Abstract
Cushing’s syndrome is caused by prolonged exposure to elevated cortisol levels. The most common form of endogenous Cushing’s syndrome is Cushing’s disease, which results from an adrenocorticotropic hormone-secreting pituitary tumour. Cushing’s disease is associated with increased mortality, mostly attributable to cardiovascular complications, and a host of comorbidities such as metabolic and skeletal disorders, infections and neuropsychiatric disturbances. As a consequence, Cushing’s disease substantially impairs health-related quality of life. It is crucial that the condition is diagnosed as early as possible, and that rapid and effective treatment is initiated in order to limit long-term morbidity and mortality. The initial treatment of choice for Cushing’s disease is selective transsphenoidal pituitary surgery; however, the risk of recurrence after initial surgery is high and remains so for many decades after surgery. A particular concern is the growing body of evidence indicating that the negative physical and psychosocial sequelae of chronic hypercortisolism may persist in patients with Cushing’s disease even after long-term surgical ‘cure’. Current treatment options for post-surgical patients with persistent or recurrent Cushing’s disease include second surgery, radiotherapy, bilateral adrenalectomy and medical therapy; however, each approach has its limitations and there is an unmet need for more efficacious treatments. The current review provides an overview of the burden of illness of Cushing’s disease, underscoring the need for prompt diagnosis and effective treatment, as well as highlighting the need for better therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cushing’s syndrome is a rare, chronic and systemic disease caused by endogenous or exogenous hypercortisolism. The most common form of endogenous Cushing’s syndrome is Cushing’s disease, which results from an adrenocorticotropic hormone (ACTH)-secreting pituitary tumour; it is responsible for 70 % of all cases of ACTH-dependent Cushing’s syndrome [1–4]. In Cushing’s disease, autonomous ACTH secretion by the tumour and disturbance of the normal cortisol feedback mechanism within the hypothalamic–pituitary–adrenal axis leads to a loss of the circadian rhythm of cortisol production, resulting in excess cortisol levels. Prolonged exposure to elevated cortisol levels in patients with Cushing’s disease results in significant clinical burden [1–6]. Patients have excess morbidities (Fig. 1), impaired health-related quality of life (HRQoL) and increased mortality (Fig. 2). Early diagnosis and treatment is therefore of paramount importance in these patients. However, the rare incidence and low prevalence of the disease, combined with the widespread presence of common overlapping symptoms such as obesity, hypertension and diabetes in the general population, means that there is often a considerable delay between the onset of symptoms and diagnosis [7].
Prevalence of morbidities in patients with Cushing’s disease compared with the general population in the USA (2006) [74]. Prevalence rates in the general population are derived from Centers for Disease Control and Prevention statistics and National Health and Nutrition Examination Survey III findings
The goal of treatment in Cushing’s disease is to normalize cortisol levels in order to ameliorate disease-associated signs and symptoms, as well as comorbidities [8]. In general, the initial treatment of choice is selective transsphenoidal pituitary surgery (TSS) [8]. In a review of 74 studies involving over 6000 patients with Cushing’s disease who underwent initial pituitary surgery (follow-up duration, 1–444 months with a mean of 64.3 months), the overall initial mean remission rate was 77.8 % [3]. Unfortunately, the risk of recurrence after surgery persists for up to 10 years, with reported rates of long-term failure of pituitary surgery averaging at approximately 32 % [3].
Current treatment options for post-surgical patients with persistent or recurrent Cushing’s disease include second surgery, radiotherapy, bilateral adrenalectomy and medical therapy [8].
The severity and range of the clinical scenario and associated morbidities in patients with Cushing’s disease continue to present a management challenge. Although the literature has often focused on the cardiovascular burden of Cushing’s disease, the actual burden includes numerous additional comorbidities (Fig. 1). The current review provides an overview of this burden of illness in patients with Cushing’s disease, underscoring the need for prompt diagnosis and better therapies.
Challenges in the diagnosis of Cushing’s disease
A diagnosis of Cushing’s syndrome must first be established before entertaining a differential diagnosis between different forms of Cushing’s syndrome, which includes Cushing’s disease [9]. The varying nature of the disease signs and symptoms makes this task especially challenging. Clinical manifestations may include distinct physical features, such as purple striae, facial plethora and proximal myopathy, as well as common conditions such as hypertension, obesity, diabetes and menstrual irregularity in women or sexual dysfunction in men [1, 2]. Clinical suspicion based on these physical signs is most likely to stimulate further investigation. Evidence-based guidelines currently recommend at least one of the three diagnostic tests for Cushing’s syndrome: late-night salivary cortisol (LNSC), 24-h urinary cortisol (UC) and low-dose dexamethasone suppression [10]. However, because of differences in assay methodologies, sensitivities and specificities, no single test is definitive and more than one type of test is usually required [10]. Recognition of Cushing’s disease, with its broad spectrum of clinical presentation combined with the challenges and limitations of screening tests, can be complicated and diagnosis is often delayed, in some cases for more than a decade [11].
When considering how rarely a diagnosis of Cushing’s disease is made, a screening procedure is difficult to justify. Any benefits of widespread screening of high-risk patient populations would likely be outweighed by the drawbacks of screening, such as cost, acceptability and the use of unnecessary procedures. Indeed, clinical guidelines recommend against widespread testing for Cushing’s syndrome/disease [10]. Instead, an appreciation of the more characteristic physical signs of Cushing’s disease could lead to earlier diagnosis and intervention, which would mitigate some of the negative consequences of chronic hypercortisolism.
Consequences of untreated or uncontrolled hypercortisolism
Increased mortality
Historical studies in patients with Cushing’s disease have reported standardized mortality ratios (SMRs) either similar to or significantly higher than those in the general population (reported ranges 0.98–9.3) [6]. In a Danish population-based study, patients with Cushing’s disease cured by successful surgery did not have an increased mortality rate compared with the general population, whereas those who had a recurrence of Cushing’s disease after unsuccessful surgery had a mortality rate five times higher than that of the general population [12].
Recent systematic analyses of mortality studies in patients with Cushing’s syndrome have been reported [13, 14]. An analysis by Graversen et al. showed that the weighted mean SMR for patients with Cushing’s disease was 1.84 [13]. The overall increased mortality was attributed to patients with persistent disease after initial surgery; these patients had an SMR of 3.73. By contrast, others have reported an excess mortality risk for patients with Cushing’s disease regardless of treatment success or failure, with most deaths related to cardiovascular causes or infections [14–17]. A recent global retrospective analysis—the MISSION study—investigated mortality rate in almost 5000 patients with Cushing’s syndrome and showed a crude SMR of 4.25 in those with Cushing’s disease [18].
Several studies have aimed to characterize the risk factors contributing to increased mortality among patients with treated Cushing’s disease in order to stratify patients and develop treatment strategies. In a Spanish population, persistent hypertension, age and impaired glucose metabolism were found to be independent predictors of mortality [11]. In a separate retrospective chart review of 346 patients with Cushing’s disease who were treated by a single surgeon between 1980 and 2011, older age at diagnosis and duration of hypercortisolism were associated with an increased risk of death [19]. The role of excess cortisol is further exemplified by data showing that mortality in patients previously treated for Cushing’s disease was increased compared with patients treated for non-functioning pituitary adenomas (NFPAs) [15]. Preliminary data from the MISSION study indicate that the mortality rate in Cushing’s syndrome is higher in patients with hypertension, impairment of glucose metabolism, dyslipidaemia, coagulopathy, cardiopathy and infections compared with patients without specific complications [18].
Collectively, these data suggest that there is a critical need for early recognition and intervention of Cushing’s disease in order to avoid the long-term consequences of the disease.
Increased morbidity
Metabolic syndrome
Metabolic syndrome is defined as a complex of inter-related risk factors, including obesity (particularly visceral obesity), hyperglycaemia, hypertension, hypertriglyceridaemia and suppressed high-density lipoprotein cholesterol [20]. Cushing’s syndrome represents an archetype of metabolic syndrome as chronic cortisol secretion leads to muscle, liver and adipocyte insulin resistance [21]. The majority of patients with Cushing’s syndrome are overweight or obese [6], up to half of patients have diabetes mellitus [6], and hypertension and dyslipidaemia are also prevalent (up to 93 % [6] and 59 % [6], respectively). These complications all contribute to increased cardiovascular risk [20].
Several studies have shown that these cardiovascular risk factors may be reversed following normalization of cortisol levels [11, 22, 23]. For example, high blood pressure is normalized in a subset of 44–75 % of patients after successful treatment of Cushing’s disease [24, 25]. However, even after post-surgical remission, cardiovascular morbidity risk can persist in most patients. Colao et al. evaluated cardiovascular risk in 15 patients cured (defined as normal cortisol and ACTH levels, with restoration of physiological circadian rhythm) of Cushing’s disease versus control groups [23]. After 5 years, despite some improvement, 40, 33 and 40 % of patients were obese, had diabetes and remained hypertensive, respectively, versus 0, 7 and 10 % of matched controls [23]. Insulin levels following a glucose load were higher than in controls, suggesting the persistence of insulin resistance in patients cured of Cushing’s disease [23]. The same group also assessed cardiovascular risk factors in 25 patients with Cushing’s disease before and after 1 year of remission [24]. Their findings showed that body mass index, as well as systolic and diastolic blood pressure, was lower after effective treatment of Cushing’s disease than before treatment; however, with the exception of systolic blood pressure, they remained higher than in healthy subjects [24]. In addition, obesity, diabetes, hypertension, hypertriglyceridaemia and hypercholesterolaemia were still present in 63, 60, 56, 60 and 76 % of the patients, respectively [24]. Another study assessing comorbidities in 29 patients with Cushing’s disease at two different time points after resolution of hypercortisolism by TSS showed the persistence of multiple metabolic morbidities, irrespective of the initial degree of hypercortisolism [26]. Therefore, whether cured or improved, all patients with Cushing’s disease remain at risk of complications related to metabolic syndrome and the associated cardiovascular risks.
Skeletal system disorders
Glucocorticoid excess influences bone development by inhibiting bone formation and increasing bone resorption [27]. Hypercortisolism mainly affects trabecular bone, causing skeletal fracture, especially at the vertebral levels, in up to 76 % of patients [6]. Prospective bone status data are available from a large number of patients with Cushing’s disease in the European Registry on Cushing’s Syndrome (ERCUSYN) [7]. Spine osteopenia and osteoporosis were reported in 29 % (40/136) and 16 % (22/136) of Cushing’s disease patients, respectively, with hip osteopenia and osteoporosis reported in 34 % (46/134) and 9 % (12/134) of patients, respectively [7].
A number of studies have shown that the degree and duration of hypercortisolism are major determinants of bone loss and fractures, and that bone impairment is only partially reversed after cortisol normalization [28–30]. These findings further highlight the need for early diagnosis and prompt treatment.
Opportunistic infections
There are a number of reasons why patients with Cushing’s disease are more susceptible to opportunistic infections compared with healthy subjects. Firstly, glucocorticoids are potent anti-inflammatory and immunosuppressive agents. Excess cortisol has been shown to suppress cellular [31] and humoral [32, 33] immune functions, significantly increasing the risk of opportunistic infections, particularly invasive fungal infections [34, 35]. Secondly, hyperglycaemia in patients with Cushing’s disease, resulting from the increase in insulin resistance in tissues, has been shown to contribute to immunosuppression [35]. Finally, the effects of excess cortisol on the skin, in particular, atrophy and poor wound healing, may disrupt the first protective barrier of defence against the entry of pathogens [35].
Data show that extreme levels of circulating cortisol are associated with an increased chance of serious infections [36] and carry a high mortality risk [37]. A particular clinical challenge is diagnosing infection in the setting of hypercortisolism, since the effects of cortisol on the immune system make the diagnosis more difficult. For example, diminished cytokine production due to glucocorticoid excess [32, 33] interrupts the normal febrile response to infection, with no manifestation of fever [34, 35]. For patients with Cushing’s disease, normalizing cortisol levels is required to reduce the risk of infection [35].
Neuropsychiatric dysfunction
Cortisol dysregulation is a pathophysiological trait shared by both Cushing’s syndrome and major depressive disorder: 50–81 % of patients with Cushing’s disease may exhibit major depression [6, 38]. Patients with Cushing’s disease and depression also appear to suffer from a more severe form of illness, in terms of both cortisol production and clinical presentation, compared with those who are not depressed [39].
It is generally believed that depression subsides following surgical treatment of Cushing’s disease. However, it has been shown that even after long-term surgical cure of Cushing’s disease, patients exhibit an increased prevalence of psychopathology and maladaptive personality traits [40], particularly those associated with anxiety [41]. In a study of 29 patients with Cushing’s disease ‘cured’ by pituitary surgery, psychiatric comorbidity persisted after long-term remission, irrespective of the initial degree of hypercortisolism [26]. Over 80 % of patients were clinically depressed at diagnosis. Even though ~50 % experienced either a significant improvement or a resolution of their mood abnormalities when evaluated within 1 year of hypercortisolism resolution, 20 % remained significantly depressed at the late evaluation (more than 2 years postoperatively) [26]. These findings suggest irreversible effects of previous glucocorticoid excess on the central nervous system rather than a direct effect of pituitary tumours and/or their treatment in general. Dimopoulou et al. speculated that a possible explanation could be impaired cortical or subcortical integrity due to the chronic cortisol excess in Cushing’s disease, which might translate into an altered personality pattern [41].
Alongside psychiatric illness, there is also a risk of suicide for patients with Cushing’s disease as a result of depression or schizophrenia-like symptoms, hallucinations and illusions [42, 43]. In order to prevent suicide attempts, all patients with Cushing’s disease should be carefully monitored for the development of depression or schizophrenia-like symptoms and precautions should be considered, where appropriate.
Cognitive impairment
Historical studies have shown that psychological disturbances are accompanied by structural modification of the brain, namely, atrophy of specific cerebral areas [44–46]. For example, a case–control study reported a subjective loss of brain volume in 86 % of patients with Cushing’s disease [46]. The normalization of cortisol secretion following disease remission has been demonstrated to reverse, at least partially, cerebral atrophy [44, 46], suggesting that neuronal death is not the only mechanism by which glucocorticoids induce loss of brain volume.
More recently, in post-surgical patients with predominantly long-term remission of Cushing’s disease, specific abnormalities in brain structure have been noted in the presence of psychological dysfunction [47]. In addition, observations suggest that chronic hypercortisolism may have irreversible effects on the central nervous system, potentially resulting in permanent damage to intellectual function [48, 49]. This is supported by recent clinical evidence showing that patients with Cushing’s syndrome and in long-term remission following pituitary surgery exhibit global cognitive and attention deficits [50, 51]. In a cross-sectional study by Tiemensma et al. impairments in cognitive function were found in patients with Cushing’s disease in remission compared with healthy individuals and patients with NFPA [50]. The authors concluded that the effects on the central nervous system are irreversible and most likely explained by previous glucocorticoid excess [50]. The findings of Ragnarsson et al. are consistent with this conclusion. They assessed 43 patients with Cushing’s disease in long-term remission and showed impaired cognitive function in the domains of speed processing, auditory attention and working memory, verbal fluency and reading speed [51]. In addition, the basic attentional mechanisms of spatial orientation and alertness were negatively affected [51]. The persistence of deleterious effects of chronic hypercortisolism on neuropsychiatric function and cognition in some Cushing’s disease patients, even after remission, exemplifies the need for early diagnosis and intervention.
Health-related quality of life
The above-mentioned clinical complications likely play a role in the significant impairments in HRQoL reported by patients with Cushing’s disease [52–54]. Indeed, HRQoL in Cushing’s disease, as determined by the standard Short Form 36 (SF-36) health survey, is significantly worse than in the general population and in patients with other pituitary tumours (Fig. 3) [53]. In an observational study of 107 patients with Cushing’s disease, Webb et al. further showed that active hypercortisolism was associated with a worse score in HRQoL compared with those patients who no longer had hypercortisolism [54].

Figure adapted from Johnson et al. [49]
Mean SF-36 scores according to tumour type. In patients with untreated Cushing’s disease, SF-36 scores were all significantly lower (P > 0.05) than the normal population score of 50 (except role emotional) and for patients with acromegaly (except bodily pain) and NFPA
Data from long-term studies show that patients with Cushing’s disease continue to experience impaired HRQoL even after post-operative remission [55, 56]. In a study conducted by Lindsay et al., HRQoL improved after surgical treatment, although levels remained below that of age- and gender-matched subjects for up to 15 years [56]. As described above, multiple dimensions, including cognition and body composition, do not normalize after endocrine cure [56]. It is, therefore, not surprising that patients experience impaired HRQoL even after post-operative remission. In addition to the potential irreversible effects of hypercortisolism on HRQoL, this assessment also needs to take into account the potential development of novel clinical manifestations induced by the fall in cortisol levels [57].
Alleviating the burden of Cushing’s disease
The treatment of Cushing’s disease is complex and represents a challenge for clinicians, requiring a multidisciplinary and individualized approach. TSS is the initial treatment of choice for most patients, and the overall goal is complete resection of the pituitary tumour and correction of hypercortisolism without inducing permanent pituitary deficiencies [3, 7]. Several treatment options are available for post-surgical patients who fail to achieve remission or who relapse; however, these are not without drawbacks [3, 7]. Repeat pituitary surgery is sometimes used for patients with persistent hypercortisolism after initial TSS. However, this is associated with a significant risk of hypopituitarism and is recommended only when evidence of a remaining pituitary tumour is established [3, 7]. Conventional radiotherapy can result in mean remission rates of ~64 % when used exclusively, increasing to ~70 % when used after pituitary surgery [3, 7]. However, the main disadvantages of radiotherapy are that it may not be appropriate in patients with severe symptoms in need of immediate resolution because of delayed efficacy (typically 2–3 years) and that significant pituitary deficiency may develop after treatment [8]. Bilateral adrenalectomy provides an immediate permanent treatment in cases where other therapies have failed [8]; however, in addition to the surgical risks, patients require lifelong mineralocorticoid and glucocorticoid replacement therapy [58].
Medical therapy
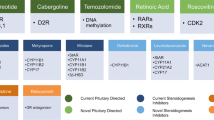
There is now a wide range of medical therapies available to treat Cushing’s disease (Fig. 4), which provides clinicians with a greater opportunity to alleviate the burden of illness associated with the condition. These agents may act at the hypothalamic–pituitary level by decreasing ACTH secretion (neuromodulatory agents), at the adrenal level by inhibiting cortisol synthesis (steroidogenesis inhibitors), or at the peripheral level by competing with cortisol (glucocorticoid receptor antagonists) [8].
Steroidogenesis inhibitors used to treat patients with Cushing’s disease include ketoconazole, metyrapone and mitotane. Ketoconazole and metyrapone have been approved in Europe for the treatment of Cushing’s syndrome [59, 60]. A review of clinical studies reporting the efficacy of ketoconazole in patients with Cushing’s disease revealed a mean response rate of 64.3 % [3]. Ketoconazole, however, also inhibits androgen synthesis and may cause liver damage [61]. The approved agent ketoconazole is a racemic mixture of two enantiomers. Levoketoconazole, the single 2S,4R enantiomer, is hypothesized to have an improved benefit–risk profile compared with racemic ketoconazole. The efficacy and safety of levoketoconazole is currently being investigated in patients with Cushing’s syndrome in the global Phase III SONICS trial (NCT01838551). Osilodrostat (LCI699) is another steroidogenesis inhibitor in Phase III clinical development for the treatment of Cushing’s disease (NCT02180217). In a previous Phase II study, osilodrostat, an oral inhibitor of 11β-hydroxylase (the final enzyme in the cortisol synthesis pathway), normalized UC in 78.9 % of patients who completed 22 weeks of treatment [62].
Mifepristone, a glucocorticoid receptor antagonist, is approved in the USA for control of hyperglycaemia secondary to hypercortisolism in patients with endogenous Cushing’s syndrome who have type 2 diabetes or glucose intolerance and have failed, or are not candidates for, surgery [63]. Mifepristone has demonstrated sustained weight loss [64], improved glucose tolerance in 60 % of glucose-intolerant patients and lowering of diastolic blood pressure in 43 % of hypertensive patients [65]. Mifepristone does not reduce cortisol levels; consequently, physicians must rely on changes in signs and symptoms to gauge treatment efficacy [66].
Pasireotide, a multireceptor-targeted somatostatin analogue, is currently the only pituitary-directed medical therapy approved in the EU and the USA for the treatment of Cushing’s disease; it is licensed for use in adult patients in whom surgery is not an option or has failed [67, 68]. In a Phase III study of pasireotide, administered subcutaneously, a decrease in UC by 6 months was achieved in most patients, as well as normalization of UC in 20 % of patients [69, 70]. The safety profile of pasireotide was similar to that of other somatostatin analogues, except for a higher frequency and degree of hyperglycaemia [69]. Blood glucose levels should be monitored during pasireotide treatment, and prompt intervention is warranted if hyperglycaemia occurs.
The dopamine agonist cabergoline is another neuromodulatory agent used off-label for the treatment of Cushing’s disease that has shown promise in an open-label trial and a retrospective trial [71, 72]. However, randomized, controlled studies with this agent are yet to be conducted.
Combination therapy for treatment of Cushing’s disease has also been proposed. In a study of 17 patients with Cushing’s disease, pasireotide monotherapy (100–250 µg three times a day) normalized UC levels in five patients and addition of cabergoline (0.5–1.5 mg every other day) normalized UC levels in four more patients [73]. Among the remaining eight patients, addition of ketoconazole (200 mg three times a day) normalized UC levels in six of these, leaving two patients with elevated UC levels. Therefore, sequential therapy with multiple treatments may be one approach to treatment, although this requires further investigation.
Conclusions
Cushing’s disease is caused by prolonged exposure to elevated cortisol levels and is associated with significant clinical burden due to numerous morbidities, increased mortality and impaired quality of life. The condition therefore requires early detection, as well as rapid treatment and effective disease control, in order to limit its long-term mortality and morbidity. A growing body of evidence indicates that the negative physical and psychosocial sequelae of chronic hypercortisolism may persist in patients with Cushing’s disease even after long-term surgical ‘cure’. Post-surgical patients who fail to achieve remission or who relapse may benefit from second-line treatments such as repeat surgery, radiation therapy and bilateral adrenalectomy; however, each procedure is not without risk. An unmet clinical need exists for more efficacious medications. With the recent approvals of pasireotide and mifepristone, along with other agents in late-stage clinical development, medical therapies may become more prominent in future patient management and may help to alleviate the burden of illness associated with Cushing’s disease.
References
R. Pivonello, M.C. De Martino, M. De Leo, G. Lombardi, A. Colao, Cushing’s syndrome. Endocrinol. Metab Clin. North Am. 37, 135–149 (2008)
J. Newell-Price, X. Bertagna, A.B. Grossman, L.K. Nieman, Cushing’s syndrome. Lancet 367, 1605–1617 (2006)
R. Pivonello, M. De Leo, A. Cozzolino, A. Colao, The treatment of Cushing’s disease. Endocr. Rev. 36, 385–486 (2015)
C. Steffensen, A.M. Bak, K.Z. Rubeck, J.O. Jorgensen, Epidemiology of Cushing’s syndrome. Neuroendocrinology 92(Suppl 1), 1–5 (2010)
A. Lacroix, R.A. Feelders, C.A. Stratakis, L.K. Nieman, Cushing’s syndrome. Lancet 386, 913–927 (2015)
R. Pivonello, A.M. Isidori, M.C. De Martino et al., Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol. 2016 (in press)
E. Valassi, A. Santos, M. Yaneva et al., The European Registry on Cushing’s syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur. J. Endocrinol. 165, 383–392 (2011)
L.K. Nieman, B.M. Biller, J.W. Findling et al., Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 100, 2807–2831 (2015)
J. Newell-Price, P. Trainer, M. Besser, A. Grossman, The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr. Rev. 19, 647–672 (1998)
L.K. Nieman, B.M. Biller, J.W. Findling et al., The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 93, 1526–1540 (2008)
J. Etxabe, J.A. Vazquez, Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin. Endocrinol. (Oxf) 40, 479–484 (1994)
J. Lindholm, S. Juul, J.O. Jorgensen et al., Incidence and late prognosis of Cushing’s syndrome: a population-based study. J. Clin. Endocrinol. Metab. 86, 117–123 (2001)
D. Graversen, P. Vestergaard, K. Stochholm, C.H. Gravholt, J.O. Jorgensen, Mortality in Cushing’s syndrome: a systematic review and meta-analysis. Eur. J. Intern. Med. 23, 278–282 (2012)
G. Ntali, A. Asimakopoulou, T. Siamatras et al., Mortality in Cushing’s syndrome: systematic analysis of a large series with prolonged follow-up. Eur. J. Endocrinol. 169, 715–723 (2013)
O.M. Dekkers, N.R. Biermasz, A.M. Pereira et al., Mortality in patients treated for Cushing’s disease is increased, compared with patients treated for nonfunctioning pituitary macroadenoma. J. Clin. Endocrinol. Metab. 92, 976–981 (2007)
G.D. Hammer, J.B. Tyrrell, K.R. Lamborn et al., Transsphenoidal microsurgery for Cushing’s disease: initial outcome and long-term results. J. Clin. Endocrinol. Metab. 89, 6348–6357 (2004)
Z.K. Hassan-Smith, M. Sherlock, R.C. Reulen et al., Outcome of Cushing’s disease following transsphenoidal surgery in a single center over 20 years. J. Clin. Endocrinol. Metab. 97, 1194–1201 (2012)
R. Pivonello, P. Vitale, L. Mantovani et al., MISSION study. A worldwide epidemiological study on the mortality associated with Cushing’s syndrome performed in nearly 5000 patients. 95th annual meeting of The Endocrine Society. San Francisco, CA, USA, 15–18 Jun abst SAT-LB-07 (2013)
J.K. Lambert, L. Goldberg, S. Fayngold et al., Predictors of mortality and long-term outcomes in treated Cushing’s disease: a study of 346 patients. J. Clin. Endocrinol. Metab. 98, 1022–1030 (2013)
H. Prasad, D.A. Ryan, M.F. Celzo, D. Stapleton, Metabolic syndrome: definition and therapeutic implications. Postgrad. Med. 124, 21–30 (2012)
R. Pivonello, M. De Leo, P. Vitale et al., Pathophysiology of diabetes mellitus in Cushing’s syndrome. Neuroendocrinology 92(Suppl 1), 77–81 (2010)
T. Mancini, B. Kola, F. Mantero, M. Boscaro, G. Arnaldi, High cardiovascular risk in patients with Cushing’s syndrome according to 1999 WHO/ISH guidelines. Clin. Endocrinol. (Oxf) 61, 768–777 (2004)
A. Colao, R. Pivonello, S. Spiezia et al., Persistence of increased cardiovascular risk in patients with Cushing’s disease after five years of successful cure. J. Clin. Endocrinol. Metab. 84, 2664–2672 (1999)
A. Faggiano, R. Pivonello, S. Spiezia et al., Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing’s disease during active disease and 1 year after disease remission. J. Clin. Endocrinol. Metab. 88, 2527–2533 (2003)
R.M. Gomez, N.M. Albiger, A.G. Diaz et al., Effect of hypercortisolism control on high blood pressure in Cushing’s syndrome. Medicina (B Aires) 67, 439–444 (2007)
A.L. Espinosa-de-los-Monteros, E. Sosa, N. Martinez, M. Mercado, Persistence of Cushing’s disease symptoms and comorbidities after surgical cure: a long-term, integral evaluation. Endocr. Pract. 19, 252–258 (2013)
G. Kaltsas, P. Makras, Skeletal diseases in Cushing’s syndrome: osteoporosis versus arthropathy. Neuroendocrinology 92(Suppl 1), 60–64 (2010)
A. Faggiano, R. Pivonello, M. Filippella et al., Spine abnormalities and damage in patients cured from Cushing’s disease. Pituitary 4, 153–161 (2001)
M.J. Barahona, N. Sucunza, E. Resmini et al., Deleterious effects of glucocorticoid replacement on bone in women after long-term remission of Cushing’s syndrome. J. Bone Miner. Res. 24, 1841–1846 (2009)
C. di Somma, R. Pivonello, S. Loche et al., Effect of 2 years of cortisol normalization on the impaired bone mass and turnover in adolescent and adult patients with Cushing’s disease: a prospective study. Clin. Endocrinol. (Oxf) 58, 302–308 (2003)
L.A. Cohn, Glucocorticosteroids as immunosuppressive agents. Semin. Vet. Med. Surg. (Small Anim) 12, 150–156 (1997)
N. Auphan, J.A. DiDonato, C. Rosette, A. Helmberg, M. Karin, Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 270, 286–290 (1995)
R.I. Scheinman, P.C. Cogswell, A.K. Lofquist, A.S. Baldwin Jr, Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science 270, 283–286 (1995)
M.S. Lionakis, D.P. Kontoyiannis, Glucocorticoids and invasive fungal infections. Lancet 362, 1828–1838 (2003)
G.G. Fareau, R. Vassilopoulou-Sellin, Hypercortisolemia and infection. Infect. Dis. Clin. North Am. 21, 639–657 (2007)
N.J. Sarlis, S.J. Chanock, L.K. Nieman, Cortisolemic indices predict severe infections in Cushing syndrome due to ectopic production of adrenocorticotropin. J. Clin. Endocrinol. Metab. 85, 42–47 (2000)
R.C. Bakker, P.R. Gallas, J.A. Romijn, W.M. Wiersinga, Cushing’s syndrome complicated by multiple opportunistic infections. J. Endocrinol. Invest. 21, 329–333 (1998)
R. Pivonello, C. Simeoli, M.C. De Martino et al., Neuropsychiatric disorders in Cushing’s syndrome. Front. Neurosci. 9, 129 (2015)
N. Sonino, G.A. Fava, A.R. Raffi, M. Boscaro, F. Fallo, Clinical correlates of major depression in Cushing’s disease. Psychopathology 31, 302–306 (1998)
J. Tiemensma, N.R. Biermasz, H.A. Middelkoop et al., Increased prevalence of psychopathology and maladaptive personality traits after long-term cure of Cushing’s disease. J. Clin. Endocrinol. Metab. 95, E129–E141 (2010)
C. Dimopoulou, M. Ising, H. Pfister et al., Increased prevalence of anxiety-associated personality traits in patients with Cushing’s disease: a cross-sectional study. Neuroendocrinology 97, 139–145 (2013)
A.A. Kasperlik-Zaluska, J. Slowinska-Srzednicka, W. Zgliczynski, A woman who gained weight and became schizophrenic. Lancet 361, 705 (2003)
E. Singer, S. Strohm, U. Gobel et al., Cushing’s disease, hypertension, and other sequels. Hypertension 52, 1001–1005 (2008)
M.N. Starkman, B. Giordani, S.S. Gebarski et al., Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing’s disease. Biol. Psychiatry 46, 1595–1602 (1999)
N.E. Simmons, H.M. Do, M.H. Lipper, E.R. Laws Jr, Cerebral atrophy in Cushing’s disease. Surg. Neurol. 53, 72–76 (2000)
I. Bourdeau, C. Bard, B. Noel et al., Loss of brain volume in endogenous Cushing’s syndrome and its reversibility after correction of hypercortisolism. J. Clin. Endocrinol. Metab. 87, 1949–1954 (2002)
C.D. Andela, S.J. van der Werff, J.N. Pannekoek et al., Smaller grey matter volumes in the anterior cingulate cortex and greater cerebellar volumes in patients with long-term remission of Cushing’s disease: a case-control study. Eur. J. Endocrinol. 169, 811–819 (2013)
H. Forget, A. Lacroix, H. Cohen, Persistent cognitive impairment following surgical treatment of Cushing’s syndrome. Psychoneuroendocrinology 27, 367–383 (2002)
D.P. Merke, J.N. Giedd, M.F. Keil et al., Children experience cognitive decline despite reversal of brain atrophy one year after resolution of Cushing syndrome. J. Clin. Endocrinol. Metab. 90, 2531–2536 (2005)
J. Tiemensma, N.E. Kokshoorn, N.R. Biermasz et al., Subtle cognitive impairments in patients with long-term cure of Cushing’s disease. J. Clin. Endocrinol. Metab. 95, 2699–2714 (2010)
O. Ragnarsson, P. Berglund, D.N. Eder, G. Johannsson, Long-term cognitive impairments and attentional deficits in patients with Cushing’s disease and cortisol-producing adrenal adenoma in remission. J. Clin. Endocrinol. Metab. 97, E1640–E1648 (2012)
L. Pikkarainen, T. Sane, A. Reunanen, The survival and well-being of patients treated for Cushing’s syndrome. J. Intern. Med. 245, 463–468 (1999)
M.D. Johnson, C.J. Woodburn, M.L. Vance, Quality of life in patients with a pituitary adenoma. Pituitary 6, 81–87 (2003)
S.M. Webb, X. Badia, M.J. Barahona et al., Evaluation of health-related quality of life in patients with Cushing’s syndrome with a new questionnaire. Eur. J. Endocrinol. 158, 623–630 (2008)
M.O. van Aken, A.M. Pereira, N.R. Biermasz et al., Quality of life in patients after long-term biochemical cure of Cushing’s disease. J. Clin. Endocrinol. Metab. 90, 3279–3286 (2005)
J.R. Lindsay, T. Nansel, S. Baid, J. Gumowski, L.K. Nieman, Long-term impaired quality of life in Cushing’s syndrome despite initial improvement after surgical remission. J. Clin. Endocrinol. Metab. 91, 447–453 (2006)
R. Pivonello, M.C. De Martino, M. De Leo et al., Cushing’s syndrome: aftermath of the cure. Arq. Bras. Endocrinol. Metabol. 51, 1381–1391 (2007)
J.T. Chow, G.B. Thompson, C.S. Grant et al., Bilateral laparoscopic adrenalectomy for corticotrophin-dependent Cushing’s syndrome: a review of the Mayo Clinic experience. Clin. Endocrinol. (Oxf) 68, 513–519 (2008)
Ketoconazole HRA summary of product characteristics. 2015. http://www.medicines.org.uk/emc/medicine/30077
Metopirone summary of product characteristics. 2015. http://www.medicines.org.uk/emc/medicine/26460
E. Daniel, J.D. Newell-Price, Therapy of endocrine disease: steroidogenesis enzyme inhibitors in Cushing’s syndrome. Eur. J. Endocrinol. 172, R263–R280 (2015)
M. Fleseriu, R. Pivonello, J. Young et al., Osilodrostat, a potent oral 11β-hydroxylase inhibitor: 22-week, prospective, Phase II study in Cushing’s disease. Pituitary 19, 138–148 (2016)
Corcept Therapeutics. Mifepristone (Korlym) prescribing information. 2012. http://www.korlym.com/docs/KorlymPrescribingInformation.pdf
H.G. Fein, T.B. Vaughan III, H. Kushner, D. Cram, D. Nguyen, Sustained weight loss in patients treated with mifepristone for Cushing’s syndrome: a follow-up analysis of the SEISMIC study and long-term extension. BMC. Endocr. Disord. 15, 63 (2015)
M. Fleseriu, B.M. Biller, J.W. Findling et al., Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J. Clin. Endocrinol. Metab. 97, 2039–2049 (2012)
N.A. Tritos, B.M.K. Biller, Advances in medical therapies for Cushing’s syndrome. Discov. Med. 13, 171–179 (2012)
Novartis Pharmaceuticals Corporation. Signifor prescribing information. 2015. http://www.pharma.us.novartis.com/product/pi/pdf/signifor.pdf
Novartis Pharma AG. Signifor summary of product characteristics. 2013. http://www.signifor.com/european-product-characteristics.jsp
A. Colao, S. Petersenn, J. Newell-Price et al., A 12-month phase 3 study of pasireotide in Cushing’s disease. N. Engl. J. Med. 366, 914–924 (2012)
R. Pivonello, S. Petersenn, J. Newell-Price et al., Pasireotide treatment significantly improves clinical signs and symptoms in patients with Cushing’s disease: results from a Phase III study. Clin. Endocrinol. (Oxf) 81, 408–417 (2014)
A.R. Lila, R.A. Gopal, S.V. Acharya et al., Efficacy of cabergoline in uncured (persistent or recurrent) Cushing disease after pituitary surgical treatment with or without radiotherapy. Endocr. Pract. 16, 968–976 (2010)
R. Pivonello, M.C. De Martino, P. Cappabianca et al., The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J. Clin. Endocrinol. Metab. 94, 223–230 (2009)
R.A. Feelders, C. de Bruin, A.M. Pereira et al., Pasireotide alone or with cabergoline and ketoconazole in Cushing’s disease. N. Engl. J. Med. 362, 1846–1848 (2010)
R.A. Feelders, S.J. Pulgar, A. Kempel, A.M. Pereira, The burden of Cushing’s disease: clinical and health-related quality of life aspects. Eur. J. Endocrinol. 167, 311–326 (2012)
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Richard Ogilvy-Stewart, PhD, Mudskipper Business Ltd, for medical editorial assistance with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RP and AC have received speaker and consultancy fees from Novartis. MCDM, MDL and CS have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Pivonello, R., De Martino, M.C., De Leo, M. et al. Cushing’s disease: the burden of illness. Endocrine 56, 10–18 (2017). https://doi.org/10.1007/s12020-016-0984-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0984-8