 3T3-L1 cells. Gene expression were analyzed by realtime RT-PCR or Western blot. a IRE1α, PERK, and ATF6; b CHOP and GRP78; c leptin, adiponectin, and resistin; d TNF-α, SIRT1, and IL-1β; e SREBP1c, PPARα and PPARγ; f Western blot of IRE1α, IL1β, GRP78, CHOP and SIRT1, 1 NC, 2 PA, 3 PA + RES; g Quantification of related protein expressions. Data are presented as mean ± SD. * P < 0.05 vs. NC group, # P < 0.05 vs. PA group
Again, we detected the influence of RES on the expressions of SIRT1, inflammatory factors, lipid metabolism, and adipokines. PA treatment obviously increased the expression of TNF-α, IL-1β, SREBP1c, and leptin, while resistin expression increased, but there were no differences between the two groups. PPARα mRNA expression decreased significantly in the PA group. RES pretreatment obviously decreased the mRNA expression of leptin, resistin, TNF-α, IL-1β and SREBP1c, while the mRNA expression of adiponectin, SIRT1, and PPARα increased significantly (Figs. 4c–e). Changes of protein expression of IRE1α, GRP78, CHOP, SIRT1, and IL1β were similar with their mRNA expression in mature 3T3-L1 cells (Figs. 4f, g).
Discussion
It is well known that unfavorable diets with excess calories are associated with overweight and obesity-associated diseases, such as diabetes, cardiovascular disease, and non-alcoholic fatty liver disease, which can lead to metabolic damage through complex pathophysiological mechanisms. RES is one of the limited viable drug candidates for obesity treatment. In the present study, RES was shown to reverse adipocyte hypertrophy and IR by attenuating ER stress and inflammation, thus increasing the expression of SIRT1 and inverting the expression of adipokines in vivo and in vitro. The results showed that RES plays a novel role in obesity-related metabolic syndrome.
The therapeutic dose of RES was determined based on previous reports [16] that determined the safe dose by examining animals fed a standard diet and RES. High doses of RES, up to 750 mg/kg/day for 3 months, were investigated in vivo in rabbits and rats, and it was concluded that RES is well-tolerated and non-toxic [20]. We used RES 400 mg/kg/day to treat the HCD-fed mice.
The preventive effect of RES on body fat accumulation has been demonstrated in various models, including rodents, primates, and humans [21–23]. Our results suggested that RES supplementation alleviated HCD-induced increases in adipose weight, adipocyte size, and macrophage infiltration, as indicated by elevated serum MCP-1 levels and heightened TNF-α expression in SAT and VAT, respectively. Serum TG, TC, LDL-C, FFA, and PA levels were also decreased after RES treatment. Reduced uptake of fatty acids for triacylglycerol formation and increased capacity for triacylglycerol mobilization may be the mechanisms responsible for the metabolic changes, at least in part, resulting in the body-fat-lowering effect of RES.
RES improved adipose insulin signaling and reduced the inflammatory response in the adipose tissue of rhesus monkeys on a high-fat, high-sugar diet [24]. Our study also showed that RES could improve glucose disposal, restore insulin sensitivity, and decrease serum TNF-α, IL-6, and MCP-1 levels through increasing SIRT1 expression. Pfluger et al. reported that transgenic SIRT1 mice fed a high-fat diet showed lower lipid-induced inflammation along with better glucose tolerance [25], which was consistent with the results of our study.
PPARα mainly participates in fatty acid oxidation, while SREBP1c is responsible for fatty acid and TG synthesis. Our results showed that HCD feeding or PA treatment regulated the expression of PPARα and SREBP1c to increase TG synthesis and decrease fatty acid oxidation in adipose tissue and adipocytes. RES decreased adipocyte size (indicated by decreased PPARγ expression), as well as body weight and adipose tissue mass, and it inverted mRNA expression of SREBP1c and PPARα in HCD-fed mice and PA-treated 3T3-L1 cells. RES had been shown to inhibit PPARγ induction in adipocytes [26]. These results indicated that RES has a lipid-lowering effect, which was consistent with Scapagnini’s review of RES [27]. Mercader et al. reported increased oxidative capacity in white adipocytes treated with RES by increased expression of CPT1 and PGC1α [28].
Obesity-induced ER stress triggers a stress cascade that involves IR, inflammation, and cell death [29]. A recent study demonstrated that PA-induced ER stress was associated with increased protein expression of GRP78 and CHOP in 3T3-L1 cells and rat primary preadipocytes [30]. These results were consistent with those of our study, in that not only were downstream CHOP and GRP78 increased by HCD feeding or PA treatment, but that upstream ER stress signaling molecules, namely IRE1α, PERK, and ATF6, were all increased in SAT tissues and 3T3-L1 cells, while PERK and ATF6 gene expression was almost undetectable in VAT. RES treatment inverted the mRNA expression of ER stress-related molecules in adipose tissues and cells. IRE1α, GRP78, and CHOP were selected as representative markers of ER stress in adipose tissue and adipocytes. RES was able to decrease their expression induced by HCD feeding or PA treatment, again demonstrating the alleviation of ER stress by RES in adipose tissue and adipocytes.
Mitochondrial dysfunction is another key determinant of IR. Mitochondria are involved in lipolytic processes through the β-oxidation of lipids, and in anabolic processes such as lipogenesis and lipid storage in adipocytes and other tissues. RES acts on differentiating preadipocytes by inhibiting mitochondrial biogenesis and lipogenesis, and RES-induced reduction of mitochondrial biogenesis and lipid storage contributes to adipose-tissue-based weight loss in animals and humans [31].
The antilipogenic effect of RES is further modulated by expression of mitochondrial ATAD3 in adipocytes, consistent with the emerging role of this protein as an important regulator of mitochondrial biogenesis and lipogenesis. Overexpression of ATAD3 counteracts RES antilipogenic effects in 3T3-L1 [15], so the expression of this protein will be investigated in future studies.
Nutrient overload, such as with the HCD in adipose tissue and PA in adipocytes, induces intracellular stress that results in activation of inflammatory cascades. Our animal model showed a chronic low-degree inflammatory state, characterized by increased proinflammatory cytokine levels in plasma and adipose tissue. Resolution of inflammation is a carefully orchestrated and complex process in which endogenously generated anti-inflammatory mediators counteract the effects of proinflammatory signaling systems. Only a limited number of studies and indices have assessed the potential anti-inflammatory effects of RES in animal models [32].
A paracrine loop involving adipocyte-derived FFA and macrophage-derived TNF-α establishes a vicious cycle that aggravates inflammatory changes and IR in adipose tissues [33]. RES decreased the expression of TNF-α and IL-1β in SAT and VAT, as well as in 3T3-L1 cells, and exhibited anti-inflammatory effects in adipose tissue and adipocytes.
Metabolically challenged adipocytes are more prevalent and the accompanying inflammatory reactions are more severe in visceral than in subcutaneous fat in obese individuals [34]. With regard to reduction of body fat, clear differences in terms of response to RES were found among adipose tissues from different anatomical locations. However, in the present study, we found that there was no difference between the level of inflammation and ER stress in SAT and VAT, which is inconsistent with previous results [35]. This discrepancy may be related to the ingredients of the HCD and the feeding times.
Our results proved that RES increases the expression of SIRT1 in adipose tissues and cells, which was also shown in reports from Lv et al. using the same dose [36]. Although there were some differences among different adipose tissues, the reason for this is unknown. Increased SIRT1 expression has been reported as a mechanism by which RES produces its beneficial health effects.
Marked changes in adipokine expression in adipose tissues strongly affect their pathophysiology [37]. As we expected, increased leptin and resistin levels were induced in adipose tissues by HCD feeding and in 3T3-L1 cells by PA treatment, while RES treatment significantly decreased the expression of these adipokines, further supporting the role that RES may play in reversing obesity-associated adipocyte dysfunction. It is possible that the initial increase in visceral adiposity and inflammation contributed to the reduced adiponectin level, but a high-fat diet enabled the mice to compensate and adiponectin levels to increase. Therefore, there was no difference in adiponectin expression between the SCD and HCD groups, while RES treatment increased the expression of adiponectin.
3T3-L1 preadipocytes must be cultured in high-sugar media. All three groups in the cell experiment were under the same conditions, with the differences among them due only to whether they were exposed to PA or to RES. Saturated palmitate is a strong inducer of the inflammatory cytokine pathways known to mediate insulin resistance in 3T3-L1 adipocytes [38]. The fact that RES effectively reduced palmitate-induced IL1β and TNFα levels in mature 3T3-L1 adipocytes lends support to the notion that RES specifically blocks palmitate-mediated inflammatory pathways.
In conclusion, our study suggests that the reduction of inflammation and ER stress due to RES treatment may have contributed to insulin sensitization in the HCD-fed mice. This mechanism is related to the reduction of body adiposity and the direct suppression of adipokine expression in adipose tissues and cells. RES also appears to reduce adipocyte dysfunction by decreasing adipocyte size and increasing SIRT1 expression. Our observations suggest a new molecular mechanism for the therapeutic efficacy of the SIRT1 activator RES.
References
R.H. Eckel, S.M. Grundy, P.Z. Zimmet, The metabolic syndrome. Lancet. 365, 1415–1428 (2005)
T. Wang, A. Jiang, Y. Guo, Y. Tan, G. Tang, M. Mai, H. Liu, J. Xiao, M. Li, X. Li, Deep sequencing of the transcriptome reveals inflammatory features of porcine visceral adipose tissue. Int. J. Biol. Sci. 9, 550–556 (2013)
B. Antuna-Puente, B. Feve, S. Fellahi, J.P. Bastard, Adipokines: the missing link between insulin resistance and obesity. Diabetes. Metab. 34, 2–11 (2008)
S.P. Weisberg, D. McCann, M. Desai, M. Rosenbaum, R.L. Leibel, A.W. Ferrante Jr., Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003)
K. Cusi, The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr. Diab. Rep. 10, 306–315 (2010)
R.A. Luvizotto, A.F. Nascimento, E. Imaizumi, D.T. Pierine, S.J. Conde, C.R. Correa, K.J. Yeum, A.L. Ferreira, Lycopene supplementation modulates plasma concentrations and epididymal adipose tissue mRNA of leptin, resistin and IL-6 in diet-induced obese rats. Br. J. Nutr. 110, 1803–1809 (2013)
M.F. Gregor, G.S. Hotamisligil, Thematic review series: adipocyte biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J. Lipid. Res. 48, 1905–1914 (2007)
N. Kawasaki, R. Asada, A. Saito, S. Kanemoto, K. Imaizumi, Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci. Rep. 2, 799 (2012)
W. Yu, Y.C. Fu, W. Wang, Cellular and molecular effects of resveratrol in health and disease. J. Cell. Biochem. 113, 752–759 (2012)
J.D. Malhotra, H. Miao, K. Zhang, A. Wolfson, S. Pennathur, S.W. Pipe, R.J. Kaufman, Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc. Natl. Acad. Sci. U. S. A. 105, 18525–18530 (2008)
T.W. Jung, K.T. Lee, M.W. Lee, K.H. Ka, SIRT1 attenuates palmitate-induced endoplasmic reticulum stress and insulin resistance in HepG2 cells via induction of oxygen-regulated protein 150. Biochem. Biophys. Res. Commun. 422, 229–232 (2012)
S.E. Chow, C.H. Kao, Y.T. Liu, M.L. Cheng, Y.W. Yang, Y.K. Huang, C.C. Hsu, J.S. Wang, Resveratrol induced ER expansion and ER caspase-mediated apoptosis in human nasopharyngeal carcinoma cells. Apoptosis. 19, 527–541 (2014)
L.Q. Liu, Z.Q. Fan, Y.F. Tang, Z.J. Ke, The resveratrol attenuates ethanol-induced hepatocyte apoptosis via inhibiting ER-related caspase-12 activation and PDE activity in vitro. Alcohol. Clin. Exp. Res. 38, 683–693 (2014)
C. Li, L. Wang, K. Huang, L. Zheng, Endoplasmic reticulum stress in retinal vascular degeneration: protective role of resveratrol. Invest. Ophthalmol. Vis. Sci. 53, 3241–3249 (2012)
S. Li, C. Bouzar, C. Cottet-Rousselle, I. Zagotta, F. Lamarche, M. Wabitsch, M. Tokarska-Schlattner, P. Fischer-Posovszky, Resveratrol inhibits lipogenesis of 3T3-L1 and SGBS cells by inhibition of insulin signaling and mitochondrial mass increase. Biochim. Biophys. Acta. 1857, 643–652 (2016)
M. Lagouge, C. Argmann, Z. Gerhart-Hines, H. Meziane, C. Lerin, F. Daussin, N. Messadeq, J. Milne, P. Lambert, P. Elliott, B. Geny, M. Laakso, P. Puigserver, J. Auwerx, Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 127, 1109–1122 (2006)
Y. Lu, L. Qian, Q. Zhang, B. Chen, L. Gui, D. Huang, G. Chen, L. Chen, Palmitate induces apoptosis in mouse aortic endothelial cells and endothelial dysfunction in mice fed high-calorie and high-cholesterol diets. Life. Sci. 92, 1165–1173 (2013)
L.D. Han, J.F. Xia, Q.L. Liang, Y. Wang, Y.M. Wang, P. Hu, P. Li, G.A. Luo, Plasma esterified and non-esterified fatty acids metabolic profiling using gas-chromatography mass spectrometry and its application in the study of diabetic mellitus and diabetic nephropathy. Anal. Chim. Acta. 689, 85–91 (2011)
Y. Lu, J. Cheng, L. Chen, C. Li, G. Chen, L. Gui, B. Shen, Q. Zhang, Endoplasmic reticulum stress involved in high-fat diet and palmitic acid-induced vascular damages and fenofibrate intervention. Biochem. Biophys. Res. Commun. 458, 1–7 (2015)
L.D. Williams, G.A. Burdock, J.A. Edwards, M. Beck, J. Bausch, Safety studies conducted on high-purity transresveratrol in experimental animals. Food. Chem. Toxicol. 47, 2170–2182 (2009)
M.T. Macarulla, G. Alberdi, S. Gómez, I. Tueros, C. Bald, V.M. Rodríguez, J.A. Martínez, M.P. Portillo, Effects of different doses of resveratrol on body fat and serum parameters in rats fed a hypercaloric diet. J. Physiol. Biochem. 65, 369–376 (2009)
A. Dal-Pan, S. Blanc, E. Aujard, Resveratrol suppresses body mass gain in a seasonal non-human primate model of obesity. BMC. Physiol. 10, 11 (2010)
S. Timmers, E. Konings, L. Bilet, R.H. Houtkooper, T. van de Weijer, G.H. Goossens, J. Hoeks, S. van der Krieken, D. Ryu, S. Kersten, E. Moonen-Kornips, M.K. Hesselink, I. Kunz, V.B. Schrauwen-Hinderling, E.E. Blaak, J. Auwerx, P. Schrauwen, Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profiles in obese humans. Cell. Metab. 14, 612–622 (2011)
Y. Jimenez-Gomez, J.A. Mattison, K.J. Pearson, A. Martin-Montalvo, H.H. Palacios, A.M. Sossong, T.M. Ward, C.M. Younts, K. Lewis, J.S. Allard, D.L. Longo, J.P. Belman, M.M. Malagon, P. Navas, M. Sanghvi, R. Moaddel, E.M. Tilmont, R.L. Herbert, C.H. Morrell, J.M. Egan, J.A. Baur, L. Ferrucci, J.S. Bogan, M. Bernier, R. de Cabo, Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell. Metab. 18, 533–545 (2013)
J. Olholm, S.K. Paulsen, K.B. Cullberg, B. Richelsen, S.B. Pedersen, Anti-inflammatory effect of resveratrol on adipokine expression and secretion in human adipose tissue explant. Int. J. Obes. 34, 1546–1553 (2010)
Z.E. Floyd, Z.Q. Wang, G. Kilroy, W.T. Cefalu, Modulation of peroxisome proliferator activated receptor gamma stability and transcriptional activity in adipocytes by resveratrol. Metabolism. 57, S32–S38 (2008)
G. Scapagnini, S. Davinelli, T. Kaneko, G. Koverech, A. Koverech, E.J. Calabrese, V. Calabrese, Dose response biology of resveratrol in obesity. J. Cell. Commun. Signal. 8, 385–391 (2014)
J. Mercader, A. Palou, M.L. Bonet, Resveratrol enhances fatty acid oxidation capacity and reduces resistin and Retinol-Binding Protein 4 expression in white adipocytes. J. Nutr. Biochem. 22, 828–834 (2011)
U. Ozcan, Q. Cao, E. Yilmaz, A.H. Lee, N.N. Iwakoshi, E. Ozdelen, G. Tuncman, C. Görgün, L.H. Glimcher, G.S. Hotamisligil, Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004)
W. Guo, S. Wong, W. Xie, T. Lei, Z. Luo, Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am. J. Physiol. Endocrinol. Metab. 293, E576–586 (2007)
S. Li, C. Bouzar, C. Cottet-Rousselle, I. Zagotta, F. Lamarche, M. Wabitsch, M. Tokarska-Schlattner, P. Fischer-Posovszky, U. Schlattner, D.R. Rousseau, esveratrol inhibits lipogenesis of 3T3-L1 and SGBS cells by inhibition of insulin signaling and mitochondrial mass increase. Biochim. Biophys. Acta 1857, 643–652 (2016)
C.S. Andrews, S. Matsuyama, B.C. Lee, J.D. Li, Resveratrol suppresses NTHi-induced inflammation via up-regulation of the negative regulator MyD88 short. Sci. Rep. 6, 34445 (2016)
T. Suganami, J. Nishida, Y. Ogawa, A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler. Thromb. Vasc. Biol. 25, 2062–2068 (2005)
A. Tchernof, J.P. Després, Pathophysiology of human visceral obesity: an update. Physiol. Rev. 93, 359–404 (2013)
J.S. Burrill, E.K. Long, B. Reilly, Y. Deng, I.M. Armitage, P.E. Scherer, D.A. Bernlohr, Inflammation and ER stress regulate branched-chain amino acid uptake and metabolism in adipocytes. Mol. Endocrinol. 29, 411–420 (2015)
Z.M. Lv, Q. Wang, Y.H. Chen, S.H. Wang, D.Q. Huang, Resveratrol attenuates inflammation and oxidative stress in epididymal white adipose tissue: implications for its involvement in improving steroidogenesis in diet-induced obese mice. Mol. Reprod. Dev. 82, 321–328 (2015)
E. Melloni, G. Zauli, C. Celeghini, I. Volpi, P. Secchiero, Release of a specific set of proinflammatory adipokines by differentiating 3T3-L1 cells. Nutrition. 1, 332–327 (2013)
K.D. McCall, D. Holliday, E. Dickerson, B. Wallace, A.L. Schwartz, C. Schwartz, C.J. Lewis, L.D. Kohn, F.L. Schwartz, Phenylmethimazole blocks palmitate-mediated induction of inflammatory cytokine pathways in 3T3L1 adipocytes and RAW 264.7 macrophages. J. Endocrinol. 207, 343–353 (2010)
Funding
This study was funded by research grants from Anhui Natural Science Foundation of China (1308085MH154 and 1608085MH208, 1608085QH205) and a special foundation of the National TCM Clinical Research Base (JDZX2015129).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state no conflict of interest. The authors alone are responsible for the content and writing of this paper.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Li Chen and Ting Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, L., Wang, T., Chen, G. et al. Influence of resveratrol on endoplasmic reticulum stress and expression of adipokines in adipose tissues/adipocytes induced by high-calorie diet or palmitic acid. Endocrine 55, 773–785 (2017). https://doi.org/10.1007/s12020-016-1212-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-1212-2