Abstract
Neck circumference is a new anthropometric index for estimating obesity. We aimed to determine the relationship between neck circumference and body fat content and distribution as well as the efficacy of neck circumference for identifying visceral adiposity and metabolic disorders. A total of 1943 subjects (783 men, 1160 women) with a mean age of 58 ± 7 years were enrolled in this cross-sectional study. Metabolic syndrome was defined according to the standard in the 2013 China Guideline. Analyses were conducted to determine optimal neck circumference cutoff points for visceral adiposity quantified by magnetic resonance imaging, and to compare the performance of neck circumference with that of waist circumference in identifying abdominal obesity and metabolic disorders. Visceral fat content was independently correlated with neck circumference. Receiver operating characteristic curves showed that the area under the curve for the ability of neck circumference to determine visceral adiposity was 0.781 for men and 0.777 for women. Moreover, in men a neck circumference value of 38.5 cm had a sensitivity of 56.1 % and specificity of 83.5 %, and in women, a neck circumference value of 34.5 cm had a sensitivity of 58.1 % and specificity of 82.5 %. These values were the optimal cutoffs for identifying visceral obesity. There were no statistically significant differences between the proportions of metabolic syndrome and its components identified by an increased neck circumference and waist circumference. Neck circumference has the same power as waist circumference for identifying metabolic disorders in a Chinese population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome (MetS) is characterized by a combination of several cardiovascular risk factors, and abdominal obesity is considered as a core component of MetS. Growing evidence has confirmed the pathogenic role of ectopic fat, with visceral fat accumulation inducing insulin resistance, and accelerating a series of metabolic disorders, such as hypertension and glucolipid metabolic disorders. Moreover, excessive visceral adiposity is associated with atherosclerosis development and incident cardiovascular disease [1, 2]. Therefore, it is important to find out reliable indices to evaluate visceral adiposity [3].
Currently, waist circumference (WC) is the most commonly used simple anthropometric indicator for evaluating abdominal adiposity in both clinical and large-scale epidemiological investigations. WC may be associated with both intra-abdominal and subcutaneous adipose tissue, however, studies found that WC was the best surrogate of visceral adiposity compared with other simple anthropometric measurements [4, 5]. Waist measurement would continue to be a useful screening tool to evaluate visceral fat content proposed by different organizations [6]. Notably, the clinically recognized definitions of abdominal obesity by the International Diabetes Federation (IDF) [7] and the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) [8] clearly note that WC was measured neither at the horizontal plane of the navel nor at the maximal circumference of waist, but should be measured at the horizontal plane between the inferior costal margin and the iliac crest on the mid-axillary line. Therefore, the anatomic landmark of waist measurement is not so obvious and could vary greatly especially in obese individuals, and being easily affected by diet, respiratory, or health conditions. Additionally, WC should be measured in conditions that preserve privacy.
As a new anthropometric index for estimating obesity, neck circumference (NC), measured at the margo inferior of the laryngeal prominence, has been proven to be closely associated with other anthropometric parameters and obesity-related cardiovascular risk factors, such as MetS and insulin resistance [9–12]. Moreover, NC has been shown to be a predictor of cardiovascular diseases [13, 14]. Several epidemiological population-based studies have demonstrated strong correlations between NC and body mass index (BMI) and WC [12–15], and NC has been found to be a better indicator for evaluating obesity compared with other anthropometric index, due to the advantages of being a stable and convenient measurement at an explicit anatomic landmark with little fluctuation related to diet and respiratory conditions. Several studies have reported that NC or neck fat content is positively correlated with visceral fat content; however, these studies were conducted in HIV-infected or severely obese patients with small sample sizes, limiting the degree to which the findings can be generalized [9, 11, 13, 16].
We adopted a magnetic resonance imaging (MRI) method to quantify the amount of visceral fat in order to determine the degree of visceral adiposity in participants of the present study. Meanwhile, total body fat and trunk fat contents were assessed accurately via a bioelectrical impedance analysis method. The goals of the present study were to determine the relationship between NC and body fat content and distribution and to determine the screening efficacy of NC for visceral adiposity as defined by MRI measurement of visceral fat content. We also assessed the value of NC as an indicator for metabolic disorders.
Subjects and methods
Subjects
The present study recruited community residents from the Zhabei area of Shanghai between October 2013 and October 2014. Each participant was required to complete a questionnaire covering all present and past illnesses, medications taken, and socioeconomic and lifestyle factors. We recruited subjects with complete questionnaire data as well as anthropometric and laboratory measurements. Individuals were excluded according to the following criteria: neck malformation, prior neck surgery, thyromegaly, thyroid dysfunction, known history of cardiovascular disease, malignant tumors, hepatic or renal dysfunction, severe disability, any infection, and current treatment with systemic corticosteroids. Finally, a total of 1943 subjects with complete body fat measurements and abdominal MRI data were available for analysis.
Anthropometric measurements
All participants underwent a complete physical examination. BMI was calculated as body weight in kilograms divided by height in meters squared. NC was measured with the subject standing and the head in the horizontal plane position. A tape was applied around the inferior margin of the laryngeal prominence and perpendicular to the long axis of the neck, and the minimum circumference was recorded to the nearest 0.1 cm. WC was measured at the horizontal plane between the inferior costal margin and the iliac crest on the mid-axillary line with the subject in the standing position. Blood pressures, including systolic blood pressure (SBP) and diastolic blood pressure (DBP), were measured three times at 3-min intervals using a mercury sphygmomanometer by appropriately sized cuff wrapped tightly on upper arm with its margo inferior 2.5 cm above chelidon.
Body fat and abdominal fat assessments
Body fat mass (FM) and trunk FM were measured by an automatic bioelectrical impedance analyzer (TBF-418B; Tanita Corp., Tokyo, Japan). Visceral and subcutaneous adipose tissue areas were assessed using a 3.0T clinical MRI scanner (Archiva; Philips Medical System, Amsterdam, The Netherlands), which imaged the abdominal region between the L4 and L5 vertebrae with the subject in the supine position. Segmentation of the images into the visceral fat area (VFA) and subcutaneous fat area (SFA) was carried out by the Slice-O-Matic image analysis software version 4.2 (Tomovision Inc., Montreal, QC, Canada) [17].
Laboratory assessments
After a 10-h overnight fast, fasting blood samples were collected to measure plasma glucose levels and lipid profiles. Participants without a validated history of diabetes underwent a 75-g oral glucose tolerance test. The 100-g carbohydrate (steamed bread meal) test was performed in diabetic patients. Fasting plasma glucose (FPG) and 2-h plasma glucose (2hPG) levels were assayed by the glucose oxidase method. Lipid profiles including serum total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured by enzymatic assays using the Hitachi 7600–120 automatic analyzer. The glycated hemoglobin (HbA1c) level was determined by high-pressure liquid chromatography (Bio-Rad Inc., Hercules, CA, USA). The serum concentration of C reactive protein (CRP) was assayed by particle-enhanced immunonephelometry using the Cardio Phase high-sensitivity-CRP reagent (Siemens Healthcare Diagnostic Inc., Newark, NJ, USA). The serum fasting insulin (FINS) concentration was measured by radioimmunoassay (Roche Diagnostics GmbH, Mannheim, Germany). Insulin sensitivity was estimated by homeostasis model assessment-insulin resistance (HOMA-IR) based on fasting glucose and insulin levels as follows: HOMA-IR = FINS (mU/L) × FPG (mmol/L)/22.5.
Diagnosis
Abdominal obesity was defined as a WC ≥ 90.0 cm for men or a WC ≥ 85.0 cm for women according to the latest 2016 China Guideline [18] and the Chinese Health Standard of Weight for Adults [19]. In addition, according to our previous study, the MRI-measured VFA was the gold standard for definitive diagnosis of abdominal obesity. Participants with a VFA of ≥ 80 cm2 were classified as having abdominal obesity [20].
Hyperglycemia, defined as a FPG ≥ 6.10 mmol/L and (or) a 2hPG ≥ 7.80 mmol/L, and (or) previously diagnosed diabetes; hypertension, defined as SBP ≥ 130 mmHg, and (or) DBP ≥ 85 mmHg, and (or) previously diagnosed hypertension; and hypertriglyceridemia, defined as a serum TG ≥ 1.70 mmol/L. A serum HDL-C less than 1.04 mmol/L was defined as low HDL-C. The above metabolic disorder definitions were in accordance with diagnostic criteria in China Guideline [18]. In the present study, MetS was defined by two or more metabolic disorders (hyperglycemia, hypertension, hypertriglyceridemia, low HDL-C) but without anthropometric indices of abdominal obesity.
Statistical analysis
All statistical analyses were performed with the Statistical Package for Social Sciences version 16.0 software (SPSS, Chicago, IL, USA), and p-values less than 0.05 were considered statistically significant. One-Sample Kolmogorov–Smirnov test was used to estimate the normality of parameters. Variables with a skewed distribution are presented as median with interquartile range (25–75 %), and the Wilcoxon rank-sum test for data with a skewed distribution were used to assess the differences between two independent groups. The Chi-square test was used to assess the differences among categorical variables. Multivariable linear regression analysis was applied to determine the factors affecting NC. Receiver operating characteristic (ROC) curve analyses and the Youden index (sensitivity + specificity − 1) were used to determine the optimal NC cutoff points for identifying visceral obesity diagnosed by VFA, and analyses were conducted to compare the performance of NC and WC cutoff points of abdominal obesity to identify MetS and its components.
Results
Clinical characteristics of the study participants
The study sample consisted of 783 men and 1160 women with a mean age of 58 ± 7 years. The prevalences of hyperglycemia, hypertension, hypertriglyceridemia, and low HDL-C among the study participants were 44.6, 62.3, 33.0 and 18.1 %, respectively. The mean NC of the total population was 35.1 cm (range 33.0–37.0 cm), and the mean value for men of 38.0 cm (range 36.5–39.5 cm) was greater than that for women at a mean of 33.6 cm (range 32.2–35.0 cm) (P < 0.001). Other body fat parameters (BMI, WC, body FM, trunk FM, VFA, SFA) of men were significantly higher than those of women (all P < 0.001), and men had significantly higher blood pressure (SBP, DBP), blood glucose levels (FPG, 2hPG), and serum TG levels than women (all P < 0.001). However, the serum TC, HDL-C, and LDL-C levels of women were greater than those of men (all P < 0.001) (Table 1).
Correlations of NC with anthropometric and laboratory parameters
A forward stepwise multivariable regression analysis was performed to determine the relationship between NC and body fat. Age, SBP, DBP, HOMA-IR, HbA1c, TG, HDL-C, LDL-C, and CRP levels as well as body fat content indices were accurately evaluated by trunk FM, and VFA and SFA were designated as independent variables. We found that both trunk FM and VFA were significantly associated with NC regardless of gender (Table 2). To further evaluate whether the association of VFA with NC was influenced by BMI, we conducted a similar multivariable regression analysis in different BMI categories (underweight/normal weight subjects vs. overweight/obese subjects). It was also found that trunk FM and VFA were independently associated with NC regardless of BMI after adjustment of several metabolic and body fat parameters (as shown in Table 3).
Optimal cutoff points of NC for identifying excessive visceral adiposity
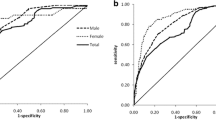
MRI was applied as the gold standard for evaluating VFA, and a VFA of ≥80 cm2 was defined as visceral obesity. As shown in Fig. 1, ROC analysis showed that the area under the curve for NC and visceral adiposity was 0.781 for men and 0.777 for women. According to the maximum value of the Youden index, the best NC cutoff points for determining visceral obesity were ≥38.5 cm for men with a sensitivity of 56.1 % and specificity of 83.5 % and ≥34.5 cm for women with a sensitivity of 58.1 % and a specificity of 82.5 %.
Efficacy of NC in discriminating MetS and its components
WC is the recognized anthropometric index for diagnosing abdominal obesity, and according to our previous study findings, a WC of ≥90.0 cm for men and a WC of ≥85.0 cm for women are appropriate cutoff points to determine abdominal obesity based on elevated visceral fat content. Therefore, we further compared the efficacies of the cutoff points for NC (men ≥ 38.5 cm, women ≥ 34.5 cm) with those for WC (men ≥ 90.0 cm, women ≥ 85.0 cm) for discriminating MetS and its components (hyperglycemia, hypertension, hypertriglyceridemia, and low HDL-C). The sensitivities, specificities, positive predictive values, and negative predictive values did not differ between NC and WC for the diagnosis of these metabolic disorders in both men and women. Therefore, NC and WC offer similar efficacies for identifying MetS and its components (Table 4).
Further analyses were conducted to determine the ability of the cutoff points for NC and WC to identify specific metabolic disorders (hyperglycemia, hypertension, hypertriglyceridemia, and low HDL-C). As shown in Fig. 2, participants were separated into different groups according to gender as well as NC and WC cutoff points. The results showed that among men with an elevated NC (NC ≥ 38.5 cm), 24.2 % had only one component of MetS, and among men with an elevated WC (WC ≥ 90.0 cm), 24.1 % had only one component of MetS, with no significant difference between these percentages (P > 0.05). Similarly, 31.9 % of men with an elevated NC had two components of MetS, and 31.3 % of men with an elevated WC had two components of MetS. Additionally, we further calculated the prevalence of each combination of two metabolic disorders in subjects with elevated NC and WC (as shown in supplementary Table 1). Moreover, 70.5 % of men with an elevated NC had MetS (two or more components of metabolic disorders but without anthropometric indices of abdominal obesity), and 72.2 % of men with an elevated WC had MetS, with no statistically significant difference between the abilities of NC and WC to identify MetS (P > 0.05). Similar results were obtained from analyses in female participants, with elevated NC and WC having the same efficacy for screening MetS and its components (Fig. 2; all P > 0.05).
Discussion
Recently, an increased NC was correlated with a higher risk of cardiovascular disease, such as glycolipid metabolic disorder, and insulin resistance [9, 21–25]. Moreover, NC was shown to be an indicator of future fatal and non-fatal cardiovascular events and all-cause mortality [13]. Evidence indicates that a higher NC is an independent risk factor for MetS and its components [12, 15, 23], and obesity, especially visceral obesity, is considered to be the central component of MetS. Therefore, the fact that obese individuals tend to have higher NC values has been fully verified. However, most previous studies adopted simple anthropometric indices, such as BMI and WC, to estimate subjects’ degree of obesity. Yuan et al. conducted a study in 3191 patients with type 2 diabetes and found that NC values of 38.5 cm for men and 34.5 cm for women were the appropriate cutoff points for identifying abdominal obesity, defined according to the diagnosis criteria of MetS recommended by the 2005 IDF (WC ≥ 90.0 cm for men, and WC ≥ 80.0 cm for women) [13]. A study by Hingorjo et al. in 150 young subjects aged 18–20 years showed that NC values of ≥35.5 cm for men and ≥32.0 cm for women were the best cutoff points for identifying overweight/obese individuals (BMI ≥ 23.0 kg/m2) [10]. In the present study, MRI, the gold standard for evaluating visceral fat content, was used to define abdominal obesity, and the corresponding optimal cutoff values of NC for diagnosing abdominal obesity were 38.5 cm for men and 34.5 cm for women. These measurements were consistent with those reported by the studies mentioned above.
Abdominal obesity is considered an important risk factor for metabolic disorders as well as cardiovascular diseases; therefore, the relationship between NC and local body fat distribution has garnered public attention recently. A study including 1053 Brazilian adults demonstrated a positive relationship between the NC and visceral fat content assessed by ultrasound [9]. Recently, a study of 177 outpatients found that the VFA was not only correlated with the NC but also associated with neck adipose area measured by computer tomography [16]. Fitch et al. [11] conducted a study of the relationship between NC and cardiometabolic disorders in HIV-infected patients and reported that NC was significantly and positively associated with VFA and trunk fat content. However, the HIV carrying status of subjects may influence body fat distribution, and a small sample size was another limitation of this study. Contrarily, the study participants in our large community-based study better represent the whole population, and our sample size is more appropriate for clarifying the association between NC and body fat.
A study of 18 nondiabetic individuals with severe obesity (BMI > 40.0 kg/m2) demonstrated that NC surpasses WC as a powerful marker of computed tomography-determined VFA [26], which is in accordance with a previous observation that the estimating effect of WC for visceral fat content may be decreased with increasing BMI [27]. Therefore, NC has the advantage of predicting visceral fat content, consistently with other studies, and we observed an independent relationship between NC and VFA regardless of BMI in the present study. However, another study by Maddaloni et al. [28] revealed that the relationship between NC and visceral fat could be interacted by BMI, this result may be confounded by its small sample size and the veracity of calculated visceral adiposity index by simple clinical parameters.
In order to investigate whether NC has the same capacity for diagnosing abdominal obesity, we adopted MRI to accurately measure the visceral fat content and determined that the best NC cutoff points for identifying visceral obesity (VFA ≥ 80 cm2) were 38.5 cm for men and 34.5 cm for women. Then, the efficacies of these NC cutoff points were compared with those of the WC cutoff points (90.0 cm for men, 85.0 cm for women) for estimating MetS and its components. In addition, these WC cutoffs values corresponded to a VFA ≥ 80 cm2 in a Chinese population, based on our previous study [20]. Men with a WC of 90.0 cm or higher, and women with a WC of 85.0 cm or higher were defined as having abdominal obesity as described in the China Guideline [18] and the Chinese Health Standard of Weight for Adults [19]. In the present study, we found no differences between the sensitivities and specificities of NC and WC for the diagnosis of metabolic disorders, and similar results are also observed for the screening proportions of MetS patients identified according to NC and WC. Therefore, NC seems to be a statistically and practically similar measurement compared with WC for discriminating MetS and its components. Moreover, NC is a more practical indicator with an obvious landmark for measurement and is easier to measure and more stable than WC. In particular, NC measurements can be made without special requirements for measurement circumstances. Thus, it is widely used to estimate obesity in large groups of individuals with different characteristics.
Adipose tissue is not only a main storage site of energy but also an active endocrine organ. Many pro-inflammatory molecules secreted by adipocytes are involved in regulating metabolic and immune functions. Previous studies showed a correlation between NC and several adipose cytokines, which suggested a role for NC in reflecting adipose tissue function and whole-body metabolic conditions. While investigating the possible correlations between inflammatory markers with anthropometric measurements, Jamar et al. [29] found that plasminogen activator inhibitor 1 is a prothrombotic adipokine involved in the coagulation cascade and fibrinolysis that may increase the risk related to obesity, and NC was showed to be an independent predictor of plasminogen activator inhibitor 1 after adjustment for gender and BMI.
There were some limitations in the present study. First, researches have revealed that NC and WC cutoff points in distinguishing metabolic disorders would be different in different races and ages on account of diverse fat distribution characteristics [30, 31], however, these ethnic specific NC cutoff points of Chinese population should be further verified in additional studies in other Asian areas. Second, further prospective studies are needed to evaluate the efficacy of the NC cutoff points for predicting atherosclerosis and cardiovascular diseases based on this cross-sectional study. Thirdly, it is noted that liver enzyme levels were closely correlated with metabolic disorders [32], however, the subjects with hepatic and renal dysfunction were excluded from our study, which eliminating the influence of those illnesses to metabolic disorders to some extent.
Conclusion
NC as a novel anthropometric indicator of adipose distribution showed good predictive ability for total body fat as well as visceral fat accumulation. NC and WC have the same power for predicting the risk of metabolic disorders, and the optimal cutoff points for NC to estimate MetS were 38.5 cm for Chinese men and 34.5 cm for Chinese women.
References
K.A. Britton, J.M. Massaro, J.M. Murabito, B.E. Kreger, U. Hoffmann, C.S. Fox, Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J. Am. Coll. Cardiol. 62(10), 921–925 (2013)
G.R. Hajer, T.W. van Haeften, F.L. Visseren, Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart. J. 29(24), 2959–2971 (2008)
A. Shuster, M. Patlas, J.H. Pinthus, M. Mourtzakis, The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 85(1009), 1–10 (2012)
A. Onat, G.S. Avci, M.M. Barlan, H. Uyarel, B. Uzunlar, V. Sansoy, Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int. J. Obes. Relat. Metab. Disord. 28(8), 1018–1025 (2004)
J.C. Seidell, A. Oosterlee, P. Deurenberg, J.G. Hautvast, J.H. Ruijs, Abdominal fat depots measured with computed tomography: effects of degree of obesity, sex, and age. Eur. J. Clin. Nutr. 42(9), 805–815 (1988)
K.G. Alberti, R.H. Eckel, S.M. Grundy, P.Z. Zimmet, J.I. Cleeman, K.A. Donato, J.C. Fruchart, W.P. James, C.M. Loria, S.C. Smith Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity, Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 120(16), 1640–1645 (2009)
P. Zimmet, D. Magliano, Y. Matsuzawa, G. Alberti, J. Shaw, The metabolic syndrome: A global public health problem and a new definition. J. Atheroscler. Thromb. 12(6), 295–300 (2005)
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and Treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 106(25), 3143–3421 (2002)
C. Stabe, A.C. Vasques, M.M. Lima, M.A. Tambascia, J.C. Pareja, A. Yamanaka, B. Geloneze, Neck circumference as a simple tool for identifying the metabolic syndrome and insulin resistance: results from the Brazilian metabolic syndrome study(BRAMS). Clin. Endocrinol. (Oxf). 78(6), 874–881 (2013)
M.R. Hingorjo, M.A. Qureshi, A. Mehdi, Neck circumference as a useful marker of obesity: a comparison with body mass index and waist. J. Pak. Med. Assoc. 62(1), 36–40 (2012)
K.V. Fitch, T.L. Stanley, S.E. Looby, A.M. Rope, S.K. Grinspoon, Relationship between neck circumference and cardiometabolic parameters in HIV-infected and non-HIV-infected adults. Diabetes Care. 34(4), 1026–1031 (2011)
G.R. Yang, S.Y. Yuan, H.J. Fu, G. Wan, L.X. Zhu, X.L. Bu, J.D. Zhang, X.P. Du, Y.L. Li, Y. Ji, X.N. Gu, Y. Li, Beijing community diabetes study group, neck circumference positively related with central obesity, overweight, and metabolic syndrome in Chinese subjects with type 2 diabetes: Beijing community diabetes study 4. Diabetes Care. 33(11), 2465–2467 (2010)
Y. Dai, X. Wan, X. Li, E. Jin, X. Li, Neck circumference and future cardiovascular events in a high-risk population—a prospective cohort study. Lipids. Health. Dis. 15, 46 (2016)
S.R. Preis, J.M. Massaro, U. Hoffmann, R.B. D’Agostino Sr, D. Levy, S.J. Robins, J.B. Meigs, R.S. Vasan, C.J. O’Donnell, C.S. Fox, Neck circumference as a novel measure of cardiometabolic risk: the framingham heart study. J. Clin. Endocrinol. Metab. 95(8), 3701–3710 (2010)
A. Onat, G. Hergenç, H. Yüksel, G. Can, E. Ayhan, Z. Kaya, D. Dursunoğlu, Neck circumference as a measure of central obesity: associations with metabolic syndrome and obstructive sleep apnea syndrome beyond waist circumference. Clin. Nutr. 28(1), 46–51 (2009)
H.X. Li, F. Zhang, D. Zhao, Z. Xin, S.Q. Guo, S.M. Wang, J.J. Zhang, J. Wang, Y. Li, G.R. Yang, J.K. Yang, Neck circumference as a measure of neck fat and abdominal visceral fat in Chinese adults. BMC Public Health 14, 311 (2014)
Y. Wang, X. Ma, M. Zhou, W. Zong, L. Zhang, Y. Hao, J. Zhu, Y. Xiao, D. Li, Y. Bao, W. Jia, Contribution of visceral fat accumulation to carotid intima-media thickness in a Chinese population. Int. J. Obes. (Lond). 36(9), 1203–1208 (2012)
J. Weng, L. Ji, W. Jia, J. Lu, Z. Zhou, D. Zou, D. Zhu, L. Chen, L. Chen, L. Guo, X. Guo, Q. Ji, Q. Li, X. Li, J. Liu, X. Ran, Z. Shan, L. Shi, G. Song, L. Yang, Y. Yang, W. Yang, Chinese diabetes society, standards of care for type 2 diabetes in China. Diabetes Metab. Res. Rev. 32(5), 442–458 (2016)
National Health and Family Planning Commission of the People’s Republic of China. Criteria of Weight for Adults. (Standards Press of China, Beijing, 2013), pp. 1–8
Y. Bao, J. Lu, C. Wang, M. Yang, H. Li, X. Zhang, J. Zhu, H. Lu, W. Jia, K. Xiang, Optimal waist circumference cutoffs for abdominal obesity in Chinese. Atherosclerosis. 201(2), 378–384 (2008)
N.G. Vallianou, A.A. Evangelopoulos, V. Bountziouka, E.D. Vogiatzakis, M.S. Bonou, J. Barbetseas, P.C. Avgerinos, D.B. Panagiotakos, Neck circumference is correlated with triglycerides and inversely related with HDL cholesterol beyond BMI and waist circumference. Diabetes Metab. Res. Rev. 29(1), 90–97 (2013)
K. Joshipura, F. Muñoz-Torres, J. Vergara, C. Palacios, C.M. Pérez, Neck circumference may be a better alternative to standard anthropometric measures. J. Diabetes Res. 2016, 6058916 (2016)
G. Cizza, L. de Jonge, P. Piaggi, M. Mattingly, X. Zhao, E. Lucassen, K.I. Rother, A.E. Sumner, G. Csako, NIDDK sleep extension study, neck circumference is a predictor of metabolic syndrome and obstructive sleep apnea in short-sleeping obese men and women. Metab. Syndr. Relat. Disord. 12(4), 231–241 (2014)
M. Khalangot, V. Gurianov, N. Okhrimenko, I. Luzanchuk, V. Kravchenko, Neck circumference as a risk factor of screen-detected diabetes mellitus: community-based study. Diabetol. Metab. Syndr. 8, 12 (2016)
L.L. Ben-Noun, A. Laor, Relationship between changes in neck circumference and cardiovascular risk factors. Exp. Clin. Cardiol. 11(1), 14–20 (2006)
L. Yang, Y.P. Sa marasinghe, P. Kane, S.A. Amiel, S.J. Aylwin, Visceral adiposity is closely correlated with neck circumference and represents a significant indicator of insulin resistance in WHO grade III obesity. Clin. Endocrinol. (Oxf). 73(2), 197–200 (2010)
T.S. Han, N. Sattar, M. Lean, ABC of obesity. Assessment of obesity and its clinical implications. BMJ. 333(7570), 695–698 (2006)
E. Maddaloni, I. Cavallari, M. De Pascalis, H. Keenan, K. Park, S. Manfrini, R. Buzzetti, G. Patti, G. Di Sciascio, P. Pozzilli, Relation of body circumferences to cardiometabolic disease in overweight-obese subjects. Am. J. Cardiol. 118(6), 822–887 (2016)
G. Jamar, L.P. Pisani, L.M. Oyama, C. Belote, D.C. Masquio, V.A. Furuya, J.P. Carvalho-Ferreira, S.G. Andrade-Silva, A.R. Dâmaso, D.A. Caranti, Is the neck circumference an emergent predictor for inflammatory status in obese adults? Int. J. Clin. Pract. 67(3), 217–224 (2013)
S. Hoebel, L. Malan, J.H. de Ridder, Determining cut-off values for neck circumference as a measure of the metabolic syndrome amongst a South African cohort: the SABPA study. Endocrine. 42(2), 335–342 (2012)
S. Hoebel, L. Malan, J. Botha, M. Swanepoel, Optimizing waist circumference cut-points for the metabolic syndrome and its components in a South African cohort at 3 year follow-up: the SABPA prospective cohort. Endocrine. 47(3), 959–961 (2014)
M. Hastedt, M. Büchner, M. Rothe, R. Gapert, S. Herre, F. Krumbiegel, M. Tsokos, T. Kienast, A. Heinz, S. Hartwig, Detecting alcohol abuse: traditional blood alcohol markers compared to ethyl glucuronide (EtG) and fatty acid ethyl esters (FAEEs) measurement in hair. Forensic Sci. Med. Pathol 9(4), 471–477 (2013)
Acknowledgments
We appreciate the efforts of all participants who contributed to sample measurements and data collections.
Funding
This work was funded by the 973 Program of China (2013CB530606), the Grant from Shanghai Health and Family Planning Commission (2013ZYJB1001), and the Translational Medicine Innovation Foundation of School of Medicine Shanghai Jiao Tong University (15ZH2010 and 15ZH4006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study protocol was reviewed and approved by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, and all procedures were performed in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
The study methods and potential risks were fully explained to all participants, and each participant provided a written informed consent prior to enrollment.
Additional information
Yuqi Luo and Xiaojing Ma contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Luo, Y., Ma, X., Shen, Y. et al. Neck circumference as an effective measure for identifying cardio-metabolic syndrome: a comparison with waist circumference. Endocrine 55, 822–830 (2017). https://doi.org/10.1007/s12020-016-1151-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-1151-y