Abstract
The prevalence of obesity and type 2 diabetes mellitus epidemics presents a great health problem worldwide. Beside the changes in diet and decreased physical activity, there is growing interest in endocrine disrupting chemicals that may have effects on these conditions. Among them, the role of certain phthalates and bisphenol A is confirmed. We have summarized the existing literature on this issue including cross-sectional, follow up epidemiological studies and in vivo and in vitro studies. Most data support the effects of bisphenol A and some phthalates, such as di-2-ethyl-hexyl phthalate, diethyl phthalate, dibuthyl phthalate, dimethyl phthalate, dibenzyl phthalate, diisononyl phthalate and others on the development obesity and type 2 diabetes mellitus. These endocrine disrupting chemicals interfere with different cell signaling pathways involved in weight and glucose homeostasis. Since the data are rather inconsistent, there is a need for new, well-designed prospective studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity and diabetes epidemics represent a real concern worldwide. There are about 2 billion overweight adults, out of which over 600 million are obese [1]. A particular problem presents the increased frequency of obesity observed in children and adolescents [2]. Based on National Health and Nutrition Examination Survey (NHANES) reports, it has been estimated that in USA only, in the 2000s, among adults older than 20, 66 % is overweight while 28 % males and 33 % females were obese. According to the reports of the Center for Disease Control (CDC) between 1960s and 2000s, body weight of both men and women has increased for about 11 kg in average, while the body mass index (BMI) has risen approximately for 3 kg/m2 for both genders. In the same period the average values of waist circumference (WC) has been increased for 6 cm among man and 2 cm among women [3]. Moreover, worldwide the number of adults with BMI over 25 kg/m2 (which is classified as overweight according to World Health Organization) has been dramatically increased in the period 1980–2013 from 28.8 to 36.9 % in men and from 29.8 to 38 % in women. Overweight and obesity, especially the visceral type, are associated with well-known cardiovascular risk factors such as hypertension, dyslipidemia, insulin resistance (IR), endothelial dysfunction and nonalcoholic fatty liver disease [4]. However, it is not simple to estimate obesity, especially on BMI only. Although it is globally accepted that general population should be classified according to the BMI values in the following categories: underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), class I obesity—overweight (BMI 25.0–29.9 kg/m2), class II obesity—obesity (BMI 30.0–39.9 kg/m2), class III obesity—extreme obesity (BMI > 40 kg/m2), the problem of defining obesity is much more complex. The percentage of body fat (PBF) is currently being recognized as a measure of obesity with possible cut-off points: 23–25 % range for men and 30–35 % for women. There are some important discrepancies in definition of obesity when BMI and PBF are taken in account. For example, 30 % of women aged between 30 and 40 can be classified as obese according to the BMI, while based on PBF 82 % can be considered obese. WC, waist-to-hip ratio and waist-to-height ratio have also been observed as quantities for measuring obesity. One of the proposals for the definition of obesity is the definition of four phenotypes of obese individuals: normal weight obese, metabolically obese normal weight, metabolically healthy obese and metabolically unhealthy obese. Each phenotype is being classified strictly based on BMI, WC, PBF, but also on triglyceride, high-density lipoprotein, low-density lipoprotein, total cholesterol, glucose and insulin serum levels as well as on high sensitivity C-reactive protein plasma values [5].
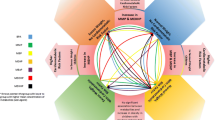
Nowadays more than 387 million people in the world suffer from type 2 diabetes mellitus (T2DM) with a prevalence of 8.3 %. By the end of 2035, 592 million people are expected to suffer from T2DM [6]. Epidemiological studies report an increased number of the patients within the younger population [2, 7]. The patients with obesity and T2DM have a considerably higher risk of cardiovascular morbidity and mortality, too. The complex network of the mechanisms involved in obesity and T2DM have not completely been defined yet. Beside the genetic predisposition and the lifestyle, today there is a growing interest in the impact of the environmental factors in the pathogenesis of both obesity and diabetes. In the last two decades, the increased importance in the development of obesity and T2DM has been attributed to the endocrine disruptors (EDCs) as epigenetic factors [8]. By definition of the US Environmental Protection Agency (EPA), EDCs are “exogenous agents that interfere with synthesis, secretion, transport, metabolism, binding action or elimination of natural blood-borne hormones that are present in the body and are responsible for homeostasis, reproduction and developmental process” [8, 9]. Some characteristics of endocrine disruption [8, 10–12] are shown in the Fig. 1. Phthalates and bisphenol A (BPA), are EDCs, shown to have a role in the development of obesity and glucose metabolism disorders [9, 12].
In this article, we have reviewed the current literature data, to highlight the newest evidences on the possible influence of phthalates and BPA on the obesity development and glucose metabolism disorders. Many papers have been published in which is examined phthalates and BPA influence on obesity, glucose metabolism and metabolic syndrome (MetS). On Pubmed database only can be found a large amount of evidence published between 2004 and 2016 that links phthalate with obesity (over 80 publications) and glucose metabolism (over 70 published results) as well as BPA with obesity (over 120 papers) and glucose metabolism (over 80 papers) conducted on laboratory animals, wildlife, and in vitro models, as well as human studies. Relevant articles were identified by searching the PubMed database, MEDLINE and EMBASE, limited to articles published in the English language but not date-restricted. Selected articles referenced in these publications were also examined. Finally, in this paper we observed only peer-reviewed epidemiological, cross-sectional, prospective cohort and randomized clinical trial studies regarding BPA and phthalate (prenatal, childhood, and adult) exposure influence on obesity and glucose metabolism. Animal and in vitro studies are also cited, but in order to explain the possible mechanism of BPA and phthalate influence on obesity development and glucose metabolism disorders. The authors have selected 25 recently published papers, which link phthalate and BPA exposure with obesity and 20 manuscripts in which phthalate and BPA exposure has been associated with glucose metabolism disorders in humans. In some of them both EDCs are examined, while in some of the papers it has been observed the influence of only phthalate or BPA but on both adverse effects, weight gain and IR and/or T2DM development.
Phthalates and BPA as EDCs
Phthalates as EDCs
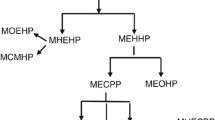
Phthalates, often called plasticizers, are diesters of 1,2-benzendicarboxylic acid. They have been used in hundreds of consumer products such as vinyl flooring, adhesives, detergents, lubricating oils, automotive plastics, toys, plastic clothes (raincoats), blood-storage containers, medical tubing, medications, personal-care products, etc. [13–16]. Phthalates’ half-life is short in outdoor environment, 50 % degradation occurs in 28 days [17]. However, the phthalates’ significant effect is the result of their mass production—11 billion pounds worldwide every year and the possible cumulative effect in the body [18, 19]. People are commonly exposed to phthalates orally, or by inhalation, intravenously, or by skin absorption. Approximately 60 % of the ingested phthalates are metabolized within 24 h and the residue in less than 48 h. The maximum amount of metabolites is excreted with the urine, and may be present in blood, saliva, feces, amniotic fluid and breast milk [20, 21]. Low molecular weight phthalates (LMW), such as diethyl phthalate, di-n-butyl phthalate (DBP) are subjected to hydrolysis, resulting in monoesters during the I phase of biotransformation, which are mainly excreted in urine. High molecular weight phthalates (MW) such as dimethyl hexyl phthalate (DEHP), di-isononyl phthalate (DINP) and di-isodecyl phthalate (DIDP) are metabolized in hydrolytic monoesters which can be excreted or enter the II phase of biotransformation forming much potent, oxidized metabolites that can be excreted via urine or glucuronized prior to excretion [20].
The epidemiological studies and animal studies have shown that phthalates, as EDCs, have a significant role in the occurrence of obesity and T2DM, as well as in the disorders of thyroid gland, liver, fertility, immune disorders, malignant diseases, etc. [8, 22, 23]. DEHP, DEP and DBP are the phthalates that have the greatest impact on the development of metabolic disorders. In 2004 Silva at al. published the NHANES results conducted in the 1999–2000, including 2540 participants between the ages of 6–80 [24]. Monoethyl phthalate (MEP), mono buthylphthalate (MBP), monobenzyl phthalate (MBzP) were detected in the urine >97 % patients, and monoethyl hexyl phthalate (MEHP) in >75 % participants, which confirmed the great exposure of the US population to DEP, DBP, BzBP and DEHP. In our research, nine urinary phthalate metabolites (MMP, MEP, MPP, MnBP, MNAP, MCHP, MBzP, MOP, MEHP) were detected above the quantification levels with 49.5 % MEP and MEHP prevalence in 305 participants (unpublished data).
Regarding the great exposure and deteriorating influence of certain phthalates on human health, WHO, US EPA and EU (European Union) defined and regulated by law the permitted concentrations of them [25–29]. The biomonitoring results of 15 urinary phthalate metabolites of the American population [16] showed that MEP was present in the greatest quantity, which is in accordance with the obtained results in our research (unpublished data). The introduction of legislation and preventive measures, has probably at least partially led to the lower exposure to certain harmful phthalate metabolites [30–32].
BPA as EDCs
BPA, a diphenylmethane derivative, with over 6 billion pounds produced each year, is one of the most produced chemicals worldwide [33, 34]. Most of the polymers as polycarbonates produced from BPA as well as epoxy resins are used as materials which are in contact with food. Medical devices, dental materials, CD-ROM, sunglasses, building materials, etc. are also sources of BPA. BPA is ubiquitous in the environment, but it has a short half-life (4 days). However, BPA is considered to possess moderate bioaccumulation properties. Usually, it reaches the human body since the residues of incomplete polymerizations migrate into the food from the resins which are used to coat the surface of food and beverage cans. BPA is also present in leaches from dentals sailings and composites, as well as indoor dust inhalation, or bathing in BPA contaminated water [35].
After oral administration, BPA reaches the maximum concentration in the blood after 80 min, while its terminal half-life is 6 h. BPA is dominantly conjugated with glucuronic acid in the liver and minor amount is conjugated as sulfate; it does not undergo enterohepatic circulation and it is mainly excreted by urine [36]. However, BPA can be measured in humans, not only in serum and urine, but also in amniotic fluid, follicular fluid, placental tissue, and fetal cord blood [37]. Although it is accepted that BPA may not partition significantly from blood to the lipophilic compartment, the BPA levels have not been reduced as rapidly as expected after fasting, suggesting that BPA either enters human body via non dietary routes or accumulates in body tissues with a long elimination time [38]. The studies conducted in the USA indicated that over 91 % of examined population was exposed to BPA, including children [39]. The increased BPA concentrations are associated with female and male infertility and polycystic ovary syndrome (PCOS) [40–45]. High BPA exposure during pregnancy resulted in shorter anogenital distance in male offspring [46]. The increased BPA levels are also associated with disturbed weight regulation, obesity promotion, lipid accumulation, IR, changes in adiponectin secretion and glucose transport in animal studies [47, 48] and increased prevalence of obesity, T2DM [49, 50], hypertension and peripheral arterial disease and in humans [51, 52]. Various biological activities of BPA have been reviewed and documented [53].
WHO, the US Food and Drug Administration, the US Department of Health & Human Services , and the CDC have expressed “some concerns” about BPA based on research [54] while in January 2015 the European Food Safety Authority, EFSA concluded that “BPA poses no health risk to consumers of any age group (including unborn children, infants and adolescents) at the current exposure levels” [55]. Hence, further researches of BPA effects on human health are desirable and warranted.
Overview of phthalates, BPA and obesity
Summary of data study on phthalate and BPA effects on obesity are shown in Table 1.
Phthalates and obesity
Baillie-Hamilton was among the first researchers to point to the connection between obesity epidemics and the increased use of industrial chemicals in the past 40 years [56]. The Expert panel identified 40–69 % probability that phthalate exposure had caused 53,900 obesity cases in older women and €15.6 billion associated cost [28].
Two big cross-sectional studies [57, 58] using NHANES 1999–2002 cycle data studied the connection between some urinary phthalate metabolites and obesity parameters. Stahlhut et al. showed that the WC was connected with the increased levels of 4 phthalates (MBzP, MEHHP, MEOHP, MEP) [57]. While the connection between the MEHP oxidative metabolites (MEHHP and MEOHP) was significant, the connection between MEHP and WC was insignificant one. That fact could be explained by shorter MEHP half-life and less potency in relation to its oxidative metabolites. Hatch et al. showed the differences according to gender and age regarding the connection between six urinary phthalate metabolites and obesity parameters (BMI and WC) [58]. There was a significant positive correlation of BMI and WC with MBzP, MEOHP, MEHHP, MEP and MBP in the adult males. BMI and WC had a significant positive correlation with MEP only in the adolescent females and insignificant one in the adult women. The correlation between MEHP, WC and BMI was negative and insignificant one in both genders. The associations of BMI and WC among 60–80 ages declined with the increased levels of phthalate metabolites.
Completely different data were obtained in our research on the correlation between urinary MEP and MEHP and BMI, WC and lipids and lipoproteins in serum [59]. In the cross-section study, the only positive significant correlation was found between MEHP and WC. MEHP also had a significant negative correlation with the age. Regarding serum lipids and lipoproteins there was no significant correlation between cholesterol triglycerides and LDL with the observed urinary metabolites. Significant negative correlation was found between MEHP and HDL. Taking into account that we conducted the study in healthy normally fed participants, our data suggested the possible influence of MEHP in the development of (MetS). Possible differences regarding the connection between MEHP and obesity parameters related to two previous studies originated from significantly higher levels of urinary MEHP in our participants which was almost ten times higher, as well as from the differences in the study design and the participant number. The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study examined 10 phthalates metabolites in serum, and positive correlations with BMI and WC, subcutaneous and visceral fat tissue were established [60]. Only MiBP, MMP, MEHP and MEP were found to be beyond the detection limits. However, only in women there was more significant connection between MiBP and subcutaneous fat tissue. Although Hatch at al. described negative correlation between MEHP and BMI and WC in elderly population, in PIVUS study positive, but weak correlation was found [58]. In Song et al. follow-up study lasting 10 years, the correlation between weight-gain and increased urinary MBzP and MBP levels was confirmed [61].
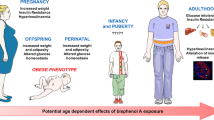
Phthalates influence the development of obesity and fat distribution in children [62, 63]. Two big cross-section studies confirmed that the exposure to phthalates contributed to the increase BW in newborns [64, 65]. Longitudinal Canadian Maternal-Infant Research on Environmental Chemicals Study (MIREC) evaluated the relationship between levels of eleven maternal urinary phthalate metabolites, leptin and adiponectin umbilical cord levels [66]. The results of this study showed significantly increased risk of high leptin among male newborns with moderate and elevated mono-(3-carboxyhexyl) phthalate (MCPP) maternal exposure levels. The exposure of fetus in utero to EDCs presents a risk for the development of the obesity later in childhood and adult period [11].
Different results on the effects of phthalates on obesity were obtained from animal studies [67]. Administration of DEP and MEP to rats during 2 weeks, but did not cause increase of BW when compared with control animals. BW of the rats treated with DBP, DINP, BBzP, MEHP and phthalate acid even decreased when compared with control animals.
BPA and obesity
The Expert panel [28] estimated that the prenatal BPA exposure caused 42,400 cases of childhood obesity with 20–69 % probability which cost €1.54 billion.
A cross-section study with NHANES 2003–2006 cycle data determined that urinary BPA was associated with general and central obesity. After multivariate adjustments, participants of the three upper quartiles had 39–62 % higher odds of being abdominally obese compared to the lowest BPA quartile participants [68]. In another cross-sectional study based on NHANES 2003–2008 cycle data, a higher urinary BPA level was positively associated with both BMI and WC in the whole group and in the subgroups determined by race and gender [69]. Wang et al. reported positive association between urinary BPA and obesity, serum BPA and obesity, as well as between urinary BPA and abdominal obesity among the Chinese aged 40+ [70]. In the study conducted in Japan higher serum BPA concentrations were observed in the obese and non-obese women with PCOS and in the obese women without PCOS [71]. Our recent study indicated higher urinary BPA concentrations among the overweight/obese subjects in comparison with the normal weight women with the highest BPA concentration detected in the obese subgroup and higher urinary BPA levels among women aged ≥40 [72]. Our results confirmed the findings of Zhao et al. who observed statistically significant linear trend between BPA exposure and fat mass and BPA exposure and leptin in healthy premenopausal women [73].
Higher urinary BPA concentrations were detected in children aged 6–11 based on Canadian Health Measure Survey 2007–2009 data [74]. Moreover, in the cross-sectional study in the children from 2003–2004, 2005–2006, and 2007–2008 NHANES cycles statistically significant linear trend was found between urinary BPA levels and BMI in gender and age adjusted models of the whole population and in non-Hispanic white boys, while non-significant among other subgroups [75]. These findings are consistent with NHANES 2003–2008 survey where after multivariate-adjustments, it was determined that participants of the three upper quartiles had 10–22 % higher odds of being obese compared to the lowest BPA quartile. When divided in subgroups, the positive statistically significant association between urinary BPA levels and obesity was found only among the white race participants [76]. A study based on the data pooled from the NHANES 2003–2010 cycles for children reported higher odds of being obese and having an abnormal waist circumference-to-height ratio in three upper quartiles in comparison with the first one [77]. In a study conducted in China on school-age children, the increased BPA levels among female students entering puberty (9–12 years) enhanced the risk of being overweight. Higher urinary BPA concentration was detected in younger children [78]. Positive linear correlation with good statistical quality was determined between urinary BPA and BMI in the Chinese children (8–15 years) and higher urinary BPA was detected in the obese group [79]. The negative association between BPA and obesity in children detected in two studies conducted in India and US, respectively due to small samples could be discussed carefully and with caution [80, 81].
Increased risk of lower birth weight, smaller size for gestational age and adverse effects of leptin and adiponectin were observed in male neonates whose mothers had the highest quartile of serum BPA levels [82]. Furthermore, the prenatal BPA exposure was associated with the decreased BMI and percent body fat in the 9 year-old girls, but not in the boys. However, the postnatal urinary BPA concentrations at the age of 9 in both genders were positively associated with the increased BMI z score, WC, and body fat and increased odds of obesity/overweight [83].
Overview of phthalates, BPA and glucose metabolism
Phthalate and BPA effects on glucose metabolism disorders are summarized in Table 2.
Phthalates and glucose metabolism
The association of some phthalate metabolites with glycemic parameters was documented in several epidemiological studies, but the data are often inconsistent.
Stahlhut et al. in the NHANES cross-section study found the higher urinary MBP, MEP and MBzP associated with increased log HOMA-IR [57]. Among the DEHP metabolites, MEHP showed lower correlation with the glycemic parameters compared to the MEHHP and MEOHP oxidized metabolites. The PIVUS results showed a correlation between MEP, MMP, MiBP and increased diabetes prevalence. Only MMP and MEP showed a significant correlation with IR. The association between MEHP and increased diabetes incidence was not found in this study [84]. Olsen et al. found a significant MiBP positive correlation and poor MEHP, MMP and MEP correlation with fasting glycemia [85]. Huang et al. using the CDC NHANES (2001 and 2008) data found a significant MnBP, MiBP, MCPP and ΣDEHP correlation with glycemia, insulinemia and IR, as well as a positive MBzP correlation with glycemia [86]. After gender and racial subgroups were formed, the significant relationship between MiBP and MBzP and insulinemia was found in female participants who otherwise had higher levels of urinary phthalate metabolites in relation to male participants. In men, there was a significant correlation between ΣDEHP and glycemia and between MCPP and IR. There were racial differences in the level and type of phthalate metabolites.
The KEEP (The Korean Elderly Environmental Panel) follow-up study 2008–2010 showed a significant correlation mole sum between MEHHP and MEOHP (ΣDEHP) and HOMA-IR [87]. This correlation was significantly higher in women than in men and in the diabetic patients compared to the non-diabetics. There was no connection between MBnP and IR. Todd et al. using NHANES study (2001 and 2008), showed that women with the highest MnBP, MiBP, MBzP, MCPP levels and the sum of three DEHP metabolites (MEHP, MEHHP and MEOHHP) had a higher probability to develop diabetes [88]. In a prospective study conducted among the US nurses only in middle-aged women the significant correlation between metabolites of butyl phthalate (MBP, MiBP, MBZP) and DEHP (MEHP, MEHHP, MECPP, MEOHP) with T2DM was found [89].
Svensson et al. found significantly higher concentrations of ΣDEHP and MECPP in the Mexican women with T2DM, but there was the correlation between ΣDEHP and IR only in the women without T2DM [90]. Hines et al. studied the American breastfeeding women and found higher MECPP concentrations in the serum and urine of the breastfeeding women with gestational diabetes [91]. Urinary DEHP metabolites (MECPP, MEHP, MEHHP and MEOHP) had a positive correlation with serum glucose, contrary to the PIVUS study [84].
The results of our study with 305 participants, aged 18–50, both genders, showed the correlation between MEP and HOMA-IR in the groups of healthy normal-weight and obese participants and T2DM patients, where only the statistically significant correlation was found in the T2DM group (r = 0.643, p = 0.018). Statistically significant correlations between MEHP and fasting glucose in T2DM group was found (r = 0.404, p = 0.030) (unpublished data).
Nowadays, because of the harmful effects on health, DEHP is increasingly replaced in products by DINP and DIDP, both HMW. Recently published data from NHANES 2009–2012 examined the correlation between LMW and HMW phthalates as well as DEHP, DIDP and DINP and IR in the adolescents [92]. The adolescents, classified as insulin resistant, had the significantly higher concentrations of HMW, DEHP and DINP metabolites. Compared the first tertile and the third tertile of the DEHP metabolites prevalence of IR was present in 20.5 and 37.7 %. Only the significant correlation between the DINP metabolites and IR was detected. Compared the first tertile and the third tertile of the DINP metabolites prevalence of IR was 23.4 and 37.7 %. Only MBP and MiBP had correlation with IR out of the LMW phthalates.
The animal study results are controversial. Martinelli et al. found a glucose tolerance disorder in the adult male Wistar rats fed a diet containing 2 % DEHP [93]. On the contrary, Feige et al. did not find the glucose metabolism disorder in the dietary DEHP-exposed mice and thus concluded that there was no DEHP influence on glucose intolerance [94]. Kwack et al. followed the short-term effects of phthalate diesters and monoesters on the male rats and showed that the glucose level was significantly higher in DEHP, MEHP MBP groups compared to the control group [67]. Harmful effects in utero exposure to phthalates was proven by Hao et al. where it was confirmed that the prenatal exposure to MEHP in mice increased blood glucose about 79 % in male offspring only and not in the female offspring [95].
BPA and glucose metabolism
A cross-section study based on NHANES 2003–2008 data reported positive association between urinary BPA levels and MetS. Although this study did not provide a direct association between BPA and disturbed glucose metabolism, glucose intolerance was one of the criteria which confirmed the association between BPA and MetS [96]. Based on NHANES 2003–2008 data positive association between BPA and T2DM after multivariate adjustments in both normal weight and overweight/obese participants was determined [49]. These data are consistent with the results reported only in NHANES 2003–2004 study where the higher urinary BPA concentrations were positively associated with T2DM [97]. Although the study of Melzer et al. which relied on the data of NHANES 2003–2006 reported no association between BPA levels in urine samples and T2DM for 2005–2006 data, an overall association was still present in the pooled data (p = 0.001) [98]. Silver et al. confirmed positive association between BPA and T2DM in the cycle NHANES 2003–2004, while no statistically significant association was found for the 2005–2006 and 2007–2008 cycles. But, the data for all three observed cycles indicated elevated glycosylated hemoglobin (HbA1c) and T2DM in the participants with the increased BPA levels. The geometric means of the urinary BPA in the observed cycles differ significantly which could influence the obtained results [50]. The study by Wang et al. reported that the fourth quartile of urinary BPA had the highest prevalence in IR after the multivariate adjustments. In the obese subgroup no significance was determined between BPA and IR, unlike with the normal weight ones [70]. The results suggested that the association between BPA and IR may be affected by BMI, since “the effect of higher BMI may overwhelm that of BPA on IR in the participants with higher BMI”. Another study observed a positive linear correlation between HbA1c and urinary BPA [99].
In the Korean National Human Biomonitoring Survey, the odds ratio of T2DM for the fourth BPA quartile was increased in comparison with the first one, but it had no statistical significance [100]. Ning et al. reported no statistically significant association between BPA and the impaired glucose regulation [101]. The odds ratios for T2DM were inconsistently and insignificantly increased in the upper urinary BPA quartiles. The main limitation of both studies was subjective approach since T2DM was self-reported. Although the NHANES 2003–2010 cycles data regarding the children found positive association between general and abdominal obesity and urinary BPA levels, no correlation was found between urinary BPA levels and IR [77].
Potential mechanism of phthalates and BPA action on obesity and glucose metabolism distrubtion
Grün and Blumberg introduced the term “obesogens” for the chemicals that directly or indirectly increase the fat accumulation and obesity incidence, among which phthalates and BPA are included [102]. The mechanisms of the action of phthalates and BPA affect obesity and glucose metabolism (Table 3.) are the result of the numerous different and insufficient in vivo and in vitro studies. Different pathways are activated by EDCs, but nuclear receptors (NR) are a primary target. In utero and non-developmental EDCs exposure may have quite different effects on these pathways [103].
Phthalates and BPA act as ligands altering NR signaling involved in the regulation of energy homeostasis and the metabolism as complete or partial agonists or antagonists. The main NR targeted by EDCs are the peroxisome proliferator-activated receptors (PPARα,γ), the androgen receptors, the estrogen receptors (ERα,β), estrogen-related receptors, the thyroid hormone receptors (TRα,β) and the pregnane X receptor (PXR) [104].
PPAR has a key role in the regulation of adipocyte differentiation and adipogenesis, lipid metabolism and glucose homeostasis by improving insulin sensitivity. The disruption of regulatory pathways controlled by PPARs may be involved in the onset of diabetes and adiposity. The activation of PPARα can lead to decrease in the fat mass and weight. The in vitro study [104] confirmed that MEHP acted on human cell lines as PPARα and PPARγ agonist while BPA had a weak influence on PPARs. Some animal studies showed a reduction in fat mass when exposed to DEHP [105], which would indicate PPARα activation. On the contrary, the epidemiological studies indicated the correlation of some phthalates (MEHP, MEP) [59] with weight-gain in their presence that suggested the mechanism of PPARγ. These differences in the phthalate mechanism on adipose tissue partly depend on the biological species, cell type and dosage which can activate different types of PPARs [105, 106]. This was also confirmed by animal studies where there was an increase in BW and white fat only on humanized, but not on wild mice after exposure to DEHP [94]. The effect of DEHP and its metabolites and DINP metabolites in increased blood glucose level and IR was demonstrated in the human and animal studies [57, 60, 107]. Although BPA is considered to have weak influence on PPARs [104] there are some evidence that BPA induces PPARγ activity [108, 109]. Both oral and injected low BPA chronic exposure, as well as low and high single dose BPA injection, induces hyperinsulinemia, hypoglycaemia, disturbed β-cell function, glucose intolerance and IR in rodents [47, 110, 111]. The in vitro studies suggested that BPA caused triglyceride content increment, enhanced lipoprotein lipase and glycerol-3-phosphate dehydrogenase activity, lipid droplets coalescence, lipid accumulation and induced differentiation of preadipocytes into adipocytes. Simultaneous activity of BPA and insulin emphasized and accelerated the adverse effects [112].
In both sexes, sexual hormones have an important role in determining the sex-dependent pattern of body fat distribution [102, 103]. Absence of androgens has obesogen effect. DEP/MEP has inconsistent, DEHP/MEHP moderate, while DBP/MBP and BBzP/MBzP have strong antiandrogen effect [103, 113, 114]. The in vitro study [104] demonstrated that butyl benzyl phthalate (BBzP) was full ERα and partial ERβ agonist, while BPA was partial ERα and ERβ agonist and full estrogen-related receptors γ (ERRγ) and PXR agonist. BPA also bound to a transmembrane ER called G protein-coupled receptor 30 (GPR30) [115, 116]. Estrogens were involved in determining the number of adult adipocytes and ERα were expressed in preadipocytes and adipocytes and ERβ antagonizes ERα mediated effects including fat reduction when co-expressed [104, 115]. Fat tissue expressed also ERRγ and GPR30 which had a significant role in regulating energy homeostasis so BPA mediated ERRγ activation might favor obesity [116–118]. PXR had an important protective role in endocrine systems from EDCs by sensing the presence of xenobiotics including EDCs and stimulating detoxification which decreased the EDCs interactions with NR. Enhanced PXR activation could increase the risk for metabolic diseases [114]. BPA was full agonist of PXP [104, 119]. Moreover, BPA in low and very high doses suppressed adiponectin release from the breast explants, subcutaneous and abdominal human fat samples more effectively than estradiol (dose-response curve was U shaped) [48].
Thyroid hormones and TSH have a significant impact on the metabolic processes and are involved in the regulation of energy homeostasis. Although the study results were inconsistent, the antagonistic effects of phthalates [120] and BPA [121] on the TR and the suppression of thyroid hormone could contribute to the development of obesity, IR and glycemic control disorders [22, 23]. Prenatal and postnatal dietary BPA exposure increased serum T4 total in the rodent offspring due to antagonizing β-TR [122].
There are also other disorders that result in obesity, IR and abnormal glucose regulation such as oxidative stress. DEHP induced ROS (reactive oxygen species) formation and lipid peroxidation, disrupted insulin signaling in adipose tissue and favored glucose intolerance [123, 124]. BPA stimulated the release of two inflammatory cytokines, IL-6 and TNFα [125]. IL-6 promoted lipolysis, suppressed adiponectin release, downregulated lipoprotein lipase activity and inhibited insulin-stimulated glucose uptake [126]. TNFα downregulated glucose transporter genes expression including Glut4, suppressed insulin receptors expression and diminished insulin sensitivity, stimulated lipolysis in concentration-dependant manner and increased the free fatty acid content [127].
There are insufficient data on how phthalates and BPA affect neuroendocrine pathways that are involved in control of appetite, obesity and glucose metabolism. Both, BPA [128] and DEHP [129] could affect neuropeptide Y (NPY) expression in the midbrain of the rats which contributed to the change in feeding behavior. Beside the influence of BPA and phthalates through classical peptidergic signals, today, little is known about their impact on the endocannabinoid system which has an orexogenic role in the hypothalamus and regulates metabolic functions and peripheral tissue [106].
In utero BPA exposure may result in obesity development via ERs. Alterations of maternal estrogen levels during gestation increased adipocyte number and impaired their function [103]. Lin et al. showed disturbed expression of key genes (Pdx-1, Ucp-2) included in the pancreatic development and β-cell function in the DEHP-treated offspring rats which could be the potential cause of T2DM in adults [130]. In female the DEHP-exposed offsprings increased blood glucose, decreased serum insulin, impaired glucose tolerance and insulin secretion were observed in adult age, while in the male DEHP-exposed offsprings only elevated serum insulin levels were detected. These and other studies showed that prenatal and perinatal phthalate exposure might induce β-cell disfunction and glucose metabolism disturbance [130, 131]. Prenatal and perinatal BPA exposure of the Wistar rats increased BW, elevated serum insulin and glucose intolerance of the offsprings, both on normal and HFD with accompanied dyslipidemia, hyperleptindemia and glucose intolerance. Regardless of the applied diet, BPA exposure reduced Pdx-1 levels which could be responsible for diminished insulin secretion, decreased Glut2 expression and structural abnormalities in the pancreatic β-cells. The adverse effects were detected only when the offsprings were exposed to the lowest dose of BPA and multiplied by HFD [132]. Prenatal and perinatal BPA exposure of rodents resulted in obesity development, hyperlipidemia and IR in the offsprings [133–135]. Moreover, BPA may cause multigenerational glucose metabolism disruption [136].
Conclusion
The role of some phthalates and BPA in the development of obesity and T2DM is indisputable, although the results of previous studies have partially been inconsistent due to study design (cross-sectional), population, gender and racial differences, but also due to the use of different methodologies.
The necessary task of the future well-designed, follow-up prospective studies is to evaluate prenatal and neonatal exposure to the individual phthalates and BPA in a better way, but at the same time, the exposure (mixture of exposures) to other EDCs. In regard to the mechanisms of action, beside the influence on the NR, it is necessary to evaluate other endocrine signaling pathways. All of these studies should contribute to the better understanding of the harmful effects of phthalate and BPA and this would lead to better prevention of the development of obesity and T2DM.
References
World Health Organization, Obesity and overweight. (2015), http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed 03 Mar 2016
S.D. De Ferranti, S.K. Osganian, Epidemiology of pediatric metabolic syndrome and type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 4, 285–296 (1995)
G.A. Bray, T. Bellanger, Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine 29, 109–117 (2006)
P. Almeda-Valdes, C.A. Aguilar-Salinas, M. Uribe, S.C. Quinteros, N. Méndez-Sánchez, Impact of anthropometric cutoff values in determining the prevalence of metabolic alterations. Eur. J. Clin. Investig. (2016). doi:10.1111/eci.12672
A. De Lorenzo, L. Soldati, F. Sarlo, M. Calvani, N. Di Lorenzo, L. Di Renzo, New obesity classification criteria as a tool for bariatric surgery indication. World J. Gastroenterol. (2016). 10.3748/wjg.v22.i2.681
L. Guariguata, D.R. Whiting, I. Hambleton, J. Beagley, V. Linnenkamp, J.E. Show, IDF Atlas. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. (2014). doi:10.1016/j.diabres.2013.11.002
R.B. Rosenbloom, J.R. Joe, R.S. Young, W.E. Winter, Emerging epidemic of type 2 diabetes in youth. Diabetes Care 22, 345–354 (1999)
E. Diamanti-Kandarakis, J.P. Bourguignon, L.C. Giudice, R. Hauser, G.S. Prins, A.M. Soto, R.T. Zoeller, A.C. Gore, Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. (2009). doi:10.1210/er.2009-0002
International Program for Chemical Safety (IPCS) in Global assessment of the state-of-the-science of endocrine disruptors Chapter 1, Executive Summary. (2010), http://www.who.int/ipcs/publications/en/ch1.pdf. Accessed 03 Mar 2016
L.N. Vandenberg, T. Colborn, T.B. Hayes, J.J. Heindel, D.R. Jacobs Jr., D.H. Lee, T. Shioda, A.M. Soto, F.S. vom Saal, W.V. Welshons, R.T. Zoeller, J.P. Myers, Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. (2012). doi:10.1210/er.2011-1050
I. Bajkin, A. Bjelica, T. Icin, V. Dobric, B.K. Zavisic, M.M. Stojanoska, Effects of phthalic acid esters on fetal health. Med. Pregl. 67, 172–175 (2014)
A.C. Gore, V.A. Chappell, S.E. Fenton, J.A. Flaws, A. Nadal, G.S. Prins, J. Toppari, R.T. Zoeller, Executive summary to EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. (2015). doi:10.1210/er.2015-1010
T. Suzuki, K. Yaguchi, S. Suzuki, T. Suga, Monitoring of phthalic acid monoesters in river water by solid-phase extraction and GC-MS determination. Environ. Sci. Technol. 35, 3757–3763 (2001)
A.O. Earls, I.P. Axford, J.H. Braybrook, Gas chromatography-mass spectrometry determination of the migration of phthalate plasticisers from polyvinyl chloride toys and childcare articles. J. Chromatogr. A 983, 237–246 (2003)
J. Bosnir, D. Puntaric, A. Galic, I. Skes, T. Dijanic, M. Klaric, M. Grgic, M. Curkovic, Z. Smit, Migration of phthalates from plastic containers into soft drinks and mineral water. Food Technol. Biotechnol. 45, 91–95 (2007)
CDC (Centers for Disease Control and Prevention) Fourth National Report on Human Exposure to Environmental Chemicals, Updates tables.(2015). http://www.cdc.gov/exposurereport/. Accessed 03 March 2016
D.W. Liang, T. Zhang, H.H. Fang, J. He, Phthalates biodegradation in the environment. Appl. Microbiol. Biotechnol. (2008). doi:10.1007/s00253-008-1548-5
Lowell Center for Sustainable Production UI. Phthalates and their Alternatives. Health and Environmental Concerns. Lowell Center for Sustainable Production, University of Massachusetts, Lowell (2011). http://www.sustainableproduction.org/downloads/DEHP%20Full%20Text.pdf. Accessed 03 Mar 2016
R. Mankidy, S. Wiseman, H. Ma, J.P. Giesy, Biological impact of phthalates. Toxicol. Lett. (2013). doi:10.1016/j.toxlet.2012.11.025
H. Fredricksen, N.E. Skakkebaek, A.M. Andersson, Metabolism of phthalates in humans. Mol. Nutr. Food Res. 51, 899–911 (2007)
ATSDR, Toxicological profile for di-(2-ethylhexyl) phthalate (DEHP). Atlanta: Agency for toxic substances and disease registry. http://www.atsdr.cdc.gov/toxprofiles Accessed 03 Mar 2016
M. Medic Stojanoska, B. Vukovic, J. Novakovic Paro, I. Bajkin, T. Icin, N. Milic, A. Milankov, B. Kovacev Zavisic, Association between urinary phthalate metabolites and diabetes mellitus: a pilot study. Diabetologia. 56, pS164 (2013)
H.D. Duntas, Chemical contamination and the thyroid. Endocrine. (2015). doi:10.1007/s12020-014-0442-4
M.J. Silva, D.B. Barr, J.A. Reidy, N.A. Malek, C.C. Hodge, S.P. Caudil, W.J. Brock, L.L. Needham, A.M. Calafat, Urinary levels of seven phthalate metabolites in the U.S population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Health Perspect. 112, 331–338 (2004)
World Health Organization (WHO), Guidelines for Drinking Water Quality. Chapter 8: Chemical Aspects. 4th edn. Geneva: World Health Organization (2011). http://whqlibdoc.who.int Accessed 05 February 2012
US Environmental Protection Agency, Water: basic information about regulated drinking water contaminants. http://water.epa.gov/drink/contaminants/basicinformation/di_2_ethyl_phthalate.com. Accessed 03 Mar 2016
ECHA, Evaluation of new scientific evidence concerning the restrictions contained in Annex XVII to regulation (EC) № 1907/2006 (REACH). http://www.echa.europa.eu. Accessed 03 Mar 2016
J. Legler, T. Fletcher, E. Govarts, M. Porta, B. Blumberg, J.J. Heindel, L. Trasande, Obesity, diabetes, and associated costs of exposure to endocrine-disrupting chemicals in the European union. J. Clin. Endocrinol. Metab. (2015). doi:10.1210/jc.2014-4326
K.E. Zimmer, A.C. Gutleb, S. Ravnum, M. Krayer von Krauss, A.J. Murk, E. Ropstad, J.U. Skaare, G.S. Eriksen, J.L. Lyche, J.G. Koppe, B.L. Magnanti, A. Yang, A. Bartonova, H. Keune, Policy relevant results from an expert elicitation on the health risks of phthalates. Environ. Health (2012). doi:10.1186/1476-069X-11-S1-S6
A. Bergman, G. Becher, B. Blumberg, P. Bjerregaard, R. Bornman, I. Brandt, S.C. Casey, H. Frouin, L.C. Giudice, J.J. Heindel, T. Iguchi, S. Jobling, K.A. Kidd, A. Kortenkamp, P.M. Lind, D. Muir, R. Ocheing, E. Ropstad, P.S. Ross, N.E. Skakkebeak, J. Toppari, L.N. Vandenberg, T.J. Woodruff, R.T. Zoller, Disrupter science—a rebuttal of industry-sponsored critical comments on the UNEP/WHO report “State of the Science of Endocrine Disrupting Chemicals 2012”. Regul. Toxicol. Pharmacol. (2015). doi:10.1016/j.yrtph.2015.07.026
T. Göen, L. Dobler, J. Koschorreck, J. Müller, G.A. Wiesmüller, H. Drexler, M. Kolossa-Gehring, Trends of the internal phthalate exposure of young adults in Germany—follow-up of a retrospective human biomonitoring study. Int. J. Hyg. Environ. Health (2011). doi:10.1016/j.ijheh.2011.07.011
A.R. Zota, A.M. Calafat, T.J. Woodruff, Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ. Health Perspect. (2014). doi:10.1289/ehp.13066811
T. Geens, D. Aerts, C. Berthot, J.P. Bourguignon, L. Goeyens, P. Lecomte, G. Maghuin-Rogister, A.M. Pironnet, L. Pussemier, M.L, Scippo, J. Van Loco, A. Covaci, A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. (2012). doi:10.1016/j.fct.2012.07.059
L.N. Vandenberg, M.V. Maffini, C. Sonnenschein, B.S. Rubin, A.M. Soto, Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr. Rev. 30, 75–95 (2009). doi:10.1210/er.2008-0021
J. Corrales, L.A. Kristofco, W.B. Steele, B.S. Yates, C.S. Breed, E.S. Williams, B.W. Brooks, Global assessment of bisphenol A in the environment: review and analysis of its occurrence and bioaccumulation. Dose-Response 13, 1–29 (2015)
W. Völkel, T. Colnot, G.A. Csanády, J.G. Filser, W. Dekant, Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem. Res. Toxicol. 15, 1281–1287 (2002)
L.N. Vandenberg, R. Hauser, M. Marcus, N. Olea, W.V. Welshons, Human exposure to bisphenol A (BPA). Reprod. Toxicol. 24, 139–177 (2007)
R.W. Stahlhut, W.V. Welshons, S.H. Swan, Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ. Health. Perspect. 117, 784–789 (2009). doi:10.1289/ehp.0800376
A.M. Calafat, X. Ye, L.Y. Wong, J.A. Reidy, L.L. Needham, Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. (2008). doi:10.1289/ehp.10753
D. Caserta, G. Bordi, F. Ciardo, R. Marci, C. La Rocca, S. Tait, B. Bergamasco, L. Stecca, A. Mantovani, C. Guerranti, E.L. Fanello, G. Perra, F. Borghini, S.E. Focardi, M. Moscarini, The influence of endocrine disruptors in a selected population of infertile women. Gynecol. Endocrinol. (2013). doi:10.3109/09513590.2012.758702
T. Takeuchi, O. Tsutsumi, Serum bisphenol A concentrations showed gender differences, possibly linked to androgen levels. Biochem. Biophys. Res. Commun. 291, 76–78 (2002)
E.S. Barrett, M. Sobolewski, Polycystic ovary syndrome: do endocrine disrupting chemicals play a role? Semin. Reprod. Med. (2014). doi:10.1055/s-0034-1371088
J.D. Meeker, A.M. Calafat, R. Hauser, Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ. Sci. Technol. (2010). doi:10.1021/es9028292
D. Li, Z. Zhou, D. Qing, Y. He, T. Wu, M. Miao, J. Wang, X. Weng, J.R. Ferber, L.J. Herrinton, Q. Zhu, E. Gao, H. Checkoway, W. Yuan, Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum. Reprod. (2010). doi:10.1093/humrep/dep381
J.D. Meeker, S. Ehrlich, T.L. Toth, D.L. Wright, A.M. Calafat, A.T. Trisini, X. Ye, R. Hauser, Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod. Toxicol. (2010). doi:10.1016/j.reprotox.2010.07.005
M. Miao, W. Yuan, Y. He, Z. Zhou, J. Wang, E. Gao, G. Li, D.K. Li, In utero exposure to bisphenol-A and anogenital distance of male offspring. Birth Defects Res. A Clin. Mol. Teratol. (2011). doi:10.1002/bdra.22845
A.B. Ropero, P. Alonso-Magdalena, E. García-García, C. Ripoll, E. Fuentes, A. Nadal, Bisphenol-A disruption of the endocrine pancreas and blood glucose homeostasis. Int. J. Androl. 31, 194–200 (2008)
E.R. Hugo, T.D. Brandebourg, J.G. Woo, J. Loftus, J.W. Alexander, N. Ben-Jonathan, Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ. Health Perspect. (2008). doi:10.1289/ehp.11537
A. Shankar, S. Teppala, Relationship between urinary bisphenol A levels and diabetes mellitus. J. Clin. Endocrinol. Metab. (2011). doi:10.1210/jc.2011-1682
M.K. Silver, M.S. O’Neill, M.R. Sowers, S.K. Park, Urinary bisphenol A and type-2 diabetes in U.S. adults: data from NHANES 2003-2008. PLoS One. (2011). doi:10.1371/journal.pone.0026868
A. Shankar, S. Teppala, Urinary bisphenol A and hypertension in a multiethnic sample of US adults. J. Environ. Public Health 2012, 481641 (2012)
A. Shankar, S. Teppala, C. Sabanayagam, Bisphenol a and peripheral arterial disease: results from the NHANES. Environ. Health Perspect. (2012). doi:10.1289/ehp.1104114
J.R. Rochester, Bisphenol A and human health: a review of the literature. Reprod. Toxicol. (2013). doi:10.1016/j.reprotox.2013.08.008
C.M. Metz, Bisphenol A: understanding the controversy. Workplace Health Saf. (2016). doi:10.1177/2165079915623790
European Food Safety Association, Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015;13(1):3978. doi:10.2903/j.efsa.2015.3978, http://www.efsa.europa.eu/en/topics/topic/bisphenol. Accessed 03 Mar 2016
P.F. Baillie-Hamilton, Chemical toxins: a hypothesis to explain global obesity epidemic. J. Altern. Complement. Med. 8, 185–192 (2002)
R.W. Stahlhut, E. van Wijngaarden, T.D. Dye, S. Cook, S.H. Swan, Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ. Health Perspect. 115, 876–882 (2007)
E.E. Hatch, J.W. Nelson, M.M. Qureshi, J. Weinberg, L.L. Moore, M. Singer, T.F. Webster, Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ. Health (2008). doi:10.1186/1476-069X-7-27
M. Medic Stojanoska, A. Milankov, B. Vukovic, D. Vukcevic, J. Sudji, I. Bajkin, N. Curic, T. Icin, B. Kovacev Zavisic, N. Milic, Do diethyl phthalate (DEP) and di-2-ethylhexyl phthalate (DEHP) influence the metabolic syndrome parameters? Pilot study. Environ. Monit. Assess. (2015). doi:10.1007/s10661-015-4754-5
P.M. Lind, V. Roos, M. Rönn, L. Johansson, H. Ahlström, J. Kullberg, L. Lind, Serum concentrations of phthalate metabolites are related to abdominal fat distribution two years later in elderly women. Environ. Health (2012). doi:10.1186/1476-069X-11-21
Y. Song, R. Hauser, F.B. Hu, A.A. Franke, S. Liu, Q. Sun, Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int. J. Obes. (Lond.) (2014). doi:10.1038/ijo.2014.63
S.L. Teitelbaum, N. Mervish, E.L. Moshier, N. Vangeepuram, M.P. Galvez, A.M. Calafat, M.J. Silva, B.L. Brenner, M.S. Wolff, Associations between phthalate metabolite urinary concentrations and body size measures in New York city children. Environ. Res. (2012). doi:10.1016/j.envres.2011.12.006
A. Smerieri, C. Testa, P. Lazzeroni, F. Nuti, E. Grossi, S. Cesari, L. Montanini, G. Latini, S. Bernasconi, A.M. Papini, M.E. Street, Di-(2-ethylhexyl) phthalate metabolites in urine show age-related changes and associations with adiposity and parameters of insulin sensitivity in childhood. PLoS One (2015). doi:10.1371/journal.pone.0117831
C. Philippat, M. Mortamais, C. Chevrier, C. Petit, A.M. Calafat, X. Ye, M.J. Silva, C. Brambilla, I. Pin, M.A. Charles, S. Cordier, R. Slama, Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ. Health Perspect. (2012). doi:10.1289/ehp.1103634
J.H. Kim, H. Park, J. Lee, G. Cho, S. Choi, G. Choi, S.Y. Kim, S.H. Eun, E. Suh, S.K. Kim, H.J. Kim, G.H. Kim, J.J. Lee, Y.D. Kim, S. Eom, S. Kim, S. Kim, Association of diethylhexyl phthalate with obesity-related markers and body mass change from birth to 3 months of age. J. Epidemiol. Community Health (2016). doi:10.1136/jech-2015-206315
J. Ashley-Martin, L. Dodds, T.E. Arbuckle, A.S. Ettinger, G.D. Shapiro, M. Fisher, A.S. Morisset, S. Taback, M.F. Bouchard, P. Monnier, R. Dallaire, W.D. Fraser, A birth cohort study to investigate the association between prenatal phthalate and bisphenol A exposures and fetal markers of metabolic dysfunction. Environ. Health (2014). doi:10.1186/1476-069X-13-84
S.J. Kwack, E.Y. Han, J.S. Park, J.Y. Bae, I.Y. Ahn, S.K. Lim, D.H. Kim, D.E. Jang, L. Choi, H.J. Lim, T.H. Kim, N. Patra, K.L. Park, H.S. Kim, B.M. Lee, Comparison of the short term toxicity of phthalate diesters and monoesters in sprague-dawley male rats. Toxicol. Res. (2010). doi:10.5487/TR.2010.26.1.075
J.L. Carwile, K.B. Michels, Urinary bisphenol A and obesity: NHANES 2003–2006. Environ. Res. (2011). doi:10.1016/j.envres.2011.05.014
A. Shankar, S. Teppala, C. Sabanayagam, Urinary bisphenol a levels and measures of obesity: results from the national health and nutrition examination survey 2003–2008. ISRN Endocrinol. (2012). doi:10.5402/2012/965243
T. Wang, M. Li, B. Chen, M. Xu, Y. Xu, Y. Huang, J. Lu, Y. Chen, W. Wang, X. Li, Y. Liu, Y. Bi, S. Lai, G. Ning, Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J. Clin. Endocrinol. Metab. (2012). doi:10.1210/jc.2011-1989
T. Takeuchi, O. Tsutsumi, Y. Ikezuki, Y. Takai, Y. Taketani, Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr. J. 51, 165–169 (2004)
N. Milić, D. Četojević-Simin, M. Milanović, J. Sudji, N. Milošević, N. Ćurić, L. Abenavoli, Medić-Stojanoska, M., Estimation of in vivo and in vitro exposure to bisphenol A as food contaminant. Food Chem. Toxicol. (2015). doi:10.1016/j.fct.2015.07.003
H.Y. Zhao, Y.F. Bi, L.Y. Ma, L. Zhao, T.G. Wang, L.Z. Zhang, B. Tao, L.H. Sun, Y.J. Zhao, W.Q. Wang, X.Y. Li, M.Y. Xu, J.L. Chen, G. Ning, J.M. Liu, The effects of bisphenol A (BPA) exposure on fat mass and serum leptin concentrations have no impact on bone mineral densities in non-obese premenopausal women. Clin. Biochem. (2012). doi:10.1016/j.clinbiochem.2012.08.024
T. Bushnik, D. Haines, P. Levallois, J. Levesque, J. Van Oostdam, C. Viau, Lead and bisphenol A concentrations in the Canadian population. Health Rep. 21, 7–18 (2010)
R. Bhandari, J. Xiao, A. Shankar, Urinary bisphenol A and obesity in U.S. children. Am. J. Epidemiol. (2013). doi:10.1093/aje/kws391
L. Trasande, T.M. Attina, J. Blustein, Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 308, 1113–1121 (2012)
D.S. Eng, J.M. Lee, A. Gebremariam, J.D. Meeker, K. Peterson, V. Padmanabhan, Bisphenol A and chronic disease risk factors in US children. Pediatrics. 132, e637–e645 (2013)
D.K. Li, M. Miao, Z. Zhou, C. Wu, H. Shi, X. Liu, S. Wang, W. Yuan, Urine bisphenol-A level in relation to obesity and overweight in school-age children. PLoS One (2013). doi:10.1371/journal.pone.0065399s
H.X. Wang, Y. Zhou, C.X. Tang, J.G. Wu, Y. Chen, Q.W. Jiang, Association between bisphenol A exposure and body mass index in Chinese school children: a cross-sectional study. Environ. Health (2012). doi:10.1186/1476-069X-11-79
J. Xue, Q. Wu, S. Sakthivel, P.V. Pavithran, J.R. Vasukutty, K. Kannan, Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ. Res. (2015). doi:10.1016/j.envres.2014.12.007
M.S. Wolff, S.L. Teitelbaum, G. Windham, S.M. Pinney, J.A. Britton, C. Chelimo, J. Godbold, F. Biro, L.H. Kushi, C.M. Pfeiffer, A.M. Calafat, Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ. Health Perspect. 115, 116–121 (2007)
W.C. Chou, J.L. Chen, C.F. Lin, Y.C. Chen, F.C. Shih, C.Y. Chuang, Biomonitoring of bisphenol A concentrations in maternal and umbilical cord blood in regard to birth outcomes and adipokine expression: a birth cohort study in Taiwan. Environ. Health (2011). doi:10.1186/1476-069X-10-94
K.G. Harley, R. Aguilar Schall, J. Chevrier, K. Tyler, H. Aguirre, A. Bradman, N.T. Holland, R.H. Lustig, A.M. Calafat, B. Eskenazi, Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ. Health Perspect. (2013). doi:10.1289/ehp.1205548
P.M. Lind, B. Zethelius, L. Lind, Circulating levels of phthalate metabolites are associated with prevalent diabetes in the elderly. Diabetes Care (2012). doi:10.2337/dc11-2396
L. Olsén, L. Lind, P.M. Lind, Associations between circulating levels of bisphenol A and phthalate metabolites and coronary risk in the elderly. Ecotoxicol. Environ. Saf. (2012). doi:10.1016/j.ecoenv.2012.02.023
T. Huang, A.R. Saxena, E. Isganaitis, T. James-Todd, Gender and racial/ethnic differences in the associations of urinary phthalate metabolites with markers of diabetes risk: national health and nutrition examination survey 2001–2008. Environ. Health (2014). doi:10.1186/1476-069X-13-6
J.H. Kim, H.Y. Park, S. Bae, Y.H. Lim, Y.C. Hong, Diethylhexyl phthalates is associated with insulin resistance via oxidative stress in the elderly, a panel study. PLoS One (2013). doi:10.1371/journal.pone.0071392
T. James-Todd, R. Stahlhut, J.D. Meeker, S.G. Powell, R. Hauser, T. Huang, J. Rich-Edwards, Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ. Health Perspect. (2012). doi:10.1289/ehp.11047177
Q. Sun, M.C. Cornelis, M.K. Townsend, D.K. Tobias, A.H. Eliassen, A.A. Franke, R. Hauser, F.B. Hu, Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ. Health Perspect. (2014). doi:10.1289/ehp.1307201
K. Svensson, R.U. Hernández-Ramírez, A. Burguete-García, M.E. Cebrián, A.M. Calafat, L.L. Needham, L. Claudio, L. López-Carrillo, Phthalate exposure associated with self-reported diabetes among Mexican women. Environ. Res. (2011). doi:10.1016/j.envres.2011.05.015
E.P. Hines, A.M. Calafat, M.J. Silva, P. Mendola, S.E. Fenton, Concentrations of phthalate metabolites in milk, urine, saliva, and serum of lactating North Carolina women. Environ. Health Perspect. (2009). doi:10.1289/ehp.11610
T.M. Attina, L. Trasande, Association of exposure to di-2-ethylhexylphthalate replacements with increased insulin resistance in adolescents from NHANES 2009-2012. J. Clin. Endocrinol. Metab. (2015). doi:10.1210/jc.2015-1686
M.I. Martinelli, N.O. Mocchiutti, C.A. Bernal, Dietary di(2-ethylhexyl)phthalate-impaired glucose metabolism in experimental animals. Hum. Exp. Toxicol. 25, 531–538 (2006)
J.N. Feige, A. Gerber, C. Casals-Casas, Q. Yang, C. Winkler, E. Bedu, M. Bueno, L. Gelman, J. Auwerx, F.J. Gonzalez, B. Desvergne, The pollutant diethylhexyl phthalate regulates hepatic energy metabolism via species-specific PPARalpha-dependent mechanisms. Environ. Health Perspect. (2010). doi:10.1289/ehp.0901217
C. Hao, X. Cheng, H. Xia, X. Ma, The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci. Rep. (2012). doi:10.1042/BSR20120042
S. Teppala, S. Madhavan, A. Shankar, Bisphenol A and metabolic syndrome: Results from NHANES. Int. J. Endocrinol. 2012, 598180 (2012). Article ID
I.A. Lang, T.S. Galloway, A. Scarlett, W.E. Henley, M. Depledge, R.B. Wallace, D. Melzer, Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA (2008). doi:10.1001/jama.300.11.1303
D. Melzer, N.E. Rice, C. Lewis, W.E. Henley, T.S. Galloway, Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS One (2010). doi:10.1371/journal.pone.0008673
R. Ahmadkhaniha, M. Mansouri, M. Yunesian, K. Omidfar, M.Z. Jeddi, B. Larijani, A. Mesdaghinia, N. Rastkari, Association of urinary bisphenol a concentration with type-2 diabetes mellitus. J. Environ. Health Sci. Eng. (2014). doi:10.1186/2052-336X-12-64
K. Kim, H. Park, Association between urinary concentrations of bisphenol A and type 2 diabetes in Korean adults: a population-based cross-sectional study. Int. J. Hyg. Environ. Health (2013). doi:10.1016/j.ijheh.2012.07.007
G. Ning, Y. Bi, T. Wang, M. Xu, Y. Xu, Y. Huang, M. Li, X. Li, W. Wang, Y. Chen, Y. Wu, J. Hou, A. Song, Y. Liu, S. Lai, Relationship of urinary bisphenol A concentration to risk for prevalent type 2 diabetes in Chinese adults: a cross-sectional analysis. Ann. Intern. Med. (2011). doi:10.7326/0003-4819-155-6-201109200-00005
F. Grün, B. Blumberg, Endocrine disrupters as obesogens. Mol. Cell. Endocrinol. (2009). doi:10.1016/j.mce.2009.02.018
E.E. Hatch, J.W. Nelson, R.W. Stahlhut, T.F. Webster, Association of endocrine disruptors and obesity: perspectives from epidemiological studies. Int. J. Androl. (2010). doi:10.1111/j.1365-2605.2009.01035.x
M. Grimaldi, A. Boulahtouf, V. Delfosse, E. Thouennon, W. Bourguet, P. Balaguer, Reporter cell lines for the characterization of the interactions between human nuclear receptors and endocrine disruptors. Front. Endocrinol. (Lausanne) (2015). doi:10.3389/fendo.2015.00062
J.R. Barrett, To each his own: DEHP yields species-specific metabolic phenotypes. Environ. Health Perspect. (2010). doi:10.1289/ehp.118-a81a
B. Migliarini, C.C. Piccinetti, A. Martella, F. Maradonna, G. Gioacchini, O. Carnevali, Perspectives on endocrine disruptor effects on metabolic sensors. Gen. Comp. Endocrinol. (2011). doi:10.1016/j.ygcen.2010.11.025
J. Boberg, S. Metzdorff, R. Wortziger, M. Axelstad, L. Brokken, A.M. Vinggaard, M. Dalgaard, C. Nellemann, Impact of diisobutyl phthalate and other PPAR agonists on steroidogenesis and plasma insulin and leptin levels in fetal rats. Toxicology (2008). doi:10.1016/j.tox.2008.05.020
J. Kwintkiewicz, Y. Nishi, T. Yanase, L.C. Giudice, Peroxisome proliferator-activated receptor-gamma mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ. Health Perspect. (2010). doi:10.1289/ehp.0901161
A. Riu, M. Grimaldi, A. le Maire, G. Bey, K. Phillips, A. Boulahtouf, E. Perdu, D. Zalko, W. Bourguet, P. Balaguer, Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ. Health Perspect. (2011). doi:10.1289/ehp.1003328
P. Alonso-Magdalena, S. Morimoto, C. Ripoll, E. Fuentes, A. Nadal, The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ. Health. Perspect. 114, 106–112 (2006)
M.K. Moon, I.K. Jeong, T. Jung Oh, H.Y. Ahn, H.H. Kim, Y.J. Park, H.C. Jang, K.S. Park, Long-term oral exposure to bisphenol A induces glucose intolerance and insulin resistance. J. Endocrinol. (2015). doi:10.1530/JOE-14-0714
H. Masuno, T. Kidani, K. Sekiya, K. Sakayama, T. Shiosaka, H. Yamamoto, K. Honda, Bisphenol A in combination with insulin can accelerate the conversion of 3T3-L1 fibroblasts to adipocytes. J. Lipid. Res. 43, 676–684 (2002)
S.H. Swan, Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ. Res. 108, 177–184 (2008)
N.K. Chaturvedi, S. Kumar, S. Negi, R.K. Tyagi, Endocrine disruptors provoke differential modulatory responses on androgen receptor and pregnane and xenobiotic receptor:potential implications in metabolic disorders. Mol. Cell. Biochem. (2010). doi:10.1007/s11010-010-0583-6
F.S. Vom Saal, S.C. Nagel, B.L. Coe, B.M. Angle, J.A. Taylor, The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol. Cell. Endocrinol. (2012). doi:10.1016/j.mce.2012.01.001
P. Thomas, J. Dong, Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 102, 175–179 (2006)
H. Okada, T. Tokunaga, X. Liu, S. Takayanagi, A. Matsushima, Y. Shimohigashi, Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma. Environ. Health Perspect. (2008). doi:10.1289/ehp.10587
É. Audet-Walsh, V. Giguére, The multiple universes of estrogen-related receptor α and γ in metabolic control and related diseases. Acta Pharmacol. Sin. (2015). doi:10.1038/aps.2014.121
Y. Sui, N. Ai, S.H. Park, J. Rios-Pilier, J.T. Perkins, W.J. Welsh, C. Zhou, Bisphenol A and its analogues activate human pregnane X receptor. Environ. Health Perspect. (2012). doi:10.1289/ehp.1104426
K. Ibhazehiebo, N. Koibuchi, Thyroid hormone receptor-mediated transcription is suppressed by low dose phthalate. Niger. J. Physiol. Sci. 26, 143–149 (2006)
Z.G. Sheng, Y. Tang, Y.X. Liu, Y. Yuan, B.Q. Zhao, X.J. Chao, B.Z. Zhu, Low concentrations of bisphenol a suppress thyroid hormone receptor transcription through a nongenomic mechanism. Toxicol. Appl. Pharmacol. (2012). doi:10.1016/j.taap.2011.12.018
R.T. Zoeller, R. Bansal, C. Parris, Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 146, 607–612 (2005)
P. Rajesh, S. Sathish, C. Srinivasan, J. Selvaraj, K. Balasubramanian, Phthalate is associated with insulin resistance in adipose tissue of male rat: role of antioxidant vitamins. J. Cell Biochem. (2013). doi:10.1002/jcb.24399
W. Wang, Z.R. Craig, M.S. Basavarajappa, K.S. Hafner, J.A. Flaws, Mono-(2ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol. Reprod. (2012). doi:10.1095/biolreprod.112.102467
N. Ben-Jonathan, E.R. Hugo, T.D. Brandebourg, Effects of bisphenol A on adipokine release from human adipose tissue: implications for the metabolic syndrome. Mol. Cell. Endocrinol. (2009). doi:10.1016/j.mce.2009.02.022
D. Kamimura, K. Ishihara, T. Hirano, IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev. Physiol. Biochem. Pharmacol. 149, 1–38 (2003)
M. Rydén, P. Arner, Tumour necrosis factor-alpha in human adipose tissue—from signalling mechanisms to clinical implications. J. Intern. Med. 262, 431–438 (2007)
Y. Masuo, M. Ishido, M. Morita, S. Oka, Effects of neonatal treatment with 6-hydroxydopamine and endocrine disruptors on motor activity and gene expression in rats. Neural Plast. 11, 59–76 (2004)
S. Singh, S.S. Li, Phthalates: toxicogenomics and inferred human diseases. Genomics (2011). doi:10.1016/j.ygeno.2010.11.008
Y. Lin, J. Wei, Y. Li, J. Chen, Z. Zhou, L. Song, Z. Wei, Z. Lv, X. Chen, W. Xia, S. Xu, Developmental exposure to di(2-ethylhexyl) phthalate impairs endocrine pancreas and leads to long-term adverse effects on glucose homeostasis in the rat. Am. J. Physiol. Endocrinol. Metab. (2011). doi:10.1152/ajpendo.00233.2011
W.J. Crinnion, Toxic effects of the easily avoidable phthalates and parabens. Altern. Med. Rev. 15, 190–196 (2010)
J. Wei, Y. Lin, Y. Li, C. Ying, J. Chen, L. Song, Z. Zhou, Z. Lv, W. Xia, X. Chen, S. Xu, Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology. (2011). doi:10.1210/en.2011-0045
J. Miyawaki, K. Sakayama, H. Kato, H. Yamamoto, H. Masuno, Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J. Atheroscler. Thromb. 14, 245–252 (2007)
E. Somm, V.M. Schwitzgebel, A. Toulotte, C.R. Cederroth, C. Combescure, S. Nef, M.L. Aubert, P.S. Hüppi, Perinatal exposure to bisphenol A alters early adipogenesis in the rat. Health Perspect. (2009). doi:10.1289/ehp.11342
B.M. Angle, R.P. Do, D. Ponzi, R.W. Stahlhut, B.E. Drury, S.C. Nagel, W.V. Welshons, C.L. Besch-Williford, P. Palanza, S. Parmigiani, F.S. vom Saal, J.A. Taylor, Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod. Toxicol. (2013). doi:10.1016/j.reprotox.2013.07.0177
G. Li, H. Chang, W. Xia, Z. Mao, Y. Li, S. Xu, F0 maternal BPA exposure induced glucose intolerance of F2 generation through DNA methylation change in Gck. Toxicol. Lett. (2014). doi:10.1016/j.toxlet.2014.04.012
Funding
This article was funded by the project No. 175033 by the Ministry of Education, Science and Technological Development of the Republic of Serbia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Stojanoska, M.M., Milosevic, N., Milic, N. et al. The influence of phthalates and bisphenol A on the obesity development and glucose metabolism disorders. Endocrine 55, 666–681 (2017). https://doi.org/10.1007/s12020-016-1158-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-1158-4