Abstract
Betatrophin is a newly identified hormone determined to be a potent inducer of pancreatic beta cell proliferation in response to insulin resistance in mice. Polycystic ovary syndrome (PCOS) is an inflammatory-based metabolic disease associated with insulin resistance. However, no evidence is available indicating whether betatrophin is involved in women with PCOS. The objective of this study was to ascertain whether betatrophin levels are altered in women with PCOS. This study was conducted in secondary referral center. This cross-sectional study included 164 women with PCOS and 164 age- and BMI-matched female controls. Circulating betatrophin levels were measured using ELISA. Metabolic and hormonal parameters were also determined. Circulating betatrophin levels were significantly elevated in women with PCOS compared with controls (367.09 ± 55.78 vs. 295.65 ± 48.97 pg/ml, P < 0.001). Betatrophin levels were positively correlated with insulin resistance marker homeostasis model assessment of insulin resistance (HOMA-IR), free-testosterone, high-sensitivity C-reactive protein (hs-CRP), atherogenic lipid profiles, and BMI in PCOS. Multivariate logistic regression analyses revealed that the odds ratio for PCOS was 2.51 for patients in the highest quartile of betatrophin compared with those in the lowest quartile (95 % CI 1.31–4.81, P = 0.006). Multivariate regression analyses showed that HOMA-IR, hs-CRP, and free-testosterone were independent factors influencing serum betatrophin levels. Betatrophin levels were increased in women with PCOS and were associated with insulin resistance, hs-CRP, and free-testosterone in these patients. Elevated betatrophin levels were found to increase the odds of having PCOS. Further research is needed to elucidate the physiologic and pathologic significance of our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrinopathy affecting 5–10 % of women of reproductive age and is characterized by ovulatory dysfunction, polycystic ovaries, biochemical (elevated androgens), and/or clinical (hirsutism and/or acne) hyperandrogenism. PCOS is associated with low-grade chronic inflammation, insulin resistance, glucose intolerance, metabolic syndrome, obesity, hyperlipidemia, hypertension, and an increased risk of developing type 2 diabetes [1–4]. Although the etiopathogenesis of PCOS is not yet well established, insulin resistance and consequent development of hyperinsulinemia are known to play important roles. Despite extensive research, the mechanisms underlying insulin resistance in PCOS patients are not completely understood. Overall, insulin resistance and compensatory hyperinsulinemia affect up to 70 % of women with PCOS [5–10].
Betatrophin, a newly identified peptide hormone secreted mainly by liver and adipose tissue, is a member of a family of angiopoietin-like proteins and is implicated in the regulation of lipid and glucose metabolism. As the peptide was discovered independently by different groups, it is known by various names that include ‘re-feeding induced fat and liver (RIFL) protein,’ ‘lipasin,’ ‘angiopoetin-like 8 protein’ (ANGPLT8), and ‘hepatocellular carcinoma-associated protein’ (TD26) [11–14]. Originally, betatrophin was determined to function in lipid metabolism by reducing triglyceride clearance through lipoprotein lipase inhibition [12]. It has been implicated that the hormone is a key mediator of the postprandial trafficking of triglyceride-fatty acids to adipose tissue [15]. In addition, betatrophin sequence variations were reported in lipid metabolism [13, 16, 17]. The link between betatrophin and glucose homoeostasis has been recently reported by Yi et al. [14]. The group demonstrated that secretion of betatrophin increases in response to insulin resistance in mice, which stimulates beta cell replication and expansion. Administration of betatrophin was shown to improve glucose homeostasis in these mice [14]. These findings indicate that betatrophin might make a promising target for new therapies in diabetes, although there are also contradictory results which show no such link between the peptide and glucose homeostasis [15, 18]. Furthermore, the results of the studies evaluating the relationship between diabetes and betatrophin are not uniform and any causal relationship between these two remains to be established. Several recent studies have reported betatrophin levels in type 2 diabetes and obesity. Different research groups have shown elevated, reduced, or unchanged betatrophin values depending on the features of the recruited population [19–22]. Controversial results have also been reported regarding the correlation between betatrophin and various metabolic parameters associated with metabolic diseases.
PCOS is a metabolic disease associated with insulin resistance. Therefore, in this study, we aimed to investigate (1) whether betatrophin metabolism is altered in women with PCOS, (2) whether the peptide is associated with the development of PCOS, and (3) whether betatrophin levels are correlated with metabolic disturbances.
Materials and methods
Ethics statement
This study was approved by the local ethics committee of the Dokuz Eylul University Medical School. The subjects gave their oral and written informed consent before their inclusion in the study. The study adhered strictly to the Declaration of Helsinki Principles as revised in 2008.
Subjects and study design
The study was designed as cross-sectional and was conducted between March 2014 and March 2015 in the Department of Obstetrics and Gynecology, Merkezefendi State Hospital, Manisa, Turkey. We consecutively recruited 164 subjects with PCOS and 164 age- and body mass index (BMI)-matched subjects with normal menstrual cycles who either presented to our clinic or volunteered via local advertisements and met the inclusion and exclusion criteria. Clinical and anthropometric variables were evaluated by a single investigator in all subjects. All the recruited women were Caucasian.
PCOS group
PCOS was diagnosed in this study based on the 2003 Rotterdam consensus criteria [23]. To limit metabolic heterogeneity within samples, all three of the following criteria were used for diagnosis (after excluding other causes of hyperandrogenism):
-
1.
Oligo- or anovulatory menstrual dysfunction (manifested as infrequent bleeding at intervals >35 days);
-
2.
Clinical and/or biochemical signs of hyperandrogenism—The Ferriman–Gallwey (FG) method was used to determine hirsutism [24]. Subjects were deemed hirsute if their FG score was ≥8. Biochemical hyperandrogenism was defined as an increase in the serum concentrations of free-testosterone (normal values: 2.9–31.8 ng/dl) and/or total testosterone (normal values: 0.15–0.7 ng/ml);
-
3.
Typical ultrasonographic finding of polycystic ovaries (with one ovary being sufficient for diagnosis), defined as the presence of ≥12 follicles measuring 2–9 mm in diameter or an ovarian volume >10 ml (without a cyst or dominant follicle in either ovary).
Control group
Control subjects were recruited from healthy women who visited the gynecology clinic for routine annual examination or from hospital workers and university students through local advertisements. All volunteers for the control group had regular menstrual cycles (28–32 days); they had no concomitant health problems or signs of hirsutism, acne, or hyperandrogenism.
Exclusion criteria
The exclusion criteria adopted during subject selection were (1) other causes of irregular menstrual cycles and/or androgen excess (i.e., Cushing’s syndrome, hyperprolactinemia, congenital adrenal hyperplasia, or other diseases of the adrenal gland, thyroid disorders, galactorrhea, breastfeeding, and pregnancy); (2) impaired glucose tolerance or type 1/type 2 diabetes; (3) hypertension, hyperlipidemia, active or chronic liver or renal failure, or congestive heart failure; (4) a history of coronary artery disease, gestational diabetes mellitus (GDM), or acute infection (within the preceding 14 days); (5) presence of any chronic inflammatory and autoimmune disease; (6) known malignancy; (7) hormonal contraception and/or anti-androgen therapy (within the preceding 6 months); and (8) BMI ≥35 kg/m2, age <18 or >35 years. The use of medications for dyslipidemia, hypertension, hyperglycemia, insulin resistance, or obesity was an exclusion criterion for this study. None of the subjects used tobacco or alcohol.
Anthropometric evaluation
A detailed history was obtained from each of the study participants. Following general physical examination of the subjects, anthropometric measurements were performed (age, weight, height, and waist circumference). Height (in centimeters) and weight (in kilograms) were measured with the subject barefoot in light daily clothes. Body mass index (BMI) was calculated using the formula weight (kg)/square meter of height (m2). Waist circumference (in cm) was measured midway between the lower rib margin and the iliac crest at the end of a gentle expiration. Blood pressure was measured in the sitting position after a rest period of at least 15 min. The average of three measurements was calculated.
Biochemical evaluation
Venous blood samples were collected from the antecubital veins of the subjects during the early follicular phase of menstrual bleeding (3rd to 5th days) in the morning (8:00–9:00 a.m.), after the onset of early spontaneous or progesterone-induced menses, following at least 12 h of fasting. Samples were separated by centrifugation for 15 min at 2000×g after clotting for 30 min at room temperature. The serum samples were subsequently stored in aliquots at −80 °C prior to the analysis of betatrophin.
Fasting blood glucose (FBG), serum insulin, hs-CRP, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), free-testosterone, total testosterone, dehydroepiandrosterone sulfate (DHEA-S), follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), and betatrophin levels were measured.
FBG, serum hs-CRP, total cholesterol, total HDL-C, and triglyceride levels were determined using an auto-analyzer (Abbott Architect C 16000, IL, USA) with its dedicated kits (Abbott Diagnostics, Wiesbaden, Germany). LDL-C was calculated using the Friedewald equation: LDL-C = total cholesterol-(HDL-C + Triglyceride/5). Non-HDL-C was calculated as HDL-C subtracted from total cholesterol. Serum insulin levels were measured by an auto-analyzer (Abbott Architect I2000, IL, USA) using chemiluminescent microparticle immunoassay (CMIA) with its dedicated kits (Abbott Diagnostics, Wiesbaden, Germany). Serum FSH, LH, E2, total testosterone, and DHEA-S levels were measured by CMIA (Beckman Coulter Inc., Brea, CA, USA). Serum free-testosterone levels were measured using the radioimmunoassay (RIA) method (DSL 4900 Gamma Counter, Beckman Coulter Inc., Fullerton, CA, USA).
Insulin resistance was calculated for each participant using the homeostasis model assessment of insulin resistance: HOMA-IR = fasting insulin (µU/ml) × fasting glucose (mg/dL)/405 [25].
Betatrophin ELISA
Serum betatrophin levels (Cusabio Biotech, Hubei, China) were measured using commercially available ELISA kits. The intra-assay CV was <6 %, the inter-assay CV was <8 %. All samples were analyzed in duplicate. The assays have high sensitivity and excellent specificity for the detection of human betatrophin with no significant cross-reactivity or interference.
Statistical analysis
Power analysis
The power analysis was performed using G Power 3.0.10 for Windows. According to our pilot study results for circulating betatrophin levels, the required size of the study population was calculated to be 132 subjects per group (α = 0.05 and the study power = 0.90).
All analyses were performed using the Statistical Package for the Social Sciences software version 18.0 (SPSS Inc. Chicago, IL, USA). Distribution of the data was tested using the Kolmogorov–Smirnov test. Triglycerides, DHEA-S, and FSH required logarithmic transformation prior to statistical analysis. Continuous variables were presented as mean ± standard deviation (SD). Demographic and laboratory characteristics of the studied women with and without PCOS were compared using independent samples t test (two-tailed). Subjects in the PCOS and control groups were further divided into two groups according to their BMI levels (BMI <25 kg/m2 and BMI ≥25 kg/m2). Betatrophin levels were then compared using independent samples t test (two-tailed). Relationships between betatrophin and other demographic and laboratory characteristics were evaluated using Pearson’s correlation analysis. Subjects were divided into four groups according to their betatrophin levels. To assess the association between betatrophin levels (quartiles) and the development of PCOS, odds ratio (OR) was calculated using multivariate logistic regression analysis. The model was adjusted for potential confounders: age, BMI, HOMA-IR, free-testosterone, hs-CRP, and lipids. Model compatibility analysis by Hosmer and Lemeshow test showed that all the models were compatible (P > 0.05). Multiple linear regression analyses were performed to adjust the covariates and identify independent relationships between betatrophin levels and age, BMI, HOMA-IR, free-testosterone, lipids, and hs-CRP. All independent variables in the multiple linear regression were tested for multicollinearity. If the variance inflation factor (VIF) exceeded 2.5, the variable was considered to be collinear. Therefore, waist circumference, FBG, insulin, and total cholesterol were not included in the models.
All reported confidence interval (CI) values are calculated at the 95 % level. A two-sided P value <0.05 was considered statistically significant.
Results
Clinical and laboratory characteristics of the study population
The comparative demographic and laboratory parameters of the studied women with and without PCOS are given in Table 1.
As subjects were matched for age and BMI, both parameters were similar in between the two groups (P > 0.05). Although mean waist circumference was slightly higher in women with PCOS, it did not reach statistical significance. There were no significant differences between the groups in terms of systolic and diastolic blood pressure.
Circulating betatrophin levels were significantly elevated in women with PCOS compared with controls (367.09 ± 55.78 vs. 295.65 ± 48.97 pg/ml, P < 0.001). Serum insulin, FBG, hs-CRP, and HOMA-IR levels were found to be increased in women with PCOS. Furthermore, PCOS patients exhibited more dyslipidemia compared to controls. There was no significant difference in total testosterone and DHEA-S levels between the two groups.
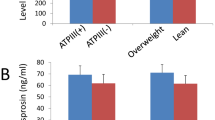
We stratified recruited subjects into two groups according to their BMI levels (<25 kg/m2 and ≥25 kg/m2). In the control group, there were 61 subjects with BMI <25 kg/m2 and 103 subjects with BMI ≥25 kg/m2; in the PCOS group, there were 49 patients with BMI <25 kg/m2 and 115 patients with BMI ≥25 kg/m2. We compared betatrophin levels in each group according to their BMI. There was no significant difference between the number of subjects in the groups (P = 0.198) (data not shown). As shown in Fig. 1, overweight/obese women (BMI ≥25 kg/m2) had significantly higher circulating betatrophin levels than lean subjects both in the control and PCOS groups (BMI <25 kg/m2).
Correlation of betatrophin with clinical parameters
Correlation coefficients between betatrophin levels and the measured clinical parameters are shown in Table 2.
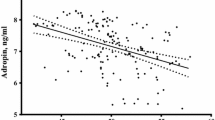
A strongly positive correlation was found between circulating betatrophin levels and free-testosterone (r = 0.324, P < 0.001) and hs-CRP (r = 0.335, P < 0.001) levels in women with PCOS (Fig. 2a, b). There was a positive correlation between HOMA-IR and betatrophin (r = 0.241, P = 0.002) (Fig. 2c). In addition, we found positive correlations between betatrophin and BMI, waist circumference, serum insulin, FBG, and atherogenic lipids profiles (non-HDL-C, triglycerides, LDL-C, and total cholesterol) in both groups. Betatrophin levels correlated with HOMA-IR and hs-CRP in the control group.
There were no significant correlations between betatrophin levels and age, blood pressure, total testosterone levels and HDL-C.
Multivariate binary logistic regression analysis
The independent association between PCOS and betatrophin levels (in quartiles) was assessed by multivariate binary logistic regression analysis for the potential confounders including age, BMI, HOMA-IR, free-testosterone, hs-CRP and lipids (Fig. 3).
Association of betatrophin with PCOS in adjusted models. Multivariate adjusted odds ratio (OR) for having PCOS according to the quartiles of betatrophin (reference, the lowest quartile). Model adjusted for age, BMI, HOMA-IR, free-testosterone, hs-CRP and lipids. OR Odds Ratio, CI Confidence Interval, Q Quartile. A P value of <0.05 was considered significant (asterisk)
In multivariate logistic regression analysis, subjects in the third (OR 1.37, 95 % CI 1.07–1.76, P = 0.014) and the highest (OR 2.51, 95 % CI 1.31–4.81, P = 0.006) quartiles of betatrophin had significantly higher odds of having PCOS compared with subjects in the lowest quartile. Odds of PCOS were not different between subjects in the second and the lowest betatrophin quartiles (OR 1.04, 95 % CI 0.72–1.55, P = 0.154). Subjects with betatrophin levels in the highest and third quartiles were approximately 2.5 and 1.4 times more likely to develop PCOS, respectively, compared with those in the lowest quartile after adjustment for potential confounders.
Multivariate regression analysis
To verify independent associations between betatrophin and metabolic profile, multiple linear regression analysis was performed (Table 3). This model explained 57.7 % of the total variability in betatrophin concentration. Multiple linear regression analysis revealed that HOMA-IR, hs-CRP, and free-testosterone independently predicted betatrophin levels.
Discussion
In the present study, we have demonstrated for the first time that circulating betatrophin levels were higher in women with PCOS when compared with control subjects. Betatrophin was positively correlated with insulin resistance marker HOMA-IR, inflammatory marker hs-CRP, free-testosterone, atherogenic lipid profiles, and BMI in PCOS. Importantly, overweight/obese women also had significantly higher circulating betatrophin levels than lean women, regardless of PCOS. Furthermore, increased circulating betatrophin levels were significantly associated with higher odds of having PCOS after adjusting for potential confounders. Subjects in the highest quartile of betatrophin were approximately 2.5 times more likely to develop PCOS compared with those in the lowest quartile. A similar result was found in subjects in the third quartile of betatrophin, who were nearly 1.4 times more likely to develop PCOS than subjects in the lowest quartile. In addition, multivariate regression analyses showed that HOMA-IR, hs-CRP, and free-testosterone were independent factors influencing serum betatrophin levels. As betatrophin is mainly secreted from the liver, our results suggest that the hepato-ovarian axis may play a role in the development of PCOS.
Upon development of insulin resistance in certain metabolic diseases (i.e., diabetes, GDM, obesity and PCOS), beta cells increase proliferation and insulin production to compensate for the increasing insulin demand. Nevertheless, insulin secretion eventually decreases as a result of the exhaustion of beta cell function [26–28]. The mechanisms of increased beta cell proliferation in response to the insulin-resistant state remain unknown. PCOS is associated with insulin resistance. Overall, insulin resistance and compensatory hyperinsulinemia effect up to 70 % of women with PCOS. Moreover, insulin resistance plays a crucial role in the pathogenesis of PCOS. PCOS subjects have a higher prevalence of glucose metabolism disorders. As many as 40 % have impaired glucose tolerance, while 10 % have T2D by their fourth decade [27, 29, 30]. A study published by Yi et al. has reported that secretion of betatrophin increases in response to insulin resistance in mice, which stimulates beta cell replication and mass expansion as well as insulin secretion [14]. Following the publication of this data, a number of studies have been conducted to determine betatrophin levels in metabolic diseases and to determine whether betatrophin levels are correlated with metabolic parameters in these disorders. However, contradictory results have been reported [20, 21, 31, 32]. Hu et al. reported that betatrophin levels were negatively associated with insulin sensitivity in patients with T2DM [31], while Gomez-Ambrossi et al. found a positive association between the two [21]. In a recently published study, betatrophin levels did not correlate with insulin resistance in a large sample size population with T2DM [32]. In addition, several groups have found elevated circulating betatrophin levels in women with GDM [33–35]. Although Ebert et al. did not find a correlation between betatrophin and insulin resistance in GDM, the other two groups reported a positive correlation. Our work here demonstrates a positive correlation between betatrophin and HOMA-IR in PCOS and control subjects. The discrepancies among published data may be related to variations in demographic and ethnic features, as well as the design and sample size of each study. Another possible explanation for the discrepancies in betatrophin levels in these inflammatory-based metabolic diseases may be the degree of inflammation, since we found a strongly positive correlation between betatrophin and the inflammatory marker, hs-CRP. Furthermore, multiple linear regression analyses revealed that hs-CRP independently influenced betatrophin levels. Therefore, the relationship between circulating levels of betatrophin and insulin resistance in humans requires further investigation.
PCOS is a metabolic disease associated with hyperandrogenism, insulin resistance, and low-grade chronic inflammation. Hyperandrogenism, insulin resistance, and inflammation all contribute to the development of the disorder [10]. In the present study, we found that HOMA-IR, hs-CRP, and free-testosterone levels were independent factors influencing betatrophin levels. Moreover, multivariate logistic regression analyses revealed that increased betatrophin levels were significantly associated with higher odds of having PCOS after adjustment for HOMA-IR, hs-CRP, and other potential confounders. Although the physiological roles of betatrophin in the ovulation system have not been elucidated yet, based on these observations, it is reasonable to speculate that betatrophin may contribute to the development of PCOS independently from associated risk factors including insulin resistance and inflammation.
In addition, betatrophin plays a role in the regulation of lipid metabolism via reduction of triglyceride clearance by lipoprotein lipase inhibition and controls postprandial trafficking of triglyceride-fatty acids to adipose tissue [12, 15]. Moreover, betatrophin sequence variations are known to affect lipid metabolism [13, 16, 17]. Although accumulating evidence indicates a strong link between betatrophin and lipid homeostasis, human studies have been inconclusive. A positive correlation has been reported between betatrophin and both LDL-C and total cholesterol [20]. Gao et al. reported a positive correlation between betatrophin and triglycerides [22]. In another study, on the other hand, circulating betatrophin levels were found to be negatively correlated with triglycerides and positively correlated with HDL-C [21]. Furthermore, Chen et al. reported a negative correlation between betatrophin and total cholesterol, LDL-C and HDL-C [36]. In the present study, PCOS patients exhibited more dyslipidemia compared with controls. Betatrophin levels were found to be positively correlated with atherogenic lipid profiles including triglycerides, LDL-C, total cholesterol, and non-HDL-C, while no significant correlation with HDL-C was detected in PCOS and control groups.
There are some limitations to the current study. Although our sample size is relatively large, the cross-sectional design of the study cannot prove causality. Another limitation of the study is the technique used to evaluate insulin resistance. Due to ethical considerations, we used the less invasive HOMA-IR method, although it is inferior to the clamp technique. Lastly, participants were not screened for betatrophin sequence variations.
In conclusion, our results reveal elevated circulating betatrophin levels in PCOS. However, it is not clear whether an increased betatrophin level is a primary activator or whether it simply results from the disorder. Betatrophin is independently associated with insulin resistance, inflammation, and free-testosterone. More importantly, increased betatrophin levels are independently associated with higher odds of having PCOS, suggesting a potential role of betatrophin in the pathophysiology of PCOS. The physiologic and pathologic significance of our findings remain to be further elucidated.
Abbreviations
- ANGPLT8:
-
Angiopoetin-like 8 protein
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CV:
-
Coefficient of variability
- DHEA-S:
-
Dehydroepiandrosterone sulfate
- E2 :
-
Estradiol
- FBG:
-
Fasting blood glucose
- FG:
-
Ferriman–Gallwey
- GDM:
-
Gestational diabetes mellitus
- HDL-C:
-
High-density lipoprotein cholesterol
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- hs-CRP:
-
High-sensitivity C-reactive protein
- LDL-C:
-
Low-density lipoprotein cholesterol
- OR:
-
Odds ratio
- PCOS:
-
Polycystic ovary syndrome
- RIFL:
-
Re-feeding induced fat and liver protein
- TD26:
-
Hepatocellular carcinoma-associated protein
- T2DM:
-
Type 2 diabetes mellitus
- VIF:
-
Variance inflation factor
References
D.A. Ehrmann, Polycystic ovary syndrome. N. Engl. J. Med. 352, 1223–1236 (2005)
R. Azziz, K.S. Woods, R. Reyna, T.J. Key, E.S. Knochenhauer, B.O. Yildiz, The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinol. Metab. 89, 2745–2749 (2004)
R.S. Legro, S.A. Arslanian, D.A. Ehrmann, K.M. Hoeger, M.H. Murad, R. Pasquali, C.K. Welt, Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 98, 4565–4592 (2013)
C.N. Jayasena, S. Franks, The management of patients with polycystic ovary syndrome. Nat. Rev. Endocrinol. 10, 624–636 (2014)
R.A. Wild, E. Carmina, E. Diamanti-Kandarakis, A. Dokras, H.F. Escobar-Morreale, W. Futterweit, R. Lobo, R.J. Norman, E. Talbott, D.A. Dumesic, Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J. Clin. Endocrinol. Metab. 95, 2038–2049 (2010)
H. Mani, M.J. Levy, M.J. Davies, D.H. Morris, L.J. Gray, J. Bankart, H. Blackledge, K. Khunti, T.A. Howlett, Diabetes and cardiovascular events in women with polycystic ovary syndrome: a 20-year retrospective cohort study. Clin. Endocrinol. (Oxf) 78, 926–934 (2013)
C.G. Solomon, F.B. Hu, A. Dunaif, J.E. Rich-Edwards, M.J. Stampfer, W.C. Willett, F.E. Speizer, J.E. Manson, Menstrual cycle irregularity and risk for future cardiovascular disease. J. Clin. Endocrinol. Metab. 87, 2013–2017 (2002)
A. Dokras, Cardiovascular disease risk in women with PCOS. Steroids 78, 773–776 (2013)
L.K. Hoffman, D.A. Ehrmann, Cardiometabolic features of polycystic ovary syndrome. Nat. Clin. Pract. Endocrinol. Metab. 4, 215–222 (2008)
M.O. Goodarzi, D.A. Dumesic, G. Chazenbalk, R. Azziz, Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 7, 219–231 (2011)
G. Ren, J.Y. Kim, C.M. Smas, Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 303, E334–E351 (2012)
R. Zhang, Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem. Biophys. Res. Commun. 424, 786–792 (2012)
F. Quagliarini, Y. Wang, J. Kozlitina, N.V. Grishin, R. Hyde, E. Boerwinkle, D.M. Valenzuela, A.J. Murphy, J.C. Cohen, H.H. Hobbs, Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA 109, 19751–19756 (2012)
P. Yi, J.-S. Park, D.A. Melton, Betatrophin: a hormone that controls pancreatic β cell proliferation. Cell 153, 747–758 (2013)
Y. Wang, F. Quagliarini, V. Gusarova, J. Gromada, D.M. Valenzuela, J.C. Cohen, H.H. Hobbs, Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc. Natl. Acad. Sci. USA 110, 16109–16114 (2013)
D. Weissglas-Volkov, C.A. Aguilar-Salinas, E. Nikkola, K.A. Deere, I. Cruz-Bautista, O. Arellano-Campos, L.L. Muñoz-Hernandez, L. Gomez-Munguia, M.L. Ordoñez-Sánchez, P.M.V.L. Reddy, A.J. Lusis, N. Matikainen, M.-R. Taskinen, L. Riba, R.M. Cantor, J.S. Sinsheimer, T. Tusie-Luna, P. Pajukanta, Genomic study in Mexicans identifies a new locus for triglycerides and refines European lipid loci. J. Med. Genet. 50, 298–308 (2013)
G.M. Peloso, P.L. Auer, J.C. Bis, A. Voorman, A.C. Morrison, N.O. Stitziel, J.A. Brody, S.A. Khetarpal, J.R. Crosby, M. Fornage, A. Isaacs, J. Jakobsdottir, M.F. Feitosa, G. Davies, J.E. Huffman, A. Manichaikul, B. Davis, K. Lohman, A.Y. Joon, A.V. Smith, M.L. Grove, P. Zanoni, V. Redon, S. Demissie, K. Lawson, U. Peters, C. Carlson, R.D. Jackson, K.K. Ryckman, R.H. Mackey, J.G. Robinson, D.S. Siscovick, P.J. Schreiner, J.C. Mychaleckyj, J.S. Pankow, A. Hofman, A.G. Uitterlinden, T.B. Harris, K.D. Taylor, J.M. Stafford, L.M. Reynolds, R.E. Marioni, A. Dehghan, O.H. Franco, A.P. Patel, Y. Lu, G. Hindy, O. Gottesman, E.P. Bottinger, O. Melander, M. Orho-Melander, R.J.F. Loos, S. Duga, P.A. Merlini, M. Farrall, A. Goel, R. Asselta, D. Girelli, N. Martinelli, S.H. Shah, W.E. Kraus, M. Li, D.J. Rader, M.P. Reilly, R. McPherson, H. Watkins, D. Ardissino, NHLBI GO Exome Sequencing Project, Q. Zhang, J. Wang, M.Y. Tsai, H.A. Taylor, A. Correa, M.E. Griswold, L.A. Lange, J.M. Starr, I. Rudan, G. Eiriksdottir, L.J. Launer, J.M. Ordovas, D. Levy, Y.D.I. Chen, A.P. Reiner, C. Hayward, O. Polasek, I.J. Deary, I.B. Borecki, Y. Liu, V. Gudnason, J.G. Wilson, C.M. van Duijn, C. Kooperberg, S.S. Rich, B.M. Psaty, J.I. Rotter, C.J. O’Donnell, K. Rice, E. Boerwinkle, S. Kathiresan, L.A. Cupples, Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 whites and blacks. Am. J. Hum. Genet. 94, 223–232 (2014)
V. Gusarova, C.A. Alexa, E. Na, P.E. Stevis, Y. Xin, S. Bonner-Weir, J.C. Cohen, H.H. Hobbs, A.J. Murphy, G.D. Yancopoulos, J. Gromada, ANGPTL8/betatrophin does not control pancreatic beta cell expansion. Cell 159, 691–696 (2014)
D. Espes, M. Martinell, P.-O. Carlsson, Increased circulating betatrophin concentrations in patients with type 2 diabetes. Int. J. Endocrinol. 2014, 323407 (2014)
A. Fenzl, B.K. Itariu, L. Kosi, M. Fritzer-Szekeres, A. Kautzky-Willer, T.M. Stulnig, F.W. Kiefer, Circulating betatrophin correlates with atherogenic lipid profiles but not with glucose and insulin levels in insulin-resistant individuals. Diabetologia 57, 1204–1208 (2014)
J. Gomez-Ambrosi, E. Pascual, V. Catalan, A. Rodriguez, B. Ramirez, C. Silva, M.J. Gil, J. Salvador, G. Fruhbeck, Circulating betatrophin concentrations are decreased in human obesity and type 2 diabetes. J. Clin. Endocrinol. Metab. 99, E2004–E2009 (2014)
T. Gao, K. Jin, P. Chen, H. Jin, L. Yang, X. Xie, M. Yang, C. Hu, X. Yu, Circulating betatrophin correlates with triglycerides and postprandial glucose among different glucose tolerance statuses-a case-control study. PLoS One 10, e0133640 (2015)
R. Tarlatzis, B. Fauser, J. Chang, R. Azziz: Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). The Rotterdam ESHRE/ASRM-. Hum. Reprod. (2004)
D. Ferriman, J.D. Gallwey, Clinical assessment of body hair growth in women. J. Clin. Endocrinol. Metab. 21, 1440–1447 (1961)
D.R. Matthews, J.P. Hosker, A.S. Rudenski, B.A. Naylor, D.F. Treacher, R.C. Turner, Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985)
M.H. Shanik, Y. Xu, J. Skrha, R. Dankner, Y. Zick, J. Roth, Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 31, S262–S268 (2008)
E. Diamanti-Kandarakis, A. Dunaif, Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr. Rev. 33, 981–1030 (2012)
P.M. Catalano, Trying to understand gestational diabetes. Diab. Med. 31, 273–281 (2014)
F. Ovalle, Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil. Steril. 77, 1095–1105 (2002)
A. Dunaif, Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis 1. Endocr. Rev. 18, 774–800 (1997)
H. Hu, W. Sun, S. Yu, X. Hong, W. Qian, B. Tang, D. Wang, L. Yang, J. Wang, C. Mao, L. Zhou, G. Yuan, Increased circulating levels of betatrophin in newly diagnosed type 2 diabetic patients. Diab. Care 37, 2718–2722 (2014)
M. Abu-Farha, J. Abubaker, I. Al-Khairi, P. Cherian, F. Noronha, F.B. Hu, K. Behbehani, N. Elkum, Higher plasma betatrophin/ANGPTL8 level in Type 2 Diabetes subjects does not correlate with blood glucose or insulin resistance. Sci. Rep. 5, 10949 (2015)
N. Wawrusiewicz-Kurylonek, B. Telejko, M. Kuzmicki, A. Sobota, D. Lipinska, J. Pliszka, B. Raczkowska, P. Kuc, R. Urban, J. Szamatowicz, A. Kretowski, P. Laudanski, M. Gorska, Increased maternal and cord blood betatrophin in gestational diabetes. PLoS One 10, e0131171 (2015)
O. Erol, H.Y. Ellidağ, H. Ayık, M.K. Özel, A.U. Derbent, N. Yılmaz, Evaluation of circulating betatrophin levels in gestational diabetes mellitus. Gynecol. Endocrinol. 31, 1–5 (2015)
T. Ebert, S. Kralisch, U. Wurst, U. Lössner, J. Kratzsch, M. Blüher, M. Stumvoll, A. Tönjes, M. Fasshauer, Betatrophin levels are increased in women with gestational diabetes mellitus compared to healthy pregnant controls. Eur. J. Endocrinol. 173, 1–7 (2015)
X. Chen, P. Lu, W. He, J. Zhang, L. Liu, Y. Yang, Z. Liu, J. Xie, S. Shao, T. Du, X. Su, X. Zhou, S. Hu, G. Yuan, M. Zhang, H. Zhang, L. Liu, D. Wang, X. Yu, Circulating betatrophin levels are increased in patients with type 2 diabetes and associated with insulin resistance. J. Clin. Endocrinol. Metab. 100, E96–E100 (2015)
Acknowledgments
M.C., G.U.K., O.Y., and O.G.C. participated in study design and performed ELISA. M.C., P.Y., Y.M.S., and T.K. participated in study design, analyzed the data, wrote, reviewed, and edited the manuscript. O.Y. and M.T. provided serum samples and contributed to discussions of data interpretation. M.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study has not been funded by any organizations.
Disclosure
The authors have nothing to disclose.
Rights and permissions
About this article
Cite this article
Calan, M., Yilmaz, O., Kume, T. et al. Elevated circulating levels of betatrophin are associated with polycystic ovary syndrome. Endocrine 53, 271–279 (2016). https://doi.org/10.1007/s12020-016-0875-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0875-z