Abstract
Breast cancer is the most common malignancy among women worldwide. There is extensive literature on the relationship between body weight and breast cancer risk but some doubts still remain about the role of adipokines per se, the role of insulin and glucose regardless of obesity, as well as the crosstalk between these players. Thus, in this study, we intend to determine the relation between body mass index (BMI), glycaemia, insulinemia, insulin-resistance, blood adipokine levels and tumour characteristics in a Portuguese group of pre- and postmenopausal overweight/obese women with breast cancer. We evaluated clinical and biochemical data in 154 participants, divided in 4 groups: (1) control with BMI <25 kg/m2, n = 29 (CT); (2) control with BMI >25 kg/m2, n = 48 (CTOb); (3) breast cancer with BMI <25 kg/m2, n = 30 (BC); and (4) breast cancer with BMI >25 kg/m2, n = 47 (BCOb). In women with breast cancer, we also performed tumour characterization. We found that BCOb present increased fasting blood glucose, insulin, resistin and monocyte chemoattractant protein 1, insulin resistance and more aggressive tumours. Notably, this profile is not correlated with BMI, proposing the involvement of other processes than adiposity. Altogether, our results suggest that glucose dysmetabolism, insulin resistance and changes in adipokine secretion, in particular resistin, may be involved in the development and progression of breast cancer in overweight/obese pre- and postmenopausal women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most common malignancy among women worldwide [1], being crucial to discover prognostic factors and therapeutic targets to fight the disease. Well-established risk factors for the development and progression of this kind of tumours are prolonged life-span, reproductive hormonal influence, family history of BC and anthropometric characteristics [2, 3]. Epidemiological studies support that obesity is positively associated with the development and progression of several types of cancer, namely breast, and also with increased cancer mortality [3, 4].
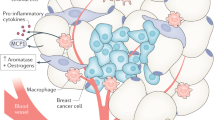
Adipose tissue, as an active endocrine organ, secretes several bioactive adipokines, regulating physiological and pathological processes such as appetite, insulin sensitivity and resistance, inflammation, immunity, haematopoiesis and angiogenesis [3]. When adipose tissue becomes dysfunctional in obesity, it triggers metabolic dysregulation, hypoxia, inflammation, impaired adipokines expression and angiogenesis, insulin resistance and macrophage recruitment [5]. Growing evidence has shown that this constellation of alterations could be implicated in breast tumorigenesis, higher recurrence and mortality [3, 4]. In fact, the breast adipose tissue produces bioactive molecules, exerting their effects on the normal and neoplastic glandular mammary tissue by endocrine, paracrine and autocrine mechanisms [3, 6].
In sum, there is extensive literature on the relationship between body weight and BC risk, but the role of adipokines per se, the role of insulin and glucose regardless of obesity, as well as the crosstalk between these players remains controversial. The metabolic dysregulation tends to be devalued and masked, being crucial to clarify their role in carcinogenesis. Hence, in this study, we intended to determine the relation between body mass index (BMI), glycaemia, insulinemia, insulin-resistance, blood adipokine levels and tumour characteristics in a group of overweight/obese Portuguese pre- and postmenopausal women with BC.
Materials and methods
Participants and study design
A total number of 154 Portuguese women were recruited from the University Hospital Centre of Coimbra (CHUC) between 2009 and 2013. They were divided into four experimental groups according to their BMI and the presence or absence of BC: Control with BMI <25 kg/m2 (CT group, n = 29); Control with BMI >25 kg/m2 (CTOb group, n = 48); BC with BMI <25 kg/m2 (BC group, n = 30); and BC with BMI >25 kg/m2 (BCOb group; n = 47).
The control subjects (CT group) were selected at the Internal Medicine Department in annual check-up of the aforementioned hospital. Women of the CTOb group were also selected at this Department, in their first Nutrition consultation. All of them were included in the study if they had never been diagnosed with benign or malignant disease nor have family history of BC. Patients of BC and BCOb groups were selected and surgically treated at the Gynaecology Department of CHUC. They had been newly diagnosed with cancer from a positive mammography and had histologically confirmed BC without no prior cancer treatment. All participants were free from any infection or other acute disease at the time of enrolment in the study.
Clinical information (personal and family medical history) as well as anthropometric data (height and weight) were collected for all participants under similar conditions by the same research physician during the first consultation. BMI was calculated as weight in kilograms divided by squared height in meters (kg/m2). Women were considered postmenopausal at the time of blood collection if they were at least 12 months after menopause or reported a bilateral oophorectomy.
Also at the first consultation, fasting blood samples (at least 8 h) were collected by venous puncture for later biochemical analysis. This was always performed by the same nurse and immediately delivery to the Laboratory of Physiology of the Faculty of Medicine.
The study has been approved by the Ethical Committee of CHUC and written informed consent was obtained from all participants.
Biochemical analysis
For the determination of plasma and serum parameters, fasting blood was centrifuged (2500 g) at 4 °C and stored at −80 °C.
Serum glucose levels were determined by an automatic analyser using a commercial kit (Olympus—Diagnóstica Portugal, Produtos de Diagnóstico SA, Portugal).
Serum values of the studied adipokines, leptin, adiponectin and resistin, and the chemokine monocyte chemoattractant protein 1 (MCP1) were determined using the following commercial enzyme-linked immunosorbent assay kits: Duo Set ELISA Development System Human Leptin, Duo Set ELISA Development System Human Adiponectin, Duo Set ELISA Development System Human Resistin (inter-assay CV <10; intra-assay CV <6; detection range = 31.20–2000 pg/mL) all from R&D System, UK, and Human MCP1 ELISA Set, BD Biosciences Pharmingen, CA, EUA. Plasma levels of insulin were also measured by ELISA kit using Mercodia Insulin ELISA, Mercodia AB, Sweden.
Insulin resistance was evaluated through the homeostasis model assessment (HOMA) index. HOMA was calculated as logarithm of ((If) × (Gf))/22.5, where (If) is the fasting insulin level (μU/mL) and (Gf) is the fasting glucose level (mmol/L).
Tumour characterization
Tumour tissue was obtained by mastectomy or tumourectomy. Tumour type, grade and size and lymph node involvement were evaluated by a trained pathologist at the Anatomopathology Department of CHUC. For cancer staging notation, the TNM classification of malignant tumours was applied. The status of oestrogen and progesterone receptors and HER-2 protein was evaluated by immunohistochemistry following routine diagnostic techniques, at the same department. When the results were ambiguous for HER-2 protein, the confirmation was made by FISH/SISH technique.
Statistical analysis
The statistical analysis was performed using R statistical software, version 3.0.2 and IBM-SPSS version 21 for Windows. A significance level of 0.05 was adopted.
An univariate analysis was performed in which each variable was compared between the four groups (CT, CTOb, BC, BCOb). Quantitative variables were first checked for normality using Kolmogorov–Smirnov tests together with an inspection of their graphical representations. Upon verifying the normality requirements not being met in most cases, Kruskal–Wallis tests were consistently used to assess differences between groups. Post hoc Mann–Whitney U tests, which allowed for pairwise comparisons, were corrected for multiple comparisons using Tukey–Kramer adjustments. Categorical variables were assessed using χ 2 tests. Correlations between quantitative variables were evaluated using Spearman correlation coefficient. The statistical power of this study is 0.98. This was computed by considering the comparison of the resistin values between the groups of CT versus BCOb, using a Mann–Whitney U test, with a significance level of 0.05.
Results
Anthropometric data
Women were divided in four experimental groups according to their BMI and the presence or absence of BC, as mentioned in Sect. 2. BMI distribution between groups is shown in Table 1. According to the World Health Organization BMI classification, CTOb and BCOb women are obese or overweight. Their BMI values are significantly higher (p < 0.001) than CT and BC experimental groups. Women's age is equally distributed across groups as well as the presence of menopause. No statistically significant differences in these two variables are found between groups (p = 0.314 and p = 0.953, respectively) (Table 1).
Glucose metabolism and insulin resistance
Women included in the BCOb group show significantly higher levels of fasting blood glucose and insulinemia as well as HOMA index than healthy controls (CT) (p < 0.001). Moreover, glycaemia was also higher than in CTOb group (p < 0.001) and insulin and HOMA index higher than in BC group (p < 0.05) (Table 2). This metabolic profile indicates that overweight/obese women with BC consistently show insulin resistance and a deregulation of glucose metabolism. Importantly, BC women also show high fasting glucose levels when comparing with healthy controls (p < 0.05). An elevation of insulin levels and HOMA index are also observed in CTOb group (p < 0.001), but less pronounced than in BCOb women (Table 2). Interesting to note is the percentage of women with prediabetes (impaired fasting glucose—IFG) and diabetes, which is different across groups. In CT group, 6 % have IGF while in CTOb, this percentage increases to 20 % and in BC to 33 %. In CTOb group, only one woman presents diabetes. Notably, 50 % of BCOb women have hyperglycaemia: 25 % have IFG and 25 % diabetes.
Systemic adipokine levels
In order to evaluate adipose tissue endocrine function, systemic levels of main adipokines, leptin, adiponectin, resistin and the inflammatory chemokine MCP1 were evaluated (Table 2). When compared with CT group, higher levels of leptin are observed in both overweight/obese groups (CTOb and BCOb) (p < 0.001), which is explained by adipose tissue expansion. Although no significant differences are observed in circulating adiponectin levels between groups (p = 0.148), they tend to be decreased in CTOb and both cancer groups when compared to CT group. In contrast, a trend to increased resistin serum levels is observed in CTOb and BC groups, but their levels are only significantly higher in BCOb women (p < 0.001) compared to CT.
Such results are in accordance with systemic values of MCP1. Although post hoc tests could not elucidate which groups were driving the found differences between groups (p = 0.029), the lowest p value was found for the comparison between CT and BCOb (p = 0.084).
Radar plots and correlations of physiological parameters
Radar plots in Fig. 1 illustrate clearly the different profiles across groups. Only variables for which statistically significant differences between groups were found in the univariate analysis were considered. Accordingly with what is depicted in Table 2, radar plots show the highest values in all parameters in BCOb women and the lowest ones in CT group. Comparing all experimental groups, it is evident the link between overweight/obesity with MCP1, leptin and, with less strength, insulin values. Slight differences were found when glucose and HOMA are represented with this method. Although resistin increases in BC and BCOb groups, only when cancer and BMI >25 kg/m2 are combined the highest levels are attained.
Radar plots of variables body mass index (BMI), glucose, insulin, homeostasis model assessment for insulin resistance (HOMA), leptin, resistin and monocyte chemoattractant protein-1 (MCP-1). Only variables for which statistically significant differences between groups were found in the univariate analysis are considered. CT control with BMI <25 kg/m2 (n = 29), CTOb control with BMI >25 kg/m2 (n = 48), BC breast cancer with BMI <25 kg/m2 (n = 30) and BCOb breast cancer with BMI >25 kg/m2 (n = 47)
Spearman correlation coefficients between BMI and all systemic parameters were calculated to investigate the involvement of overweight/obesity in cancer (Table 3). In CTOb group, significant correlations are found for insulin (r = 0.36, p = 0.01), HOMA index (r = 0.35, p = 0.02), leptin (r = 0.61, p < 0.001), resistin (r = 0.51, p < 0.001) and MCP1 (r = 0.47, p = 0.001). In the BCOb group only leptin maintains a significant correlation with BMI (r = 0.38, p = 0.01). All the other parameters lose their correlation when cancer is present, suggesting that other players than BMI are contributing to hyperresistinemia and metabolic dysregulation. With this scenario, we performed a Spearman correlation test between resistin and all systemic parameters and also a partial Spearman correlation test, with BMI adjustment (Table 4). In CTOb group, we observed positive correlations between resistin and leptin (r = 0.49, p < 0.01) and between resistin and MCP-1 (r = 0.4, p = 0.01) without BMI adjustment. In BC group, resistin correlates with MCP-1 (r = 0.4, p = 0.04), only after BMI adjustment. In BCOb group, resistin negatively correlates with adiponectin both without (r = −0.33, p = 0.03) and with (r = −0.37, p = 0.01) BMI adjustment. In this group, resistin also correlates with MCP-1 both without (r = 0.45, p < 0.01) and with (r = 0.46, p < 0.01) BMI adjustment.
Tumour data
Regarding the tumour types, the majority of women in both cancer groups have invasive ductal carcinoma (80 % in BC and 79 % in BCOb). In BC group, 10 % have ductal carcinoma in situ and 10 % invasive lobular carcinoma. In BCOb group 13 % have ductal carcinoma in situ and 4 % invasive lobular carcinoma and the same percentage for mucinous carcinoma (Table 5). Furthermore, the majority of women in both cancer groups present the oestrogen and progesterone receptors (96 % in BC and 88 % in BCOb). Of these, 23 % of BC women and 29 % of BCOb women present HER-2 (Table 5). Depicted in Fig. 2a, the tumour characterization in both cancer groups indicates that overweight/obese women have more aggressive tumours. In BCOb group the percentage of women with tumours in grade 3 (17 %), size bigger than 2 cm (21 %), lymph node involvement (45 %) and tumour staging II (49 %) is higher than in BC women (3, 7, 33 and 34 %, respectively). None of these parameters were statistically significantly different between groups. Furthermore, for each tumour histopathological category (tumour grades: G1, G2 and G3; tumour stage: 0, I and II; involvement of lymph node: yes or no; tumour size: ≤2 cm and >2 cm), we assessed differences in levels of leptin, adiponectin, resistin and monocyte chemoattractant protein-1 (MCP-1). We only observed in BCOb group a statistically significant difference in leptin (p = 0.025) between women with and without lymph node involvement (Fig. 2b).
Tumour histopathological characterization. a Graphics show the percentage of different tumour grades (G1, G2 and G3) and stage (0, I and II), of involvement of lymph node (yes or no) and of tumour size (≤2 cm and >2 cm) in each group. b Differences in levels of leptin, adiponectin, resistin and monocyte chemoattractant protein-1 (MCP-1) across the categories of tumour histopathological characteristics. BC Breast cancer with BMI <25 kg/m2 (n = 27–30) and BCOb breast cancer with BMI >25 kg/m2 (n = 46–47)
Discussion
Growing evidence links overweight/obesity with high risk of BC development and worse prognosis [2, 3]. In our study, we observed in overweight/obese women with BC an adverse metabolic profile with glucose metabolism disorder, hyperinsulinemia and insulin resistance, as well as an altered profile in circulating chemokines and adipokines, especially resistin. These observations could be associated with more malignant clinicopathological tumour status also observed in these women.
Epidemiological evidences indicate that both pre- and postmenopausal women with obesity, insulin resistance, metabolic syndrome and type 2 diabetes (T2D) are at increased risk for BC development [3]. However, previous studies propose that the link between obesity and BC is stronger in postmenopausal women and show discrepant results when premenopausal women were considered [7]. In our study design, the influence of menopause in all evaluated parameters was first analysed. As there was no evidence of the influence of this parameter on the systemic parameters and also locally on tumour malignancy, the study was carried on with no division in pre- and postmenopausal women. Furthermore, as was already mentioned, the percentage of postmenopausal women is similar across groups (Table 1).
Hyperinsulinemia, insulin resistance and adipokine changes have been suggested to play a pivotal role in obesity-cancer association [3–5]. In obesity and T2D, insulin resistance leads to chronic hyperinsulinemia, which is linked to tumour growth [8] due to direct mitogenic effects of insulin and indirectly via increased production of insulin-like growth factor 1 (IGF1) [3, 4, 9]. In accordance, overweight/obese groups (CTOb and BCOb) show higher levels of fasting insulin and HOMA index. However, considering the cut-off values for Portuguese population (fasting insulin = 8.6 µU/mL and HOMA index = 2.33), only BCOb group reaches the values of hyperinsulinemia and insulin resistance [10] (Table 1). Moreover, Spearman correlation analysis shows a significant correlation coefficient between BMI and both HOMA index and insulinemia in CTOb group, which was not observed in BCOb women, showing that other factors than fat mass could contribute to insulin resistance and hyperinsulinemia in overweight/obese patients with BC (Table 3). Regarding glycaemia, higher values were reported in cancer groups (BC and BCOb) (Table 2). According to the American Diabetes Association criteria [11], 33 % of women in the BC group had IFG (only 1 woman has T2D) and 50 % women in BCOb group, of whom 25 % had T2D. While a positive association between obesity and cancer has been reported, high rates of cancer-related mortality have been seen in patients with prediabetes and T2D, regardless of BMI, suggesting that diabetes may be an independent risk factor for cancer [4, 12, 13]. One mechanism by which glucose could induce cancer progression is the induction of oxidative stress, leading to stimulation of inflammatory signalling pathways culminating in genomic instability and disruption of normal mechanisms of cellular signalling. All these mechanisms have already been associated with cancer [14].
Besides these metabolic abnormalities, changes of adipokine and chemokine profile were observed in overweight/obese women with cancer. Adipose tissue secretes a variety of bioactive molecules that regulate physiological and pathological processes and, in obesity, the deregulated expression of such molecules may thus be involved in the obesity-related BC [3, 5]. Recent evidences support the idea that impaired adipokine secretion results from adipose tissue dysfunction. Although the mechanisms involved are not fully understood, excessive accumulation of non-esterified fatty acids and defective vascular adaptation during adipose tissue expansion are thought to be involved. This could in turn conduce to hypoxia and a low-grade inflammatory state, mainly mediated by macrophages recruitment. Adipocyte and macrophage abnormal secretion create an inflammatory environment propitious for cancer development and progression [3, 5]. Several epidemiological studies suggested leptin, one of the classical adipokines, to be involved in cancer development and progression, although inconsistent and conflicting associations between circulating leptin and risk of BC were made [15]. Moreover, many studies did not isolate the BMI in the experimental design and others found no association between leptinemia and premenopausal or postmenopausal cancer [6, 15]. The potential pro-tumoural effects of leptin were supported by its mitogen actions, as leptin was shown to exert neoplastic effects in BC acting directly in tumour growth, migration and invasion signalling pathways or indirectly by decreasing tissue sensitivity to insulin, regulating inflammatory responses and tumour angiogenesis [15]. However, in our study, the observed increased levels in overweight/obese groups are consistent with the amount of body fat, which could be associated with hyperleptinemia and leptin resistance. Unlike leptin, adiponectin negatively correlates with body fat and has insulin-sensitizing, anti-inflammatory, anti-atherogenic and anti-neoplastic effects [16, 17]. Previous studies have shown that adiponectin could exert BC antineoplastic effects, directly by modulating survival signalling pathways or indirectly modulating insulin sensitivity and inflammation response. Although significant differences between groups were not found in our study, adiponectinemia tend to be lower in overweight/obese and cancer groups (Table 2). Apart from leptin and adiponectin, which are the most studied adipokines related to cancer pathogenesis, resistin caught our attention for having significantly high levels only in BCOb group (Table 2). Actually, in this group, resistin is significantly increased independently from BMI, as shown in Table 3 and as was already reported by other authors [18, 19]. Previous epidemiological studies support our findings, indicating that serum resistin is significantly high in patients with breast, gastric, and colorectal tumours and endometrial adenocarcinoma, oesophageal squamous cell carcinoma and lymphoma [19–22]. Furthermore, some studies highlighted that high resistin expression in BC tissue is associated with more aggressive clinicopathological status and adverse prognosis [19, 21, 22]. However, our data did not reveal any relationship between resistin and tumour histopathological characteristics (Fig. 2b).
The dissociation of increased levels of resistin from BMI led us to clarify its relationship with the others assessed parameters by assessing the partial Spearman correlations after BMI adjustment. Despite resistin had been linked with insulin resistance, we did not find a correlation between this adipokine and insulin, glucose or HOMA, neither before nor after BMI adjustment (Table 4).
Inflammation is other strong connection between resistin and cancer [19]. This adipokine leads to activation of NF-kB transcription factor, promoting the activation of pro-inflammatory genes directly involved in the initiation, promotion, and progression of carcinogenesis. NF-kB also induces the expression of some pro-inflammatory cytokines, such as IL-6, that, together with the activation of MAPK and PI3K/Akt cascades, stimulates cell proliferation, differentiation and upregulates antiapoptotic and angiogenic proteins in tumour cells [19]. Resistin also interferes with the production of endothelial adhesion molecules such as ICAM-1 and VCAM-1 and matrix metalloproteinases MMP-1 and MMP-2, crucial for metastasis [3, 19]. Our data corroborate this strong link between resistin and inflammation and its influence in carcinogenesis. Without BMI adjustment, resistin correlates with MCP-1 in both overweight/obese groups (CTOb and BCOb), whereas after the adjustment the correlation changes to cancer groups (BC and BCOb) (Table 4). As mentioned before, in unhealthy obesity state as well in the tumour microenvironment, inflammation is present, characterized for increased production of pro-inflammatory and chemoattractant molecules such as MCP-1 [5, 19] that, in the present study, reach the highest value in BCOb (Table 2). Partial Spearman correlations show that even without the chronic inflammation influence induced by obesity, MCP-1 is an important link between resistin and cancer. Moreover, in BCOb group, resistin is negatively correlated with adiponectin, an anti-inflammatory cytokine, both before and after BMI adjustment (Table 4).
Results depicted in Table 3 also make more evident that high levels of resistin, MCP-1, insulin and HOMA found in BCOb group could not be only related with body weight. Whereas leptin, resistin, MCP-1, insulin and HOMA are positively correlated with BMI in CTOb group, in BCOb group, regardless of overweight/obesity, only leptin keeps the positive correlation with BMI.
Our results suggest that, instead of an exclusive influence of body weight/fat mass, breast cancer may in part be governed by adipose tissue dysfunction in overweight/obesity, being associated with insulin resistance, inflammation and impaired adipokine secretion.
In conclusion, we found in overweight/obese women with BC an adverse metabolic profile with glucose dysmetabolism, hyperinsulinemia and insulin resistance, concomitant with changes of circulating chemokines and adipokines, especially resistin. We strongly consider this crosstalk between metabolic, inflammatory and endocrine profiles as a pivotal role in the development and more aggressive clinicopathological features of breast tumour. The small sample size, the weak match between groups and the case–control design have to be taken into account. These issues should be considered in the design of future studies to confirm the presented results. Thus, our data need to be analysed further and validated in a larger independent series. Future longitudinal and mechanistic studies are needed in order to prove causality and provide further insights into the role of such parameters in breast malignancy.
References
V.G. Vogel, Epidemiology, genetics, and risk evaluation of postmenopausal women at risk of breast cancer. Menopause 15, 782–789 (2008)
A.G. Renehan, M. Tyson, M. Egger, R.F. Heller, M. Zwahlen, Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371, 569–578 (2008)
M. Dalamaga, Obesity, insulin resitance, adipocytokines and breast cancer: New biomarkers and attractive therapeutic targets. World J. Exp. Med. 20, 34–42 (2013)
D.H. Cohen, D. LeRoith, Obesity, type 2 diabetes, and cancer: the insulin and IGF connection. Endocr. Relat. Cancer 19, F27–F45 (2012)
P. Matafome, D. Santos-Silva, C.M. Sena, R. Seiça, Common mechanisms of dysfunctional adipose tissue and obesity-related cancers. Diabetes Metab. Res. Rev. 29, 285–295 (2013)
A.M. Lorincz, S. Sukumar, Molecular links between obesity and breast cancer. Endocr. Relat. Cancer 13, 279–292 (2006)
G.L. Anderson, M.L. Neuhouse, Obesity and the risk for premenopausal and postmenopausal breast cancer. Cancer Prev. Res. 5, 515–521 (2012)
L. Vona-Davis, D.P. Rose, Type 2 diabetes and obesity metabolic interactions: common factors for breast cancer risk and novel approaches to prevention and therapy. Curr. Diab. Rev. 8, 116–130 (2012)
D.P. Rose, L. Vona-Davis, The cellular and molecular mechanisms by which insulin influences breast cancer risk and progression. Endocr. Relat. Cancer 19, R225–R241 (2012)
A.T. Timóteo, F. Miranda, M.M. Carmo, R.C. Ferreira, Optimal cut-off value for homeostasis model assessment (HOMA) index of insulin- resistance in a population of patients admitted electively in a portuguese cardiology ward. Acta Med. Port. 27, 473–479 (2014)
American Diabetes Association, Classification and diagnosis of diabetes. Diabetes Care 38, S8–S16 (2015)
F. Xue, K.B. Michels, Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am. J. Clin. Nutr. 86, s823–s835 (2007)
Y. Huang, X. Cai, M. Qiu, P. Chen, H. Tang, Y. Hu, Y. Huang, Prediabetes and the risk of cancer: a meta-analysis. Diabetologia 57, 2261–2269 (2014)
N.S. Brown, R. Bicknell, Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 3(5), 323–327 (2001)
H.S. Moon, M. Dalamaga, S.Y. Kim, S.A. Polyzos, O.P. Hamnvik, F. Magkos, J. Paruthi, C.S. Mantzoros, Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr. Rev. 34, 377–412 (2013)
R.S. Ahima, Adipose tissue as an endocrine organ. Obesity 14(5), 242S–249S (2006)
N.S. Brown, R. Bicknell, Hypoxia and oxidative stress in breast cancer: Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 3, 323–327 (2001)
C.A. Sun, M.H. Wu, C.H. Chu, Y.C. Chou, G.C. Hsu, T. Yang, YuCP Chou Wy, J.C. Yu, Adipocytokine resistin and breast cancer. Breast Cancer Res. Treat. 123, 869–876 (2010)
M. Dalamaga, Resistin as a biomarker linking obesity and inflammation to cancer: potential clinical perspectives. Biomark. Med. 8(1), 107–118 (2014)
Y.C. Lee, Y.J. Chen, C.W. Chun, S. Lo, M.F. Hou, S.S. Yuan, Resistin expression in breast cancer tissue as a marker of prognosis and hormone therapy stratification. Gynecol. Oncol. 125, 742–750 (2012)
M. Dalamaga, K. Karmaniolas, E. Papadavid, N. Pelekanos, G. Sotiropoulos, A. Lekka, Hyperresistinemia is associated with postmenopausal breast cancer. Menopause 20, 845–851 (2013)
M. Dalamaga, G. Sotiropoulos, K. Karmaniolas, N. Pelekanos, E. Papadavid, A. Lekka, Serum resistin: a biomarker of breast cancer in postmenopausal women? Association with clinicopathological characteristics, tumor markers, inflammatory and metabolic parameters. Clin. Biochem. 46, 584–590 (2013)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no potential conflicts of interest.
Funding
This work was funded with a Grant (PTDC/SAU-MET/121133/2010) from the Portuguese Foundation of Science and Technology.
Rights and permissions
About this article
Cite this article
Crisóstomo, J., Matafome, P., Santos-Silva, D. et al. Hyperresistinemia and metabolic dysregulation: a risky crosstalk in obese breast cancer. Endocrine 53, 433–442 (2016). https://doi.org/10.1007/s12020-016-0893-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0893-x