Abstract
A repeat fine needle aspiration (FNA) is recommended for thyroid nodules diagnosed as atypia of undetermined significance (AUS) in a previous cytology. We evaluated the utility of NRAS codon 61 (NRAS61) mutation analysis and core needle biopsy (CNB) for the diagnosis of thyroid nodules previously diagnosed as AUS. This study enrolled 236 patients who underwent both NRAS61 mutation analysis and CNB of thyroid nodules previously diagnosed as AUS at cytology. The NRAS61 mutation was detected in 36 nodules and was more frequently detected in the AUS and follicular neoplasm (FN)/suspicious for follicular neoplasm (SFN) categories, as determined by histological analysis of CNB, than in the benign group (p = 0.005). Sixty-one patients underwent surgery, and 29 nodules were finally diagnosed as malignant after surgery. Among 61 patients who underwent surgery, nodules with the NRAS61 mutation (42–65 %) had a significantly higher malignancy rate than nodules with wild-type NRAS61 (7–37 %, p = 0.038). The association between malignancy and the NRAS61 mutation was significant after adjusting for age, sex, nodule size, and histological diagnosis of CNB (p = 0.01). NRAS61 mutation analysis together with CNB could be helpful for arriving at a clinical decision in patients with thyroid nodules showing AUS in a previous cytology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Bethesda System for Reporting Thyroid Cytopathology defined the ‘atypia of undetermined significance (AUS)/follicular lesion of undetermined significance (FLUS)’ category because some cytopathologies are difficult to classify into benign, suspicious, or malignant categories in thyroid fine needle aspiration (FNA) cytological examinations [1]. The AUS category constitutes a heterogeneous group, and malignancy rates in AUS are reported to range from 6 to 48 % [2–4]. Repeat FNA and/or molecular testing is recommended for thyroid nodules classified as AUS with further evaluation of worrisome clinical and ultrasonographic features [5, 6].
FNA is a cost effective and safe procedure for assessing thyroid nodules [7, 8]. However, 10–50 % of cases show inconclusive findings even after repeat FNA of thyroid nodules initially classified as AUS [9–12]. Core needle biopsy (CNB) is an alternative procedure that uses a large and hollow needle. CNB was reported to provide a large tissue sample and facilitate precise histological diagnosis [12, 13]. Recent studies showed that CNB resulted in a better diagnosis of thyroid malignancy than repeat FNA in nodules with nondiagnostic or AUS cytology. Inconclusive findings were reported to be 2–27 % for CNB and 49–50 % for repeated FNA in such settings [11, 12].
Molecular analysis has been used to detect thyroid malignancy in thyroid nodules. BRAF V600E, RAS point mutations, RET-PTC, and PAX8-PPARγ rearrangement are frequently associated with thyroid cancer [14, 15]. Molecular analysis of thyroid nodules with AUS cytology could improve the diagnostic value of FNA. A previous study reported that the malignancy rate of thyroid nodules with AUS cytology was 88 % in any mutation positive nodules and only 5.9 % in mutation negative nodules [16, 17]. Among various genetic alterations, RAS point mutations are the most frequent mutations found in thyroid nodules with AUS cytology [16–18], and the NRAS codon 61 (NRAS61) mutation was the most common among six hot-spot mutations of RAS genes [19]. The malignancy risk was reported to be 84 % in RAS mutation positive nodules with AUS cytology [16].
To date, there is no study to evaluate the diagnostic performance of using CNB and molecular analysis for the diagnosis of indeterminate thyroid nodules. In this study, we evaluated a diagnostic utility of performing NRAS61 mutation analysis on CNB samples of thyroid nodules previously assigned to the AUS category.
Material and methods
Patients
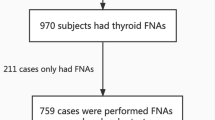
Between April 2013 and June 2014, patients who had undergone both NRAS61 mutation analysis and CNB of nodules previously diagnosed as AUS by FNA at Asan Medical Center, Seoul, Korea were enrolled in this study. Only patients who had thyroid nodules with a size of 1 cm or larger were included. A total of 236 patients were included in this study. This study protocol was approved by the institutional review board of Asan Medical Center.
Ultrasonography (US)-guided CNB procedure
All US examinations were performed using one of three US systems: iU22, HDI-5000 (Philips Healthcare, Bothell, WA), or EUB-7500 unit (Hitachi Medical Systems, Tokyo, Japan). The US systems were equipped with a linear, high-frequency probe (5–14 MHz). A comprehensive US evaluation of the neck and thyroid gland was performed in all cases, and the size, location, and composition of any nodules were evaluated. US-guided CNBs were performed using a 1.1, 1.6, or 2.0 excursion, disposable, 18-gauge, double-action, spring-activated needle (TSK Ace-cut; Create Medic, Yokohama, Japan) after local anesthesia using 1 % lidocaine as previously reported [20]. Briefly, the core needle was approached with a free hand technique from the isthmus and directed to the solid component of the nodule. The stylet and cutting cannula of the needle were fired after the tip of the biopsy needle was advanced to the edge of the nodule. An additional CNB was performed if the lesion was considered to have been inaccurately targeted or if an inadequate tissue core was obtained according to visual inspection. Tissue cores were placed in 10 % buffered formalin immediately after biopsy. After the biopsy, all patients were requested to compress the biopsy site for 10–20 min. When a patient complained of neck pain or swelling at the biopsy site, an US examination was performed to check for complications.
Histological diagnosis
CNB samples were classified histologically into six categories broadly based on those of the Bethesda System for Reporting Thyroid Cytopathology [1]: nondiagnostic, benign, AUS, follicular neoplasm (FN)/suspicious for follicular neoplasm (SFN), suspicious for malignancy, and malignancy. An experienced endocrine pathologist (D.E.S), who was blinded to the clinical information, reviewed the results of histological analysis. Nondiagnostic findings included the absence of any identifiable follicular thyroid tissue (skeletal muscle or fibrous adipose tissue only), the presence of only normal thyroid gland, and the presence of tissue containing only a few follicular cells [12, 21]. The AUS category was assigned to CNBs that included some atypical cells with nuclear and/or architectural atypia and for which there was insufficient evidence for the diagnosis of FN/SFN, suspicious for malignancy, or malignancy because of extensive secondary degeneration, equivocal presence of tumor capsule, and clinically lymphocytic thyroiditis or mutinodular goiter background [20, 22]. The FN/SFN category for CNB included nodules which revealed architectural atypia such as microfollicular/trabecular/solid growth pattern with tumor capsule in the absence of clinical background of lymphocytic thyroiditis or mutinodular goiter. The suspicious for malignancy or malignancy categories for CNB were similar to those of ‘Bethesda System for Reporting Thyroid Cytopathology [1]. A diagnostic criterion of CNB was defined as FN/SFN, suspicious for malignancy, or malignancy categories of CNB because these should be considered for thyroid surgery. The final diagnosis of the thyroid nodules was confirmed by histological examination of the surgical specimen after thyroidectomy.
Analysis of NRAS61 mutation
Genomic DNA from formalin-fixed fresh CNB tissues was extracted using the QIAamp DSP DNA Mini Kit (Qiagen, Hilden, Germany) as previously reported [23]. Polymerase chain reaction (PCR) was performed on genomic DNA to generate amplified fragments of NRAS61 with the following primers: forward, 5′-TTGCATTCCCTGTGGTTTTT-3′; reverse, 5′-TCCGCAAATGACTTGCTATT-3′) using KOD FX polymerase (Toyobo, Osaka, Japan). Genomic DNA was amplified in a 10-μL reaction volume that contains 50 ng genomic DNA, 0.3 μL of 10 μM primers with 1 μL of 10 × PCR buffer and 1 μL of MgCl2 (2 mM). The amplification protocol consisted of an initial denaturation at 94 °C for 2 min, followed by 40 cycles at 98 °C for 10 s, 55 °C for 40 s, and 68 °C for 30 s, followed by a final extension step at 68 °C for 7 min. PCR products were analyzed on 2 % agarose gels that were stained with ethidium bromide. For purification of PCR amplified product, 1 μL of exonuclease I (1 unit/μL); (USB Corp., Cleveland, OH, USA) was incubated with 1 μL of PCR amplified products in 10 μL of the reaction for 40 min at 37 °C and 15 min at 85 °C. Sequencing reactions were performed in 1 μL of treated PCR amplified products with BigDye Ready Reaction Kit (ABI PRISM BigDye Terminator version 3.1; Applied Biosystems, Foster City, CA, USA) with the forward primer. The final products were analyzed on ABI PRISM Genetic Analyzer 3100 automatic DNA sequencer, Applied Biosystems).
Statistical analysis
R version 3.0 and R libraries prodlim, car, Cairo, and survival were used to analyze data (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org). Continuous variables between two groups were compared using Student’s t test. Categorical variables were compared using the Chi-squared test or Fisher’s exact test. The multivariate analysis included age, sex, nodule size, CNB, and NRAS61 mutation analysis. We evaluated the diagnostic values of NRAS61 mutation analysis and histological analysis of CNB by calculation of sensitivity, specificity, negative predictive value, positive predictive value, and accuracy. Diagnostic values were calculated using the following criteria: sensitivity = [true positive (TP)/{TP + false negative (FN)}] × 100, specificity = [true negative (TN)/{TN + false positive (FP)}] × 100, negative predictive value (NPV) = [TN/(TN + FN)] × 100, positive predictive value (PPV) = [TP/(TP + FP)] × 100, and accuracy = [(TP + TN)/(TP + TN + FP + FN)] × 100. McNemar’s test was used to compare the diagnostic value between two kinds of diagnostic tools. All p values were two-sided, and p < 0.05 was considered statistically significant.
Results
Baseline characteristics
The mean age of the 236 patients was 54.1 ± 12.1 years, and 42 patients (18 %) were male. The mean longest diameter of the target nodules measured by US was 2.2 ± 1.1 cm. The needle was passed once into the target nodule in 187 patients (79 %), twice into the target nodule in 48 patients (20 %), and three times into the target nodule in one patient (0.4 %). The mean number of tissue cores was 1.2 ± 0.5. Eighty-nine biopsies (38 %) were obtained with a 1.1-cm excursion needle, 137 (58 %) with a 1.6-cm excursion needle, and 10 (4 %) with a 2.0-cm excursion needle.
Histological diagnosis of CNB
Thyroid nodules were classified into six categories by histological diagnosis of CNB (Table 1): nondiagnostic (n = 8, 3 %), benign (n = 90, 38 %), AUS (n = 99, 42 %), FN/SFN (n = 32, 14 %), suspicious for malignancy (n = 3, 1 %), and malignancy (n = 4, 2 %). A CNB was helpful for arriving at a clinical decision in 129 cases (55 %): 39 nodules were assigned for surgical treatment (FN/SFN, suspicious for malignancy, or malignancy), and 90 were benign nodules.
NRAS61 mutation
The NRAS61 mutation was found in 36 of 236 CNB samples (15 %, Table 1). NRAS Q61R was detected in 32 nodules (89 %), and NRAS Q61K was detected in 4 nodules (11 %). There were no differences in age, sex, nodule size, number of needle passes, number of tissue core, or type of excursion needle between patients with nodules with the NRAS61 mutation and those with nodules with wild-type NRAS61.
NRAS61 mutation analysis and histological diagnosis of CNB
The NRAS61 mutation was only found in the thyroid nodules of benign, AUS, and FN/SFN groups based on the analysis of CNB samples: seven in 90 nodules (8 %) of the benign group, 21 in 99 nodules (21 %) of the AUS group, and eight in 32 nodules (25 %) of the FN/SFN group (Table 1). It was not detected in CNB samples from patients in the inadequate, suspicious for malignancy, or malignancy groups. The NRAS61 mutation was more common in AUS and SFN/FN groups than in the benign group (p = 0.005). Seven nodules in the benign category and 21 nodules in the AUS category had the NRAS61 mutation. Therefore, in addition to CNB, NRAS61 mutation analysis was helpful at arriving at a clinical decision for surgery in these 28 patients (12 %), and 15 of these patients underwent surgery. The other 13 patients did not choose thyroid surgery even after physician’s recommendation. Most of them were closely followed up by regular US examination. In 5 patients who were diagnosed as suspicious for malignancy or malignancy by CNB, two patients did not undergo surgery. Physician recommended thyroid surgery for these two patients, but they did not undergo surgery and were not followed up anymore in our institution.
Comparison of malignancy rates according to the results of NRAS61 mutation analysis
Sixty-one patients (26 %) underwent thyroid surgery after CNB, and 29 of these patients were finally diagnosed as having a malignant tumor (Table 1).The NRAS61 mutation was present in 36 of 61 patients, and 15 of these (65 %) were finally diagnosed as having a malignant tumor. The malignancy rate was significantly higher in patients with the NRAS61 mutation (42–65 %) than in those with wild-type NRAS61 (7–37 %; OR: 3.15, p = 0.038; Table 1). The association between final diagnosis of malignancy and the presence of mutant NRAS61 was significant after adjusting for age, sex, nodule size, and histological diagnosis of CNB (OR: 6.63, p = 0.006; Table 2).
Malignancy rate according to the results of NRAS61 mutation analysis and diagnostic categories of CNB
The NRAS61 mutation was found in thyroid nodules belonging to benign, AUS, and FN/SFN groups based on CNB histological analysis. Therefore, we compared the malignancy rate of these three groups. We determined the malignancy rates both in total patients and in patients who underwent surgery (Table 1).
In the benign group, the malignancy rate of the mutant NRAS61 group (14–50 %) was similar to that of the wild-type NRAS61 group (1–25 %; p = 0.99). One follicular variant papillary thyroid carcinoma (FV-PTC) was found in the mutant NRAS61 group, and one follicular thyroid carcinoma (FTC) was found in the wild-type NRAS61 group. The mean size of mutant NRAS61 group was 2.1 ± 0.7 cm and that of wild-type NRAS61 group was 2.4 ± 1.3 cm. There was no difference in nodule size between two groups of benign category of CNB (p = 0.42).
In the AUS group, no significant difference in the malignancy rate was detected between the NRAS61 (3–54 %) mutation group and the wild-type NRAS61 group (5–31 %; p = 0.43). The mutant NRAS61 group had six FV-PTCs and one FTC. There were three FV-PTCs and one FTC in the wild-type NRAS61 group.
In the FN/SFN group, the malignancy rate of the mutant NRAS61 mutation group (88 %) was significantly higher than that of the wild-type NRAS61 group (17–27 %; OR: 16.5, p = 0.009). The mutant NRAS61 group had three FV-PTCs and four FTCs (88 %). There were three FTCs and one FV-PTC (17–27 %) in the wild-type NRAS61 group.
Diagnostic values of NRAS61 mutational analysis and CNB analysis in patients who underwent thyroid surgery
The diagnostic values of NRAS61 mutation analysis and CNB in 61 patients who underwent thyroid surgery are summarized in Table 3. There was no significant difference in sensitivity or specificity between NRAS61 mutation analysis and CNB analysis. NRAS61 mutation analysis and CNB together had better sensitivity (82.8 %) than NRAS61 mutation analysis alone (51.7 %, p = 0.02) or CNB analysis alone (55.2 %, p = 0.01).
We determined the diagnostic value of NRAS61 mutation analysis alone for thyroid nodules belonging to the AUS group of CNB in patients who underwent thyroid surgery. Eleven of twenty-six thyroid nodules (42 %) in this category were confirmed as having thyroid malignancy. The sensitivity and specificity of NRAS61 mutation analysis for the diagnosis of thyroid malignancy were 63.6 and 60 %, respectively (Table 3). Seven of thirteen thyroid nodules were diagnosed as malignant in the mutant NRAS61 group (PPV = 53.8 %), and 9 of 13 thyroid nodules were diagnosed as benign in the wild-type group (NPV = 69.2 %).
Discussion
This study demonstrated that NRAS61 mutation analysis combined with CNB histological analysis has the potential to diagnose thyroid malignancy in AUS thyroid nodules previously identified by FNA. The NRAS61 mutation was found in 15 % of nodules with AUS cytology. The NRAS61 mutation was only found in thyroid nodules belonging to benign, AUS, and FN/SFN categories of CNB. Nodules with the NRAS61 mutation had a significantly higher malignancy rate than nodules without the NRAS61 mutation. This association between malignancy and the NRAS61 mutation was significant after adjusting for age, sex, nodule size, and CNB histological analysis.
The AUS category defined according the Bethesda system is a heterogeneous group, which has a wide range of cytological features, including benign, FN, and suspicious for malignancy [1, 24]. The proportion of the AUS category among thyroid nodules is 2–18 %, and the malignancy rate in the AUS category is 5–15 % [1, 5, 24]. However, several studies reported malignancy rates of 6-48 %, which are higher than those in the original Bethesda system [2–4]. The AUS category has the highest degree of variability for malignancy rate among the six Bethesda categories, which is one reason why there is considerable variability in the malignancy rate. Several factors influence the decision to perform thyroid surgery or extent of surgery to the AUS category of thyroid nodules. Patients who have a nodule with a size over 4 cm, a family history of thyroid cancer, a past history of radiation exposure, or suspicious US features for thyroid malignancy were reported to have a high risk of malignancy in AUS nodules [25–31].
With the technical improvement of CNB provided by US guidance, CNB was recently revived for the diagnosis of thyroid cancer [32, 33]. CNB was reported to have a higher adequacy rate and better diagnostic value than a repeat FNA in nodules with AUS cytology [11, 12]. The amount of tissue obtained by CNB is larger than that obtained by FNA. Also, CNB is less dependent on operator skill than FNA for successful penetration of the nodule [12, 34]. In this regard, CNB could be more useful as a secondary diagnostic tool than repeat FNA for thyroid nodules with AUS cytology determined in a previous cytology analysis. However, the utility of CNB analysis alone is limited because a considerable number of cases (42 %) could be classified into the AUS category according to this study. Therefore, other diagnostic tools are needed to improve the diagnostic performance of CNB for thyroid nodules in this category.
The malignancy risk of thyroid nodules having both AUS cytology and a RAS mutation were reported to be 84 % [16]. The NRAS point mutation was the most commonly detected genetic alteration in FV-PTC and FTC using targeted next generation sequencing (Thyroseq), which was designed to target 12 cancer-related genes with 284 mutational hot spots [35]. The RAS mutation is involved in early carcinogenesis of thyroid cancer and cancer progression [14]. It is found not only in thyroid cancers such as FV-PTC or FTC but also in benign thyroid tumors [36, 37]. However, RAS mutations are more frequent in FTC than in FA or NH [38, 39]. Several studies also reported that RAS mutation, especially the NRAS codon 61 mutation, is associated with poor prognosis and distant metastasis of FTC [38–40]. Therefore, we considered that NRAS61 mutation analysis could be a useful diagnostic option for the diagnosis of FV-PTC or FTC in this setting. A previous study reported that benign thyroid nodules bearing RET/PTC rearrangements grew more rapidly than those nodules without RET/PTC [41]. Like benign nodules harboring RET/PTC, benign nodules harboring NRAS61 mutation could grow faster than those with wild-type NRAS61. However, there was no difference in nodule size between mutant NRAS61 group and wild-type NRAS61 group of benign category of CNB. Unfortunately, we could not evaluate the changes in size of thyroid nodules according to the NRAS61 mutational status during long-term periods, so we could not compare the rapidity of growth in benign nodules according to the NRAS61 mutation status.
This study is limited by its retrospective design. The low prevalence of suspicious for malignancy or malignancy categories in the CNB results might be related to selection bias. The results of this study could not demonstrate a role for CNB in the diagnosis of nodules with AUS cytology, because we included thyroid nodules that underwent both NRAS61 mutation analysis and CNB. Only a limited number of patients underwent thyroid surgery because physicians tend to prefer watchful observation of thyroid nodules, most of which are likely to be benign. This might be the reason for the low diagnostic rate for benign thyroid nodules and limited sensitivity or negative predictive values of NRAS61 mutation analysis. Therefore, we described the % of malignancy corresponding to total patients and patients who underwent surgery. In the AUS group, there was no significant difference in the malignancy rate between mutant NRAS61 group and the wild-type NRAS61 group. Only 26 of 99 patients (26 %) underwent surgery in AUS group. Most of patients in AUS category were not included in the analysis because they did not undergo surgery. This might cause no difference in the malignancy rate between mutant NRAS61 group and the wild-type NRAS61 group of AUS category. Four thyroid nodules with wild-type NRAS61 were diagnosed as malignant in the AUS group by histological analysis of CNB. Molecular analyses of more genes, including BRAF, RAS, RET-PTC, and PAX8-PPARγ, might provide a better diagnostic performance for detecting thyroid malignancy than NRAS61 mutation analysis alone. Combination of NRAS61 mutational analysis and CNB showed a lower specificity than CNB alone. We regarded combination of two tests as any positive result of two tests, so combination of two tests had a lower specificity than each single test.
In summary, CNB was useful for diagnosing and arriving at a clinical decision concerning thyroid nodules previously classified as having AUS cytology. The NRAS61 mutation was significantly associated with a high malignant rate in thyroid nodules. Additional NRAS61 mutation analysis along with CNB helped arrive at a clinical decision in 12 % of patients with a previous cytological diagnosis of AUS in thyroid nodules, and it improved diagnostic sensitivity. These findings indicate that performing NRAS61 mutation analysis in addition to histological analysis of CNB could be useful for the diagnosis of thyroid nodules with AUS cytology.
References
E.S. Cibas, S.Z. Ali, The Bethesda system for reporting thyroid cytopathology. Thyroid 19(11), 1159–1165 (2009). doi:10.1089/thy.2009.0274
L.J. Layfield, M.J. Morton, H.M. Cramer, S. Hirschowitz, Implications of the proposed thyroid fine-needle aspiration category of “follicular lesion of undetermined significance”: a five-year multi-institutional analysis. Diagn. Cytopathol. 37(10), 710–714 (2009). doi:10.1002/dc.21093
N.P. Ohori, K.E. Schoedel, Variability in the atypia of undetermined significance/follicular lesion of undetermined significance diagnosis in the Bethesda System for Reporting Thyroid Cytopathology: sources and recommendations. Acta Cytol. 55(6), 492–498 (2011). doi:10.1159/000334218
M. Bongiovanni, A. Spitale, W.C. Faquin, L. Mazzucchelli, Z.W. Baloch, The Bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol. 56(4), 333–339 (2012). doi:10.1159/000339959
D.S. Cooper, G.M. Doherty, B.R. Haugen, R.T. Kloos, S.L. Lee, S.J. Mandel, E.L. Mazzaferri, B. McIver, F. Pacini, M. Schlumberger, S.I. Sherman, D.L. Steward, R.M. Tuttle, Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19(11), 1167–1214 (2009). doi:10.1089/thy.2009.0110
N. Dincer, S. Balci, A. Yazgan, G. Guney, R. Ersoy, B. Cakir, G. Guler, Follow-up of atypia and follicular lesions of undetermined significance in thyroid fine needle aspiration cytology. Cytopathology 24(6), 385–390 (2013). doi:10.1111/cyt.12021
J.H. Kim, N.K. Kim, Y.L. Oh, H.J. Kim, S.Y. Kim, J.H. Chung, S.W. Kim, The validity of ultrasonography-guided fine needle aspiration biopsy in thyroid nodules 4 cm or larger depends on ultrasonography characteristics. Endocrinol. Metabol. 29, 545–552 (2014)
J.Y. Kwak, Indications for fine needle aspiration in thyroid nodules. Endocrinol. Metabol. 28(2), 81–85 (2013). doi:10.3803/EnM.2013.28.2.81
Z. Baloch, V.A. LiVolsi, P. Jain, R. Jain, I. Aljada, S. Mandel, J.E. Langer, P.K. Gupta, Role of repeat fine-needle aspiration biopsy (FNAB) in the management of thyroid nodules. Diagn. Cytopathol. 29(4), 203–206 (2003). doi:10.1002/dc.10361
J. Yang, V. Schnadig, R. Logrono, P.G. Wasserman, Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer 111(5), 306–315 (2007). doi:10.1002/cncr.22955
K.T. Park, S.H. Ahn, J.H. Mo, Y.J. Park, J. Park do, S.I. Choi, S.Y. Park, Role of core needle biopsy and ultrasonographic finding in management of indeterminate thyroid nodules. Head Neck 33(2), 160–165 (2011). doi:10.1002/hed.21414
D.G. Na, J.H. Kim, J.Y. Sung, J.H. Baek, K.C. Jung, H. Lee, H. Yoo, Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as nondiagnostic or atypia of undetermined significance by the Bethesda system for reporting thyroid cytopathology. Thyroid 22(5), 468–475 (2012). doi:10.1089/thy.2011.0185
P. Lo Gerfo, T. Colacchio, F. Caushaj, C. Weber, C. Feind, Comparison of fine-needle and coarse-needle biopsies in evaluating thyroid nodules. Surgery 92(5), 835–838 (1982)
M. Xing, Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 13(3), 184–199 (2013). doi:10.1038/nrc3431
J.S. Bae, S.K. Choi, S. Jeon, Y. Kim, S. Lee, Y.S. Lee, C.K. Jung, Impact of NRAS mutations on the diagnosis of follicular neoplasm of the thyroid. Int. J. Endocrinol. 2014, 289834 (2014). doi:10.1155/2014/289834
Y.E. Nikiforov, N.P. Ohori, S.P. Hodak, S.E. Carty, S.O. LeBeau, R.L. Ferris, L. Yip, R.R. Seethala, M.E. Tublin, M.T. Stang, C. Coyne, J.T. Johnson, A.F. Stewart, M.N. Nikiforova, Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J. Clin. Endocrinol. Metabol. 96(11), 3390–3397 (2011). doi:10.1210/jc.2011-1469
L. Yip, L.I. Wharry, M.J. Armstrong, A. Silbermann, K.L. McCoy, M.T. Stang, N.P. Ohori, S.O. LeBeau, C. Coyne, M.N. Nikiforova, J.E. Bauman, J.T. Johnson, M.E. Tublin, S.P. Hodak, Y.E. Nikiforov, S.E. Carty, A clinical algorithm for fine-needle aspiration molecular testing effectively guides the appropriate extent of initial thyroidectomy. Ann. Surg. 260(1), 163–168 (2014). doi:10.1097/sla.0000000000000215
J.H. An, K.H. Song, S.K. Kim, K.S. Park, Y.B. Yoo, J.H. Yang, T.S. Hwang, D.L. Kim, RAS mutations in indeterminate thyroid nodules are predictive of the follicular variant of papillary thyroid carcinoma. Clin. Endocrinol. (2014). doi:10.1111/cen.12579
I.A. Prior, P.D. Lewis, C. Mattos, A comprehensive survey of Ras mutations in cancer. Cancer Res. 72(10), 2457–2467 (2012). doi:10.1158/0008-5472.CAN-11-2612
Y.J. Choi, J.H. Baek, E.J. Ha, H.K. Lim, J.H. Lee, J.K. Kim, D.E. Song, Y.K. Shong, S.J. Hong, Differences in risk of malignancy and management recommendations in subcategories of thyroid nodules with atypia of undetermined significance or follicular lesion of undetermined significance: the role of ultrasound-guided core-needle biopsy. Thyroid 24(3), 494–501 (2014). doi:10.1089/thy.2012.0635
J.S. Yeon, J.H. Baek, H.K. Lim, E.J. Ha, J.K. Kim, D.E. Song, T.Y. Kim, J.H. Lee, Thyroid nodules with initially nondiagnostic cytologic results: the role of core-needle biopsy. Radiology 268(1), 274–280 (2013). doi:10.1148/radiol.13122247
R.G. Yoon, J.H. Baek, J.H. Lee, Y.J. Choi, M.J. Hong, D.E. Song, J.K. Kim, J.H. Yoon, W.B. Kim, Diagnosis of thyroid follicular neoplasm: fine-needle aspiration versus core-needle biopsy. Thyroid 24(11), 1612–1617 (2014). doi:10.1089/thy.2014.0140
H.J. Lee, J. Choi, T.S. Hwang, Y.K. Shong, S.J. Hong, G. Gong, Detection of BRAF mutations in thyroid nodules by allele-specific PCR using a dual priming oligonucleotide system. Am. J. Clin. Pathol. 133(5), 802–808 (2010). doi:10.1309/AJCPO3F2ENKMDTUS
Z.W. Baloch, V.A. LiVolsi, S.L. Asa, J. Rosai, M.J. Merino, G. Randolph, P. Vielh, R.M. DeMay, M.K. Sidawy, W.J. Frable, Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn. Cytopathol. 36(6), 425–437 (2008). doi:10.1002/dc.20830
R.S. Mehta, S.E. Carty, N.P. Ohori, S.P. Hodak, C. Coyne, S.O. LeBeau, M.E. Tublin, M.T. Stang, J.T. Johnson, K.L. McCoy, M.N. Nikiforova, Y.E. Nikiforov, L. Yip, Nodule size is an independent predictor of malignancy in mutation-negative nodules with follicular lesion of undetermined significance cytology. Surgery 154(4), 730–736 (2013). doi:10.1016/j.surg.2013.05.015
R.M. Tuttle, H. Lemar, H.B. Burch, Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle aspiration. Thyroid 8(5), 377–383 (1998)
R.E. Goldstein, J.L. Netterville, B. Burkey, J.E. Johnson, Implications of follicular neoplasms, atypia, and lesions suspicious for malignancy diagnosed by fine-needle aspiration of thyroid nodules. Ann. Surg. 235(5), 656–662 (2002)
R.T. Schlinkert, J.A. van Heerden, J.R. Goellner, H. Gharib, S.L. Smith, R.F. Rosales, A.L. Weaver, Factors that predict malignant thyroid lesions when fine-needle aspiration is “suspicious for follicular neoplasm”. Mayo Clin. Proc. 72(10), 913–916 (1997). doi:10.1016/s0025-6196(11)63360-0
H.M. Gweon, E.J. Son, J.H. Youk, J.A. Kim, Thyroid nodules with Bethesda system III cytology: can ultrasonography guide the next step? Ann. Surg. Oncol. 20(9), 3083–3088 (2013). doi:10.1245/s10434-013-2990-x
P.W. Rosario, Thyroid nodules with atypia or follicular lesions of undetermined significance (Bethesda Category III): importance of ultrasonography and cytological subcategory. Thyroid 24(7), 1115–1120 (2014). doi:10.1089/thy.2013.0650
J.H. Yoon, H.S. Lee, E.K. Kim, H.J. Moon, J.Y. Kwak, A nomogram for predicting malignancy in thyroid nodules diagnosed as atypia of undetermined significance/follicular lesions of undetermined significance on fine needle aspiration. Surgery 155(6), 1006–1013 (2014). doi:10.1016/j.surg.2013.12.035
S. Taki, K. Kakuda, K. Kakuma, Y. Annen, S. Katada, R. Yamashita, M. Kosugi, T. Michigishi, N. Tonami, Thyroid nodules: evaluation with US-guided core biopsy with an automated biopsy gun. Radiology 202(3), 874–877 (1997). doi:10.1148/radiology.202.3.9051050
W.J. Choi, J.H. Baek, Role of core needle biopsy for patients with indeterminate, fine-needle aspiration cytology. Endocrine 45(1), 1–2 (2014). doi:10.1007/s12020-013-0071-3
J.I. Son, S.Y. Rhee, J.T. Woo, W.S. Park, J.K. Byun, Y.J. Kim, J.M. Byun, S.O. Chin, S. Chon, S. Oh, S.W. Kim, Y.S. Kim, Insufficient experience in thyroid fine-needle aspiration leads to misdiagnosis of thyroid cancer. Endocrinol. Metabol. 29(3), 293–299 (2014). doi:10.3803/EnM.2014.29.3.293
M.N. Nikiforova, A.I. Wald, S. Roy, M.B. Durso, Y.E. Nikiforov, Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J. Clin. Endocrinol. Metabol. 98(11), E1852–E1860 (2013). doi:10.1210/jc.2013-2292
Y.E. Nikiforov, Thyroid carcinoma: molecular pathways and therapeutic targets. Mod. Pathol. 21(Suppl 2), S37–S43 (2008). doi:10.1038/modpathol.2008.10
J.C. Ricarte-Filho, M. Ryder, D.A. Chitale, M. Rivera, A. Heguy, M. Ladanyi, M. Janakiraman, D. Solit, J.A. Knauf, R.M. Tuttle, R.A. Ghossein, J.A. Fagin, Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 69(11), 4885–4893 (2009). doi:10.1158/0008-5472.can-09-0727
M. Fukahori, A. Yoshida, H. Hayashi, M. Yoshihara, S. Matsukuma, Y. Sakuma, S. Koizume, N. Okamoto, T. Kondo, M. Masuda, Y. Miyagi, The associations between RAS mutations and clinical characteristics in follicular thyroid tumors: new insights from a single center and a large patient cohort. Thyroid 22(7), 683–689 (2012). doi:10.1089/thy.2011.0261
E.K. Jang, D.E. Song, S.Y. Sim, H. Kwon, Y.M. Choi, M.J. Jeon, J.M. Han, W.G. Kim, T.Y. Kim, Y.K. Shong, W.B. Kim, NRAS codon 61 mutation is associated with distant metastasis in patients with follicular thyroid carcinoma. Thyroid 24(8), 1275–1281 (2014). doi:10.1089/thy.2014.0053
G. Garcia-Rostan, H. Zhao, R.L. Camp, M. Pollan, A. Herrero, J. Pardo, R. Wu, M.L. Carcangiu, J. Costa, G. Tallini, ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J. Clin. Oncol. 21(17), 3226–3235 (2003). doi:10.1200/jco.2003.10.130
M.R. Sapio, A. Guerra, V. Marotta, E. Campanile, R. Formisano, M. Deandrea, M. Motta, P.P. Limone, G. Fenzi, G. Rossi, M. Vitale, High growth rate of benign thyroid nodules bearing RET/PTC rearrangements. J. Clin. Endocrinol. Metabol. 96(6), E916–E919 (2011). doi:10.1210/jc.2010-1599
Acknowledgments
This study was supported by Grants (No. 2015-633) from the Asan Institute for Life Sciences, Seoul, Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Additional information
Dong Eun Song and Won Bae Kim have contributed equally and should be regarded as co-corresponding authors.
Rights and permissions
About this article
Cite this article
Jang, E.K., Kim, W.G., Kim, E.Y. et al. Usefulness of NRAS codon 61 mutation analysis and core needle biopsy for the diagnosis of thyroid nodules previously diagnosed as atypia of undetermined significance. Endocrine 52, 305–312 (2016). https://doi.org/10.1007/s12020-015-0773-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0773-9