Abstract
Unlike GLP-1, liraglutide is not cleared by the glomerulus and its pharmacokinetic is not altered in patients with mild renal impairment. The aim of our study was to analyze the effects of liraglutide on renal function in patients with type 2 diabetes. A twelve-month longitudinal prospective post-marketing study was performed. According to eGFR (estimated glomerular filtration rate) calculated with CKD-EPI equation, 84 consecutive patients were divided in Group A (eGFR > 90 ml/min) and Group B (eGFR < 90 ml/min). BMI, glucose, HbA1c, serum creatinine, microalbuminuria, and eGFR were evaluated at baseline and after 12 months of treatment. A reduction in fasting plasma glucose (p < 0.01), HbA1c (p < 0.003), BMI (p < 0.01), and systolic (p < 0.01) and diastolic blood pressure (p < 0.006) was recorded irrespective of eGFR category. Concerning renal function, creatinine levels had a trend to decrease in both groups. eGFR did not change in Group A, while it increased in Group B (p < 0.05) independently from the concomitant changes of other parameters. Moreover, seven out of 41 patients of Group B had increased eGFR levels which reached the normal values (>90 ml/min). At baseline, five patients had pathological microalbuminuria, but at 12 months three of them returned to normal albuminuria (p < 0.006). Total microalbuminuria levels improved in both groups (p < 0.02). According to preliminary data in animals, our study shows that liraglutide is effective in preserving eGFR in diabetic patients, increasing it in those with reduced renal function. This was associated with a decrease of frequency of patients positive to microalbuminuria. Further studies are needed to confirm these data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liraglutide is a human GLP-1 long-acting analog approved for once daily treatment for patients with type 2 diabetes.
Liraglutide, which shares 97 % structural homology with human GLP-1, has a longer half-life than the native hormone and similar pharmacokinetics, with no single organ responsible for its major removal [1]. After a single dose of liraglutide, no intact molecules of the GLP-1 analog were found in urine or feces, suggesting slow degradation into small peptides, amino acids, and fatty acid fragments eliminated through the liver or the kidney. Pharmacokinetic studies failed to identify a specific excretory organ, suggesting that liraglutide could be metabolized in a way similar to that of large proteins [1]. Unlike GLP-1, liraglutide is not cleared by the glomerulus and its pharmacokinetic is not altered in patients with mild renal impairment [2]. Those properties may offer advantages in using liraglutide in patients with impaired renal function. In fact, dose adjustment is not required in these patients, while the use of liraglutide in patients with moderate or greater renal dysfunction is not recommended because of lack of clinical experience [3].
Several studies demonstrated that liraglutide effectively lowers glycosylated hemoglobin (HbA1c) as monotherapy or in combination with oral anti-diabetic drugs [4]. A meta-analysis showed that mild renal impairment did not significantly affect the reduction of HbA1c or the percentage of patients reaching glycemic targets. Moreover, liraglutide was not associated with acute changes in renal function as measured by serum creatinine supporting data on its safety in patients with mild renal impairment [4].
However, there is a lack of data about the renal effects of liraglutide, especially in the daily clinical practice, despite evidence supporting a role of GLP-1 in modulation of renal function [5]. Different studies showed that GLP-1 is able to induce diuresis and natriuresis, but the mechanisms that regulate those effects are still unknown. GLP-1 receptor has been recently detected in porcine proximal tubular cells, where it reduces sodium reabsorption [6]. Moreover, the infusion of GLP-1 activates an intracellular pathway mediated by cAMP/PKA which causes an increase in glomerular filtration rate (GFR), renal plasma flow, and bicarbonate and fractional potassium excretion [7] in addition to its diuretic and natriuretic effects. The evidence that this peptide increases both GFR and renal plasma flow suggests that GLP-1 might exert a direct effect on renal vasculature, most likely by decreasing the resistance of the pre-glomerular capillaries [8, 9].
Based on the above, the aim of our study was to analyze the effects of 12-month therapy with liraglutide on renal function in a group of patients with type 2 diabetes.
Research design and methods
We performed a longitudinal, prospective, observational study. Study quality was assessed using the checklist “STROBE” (for ‘STrengthening the Reporting of OBservational studies in Epidemiology’; additional file 1) (Supplemental Table 1). We consecutively recruited patients with type 2 diabetes, referred to the Diabetes and Endocrine Unit of the “Maggiore della Carità” hospital in Novara from August 2010 to April 2013, and all patients were evaluated at baseline and after 12 months of treatment.
We considered all eligible patients with type 2 diabetes not on target with common oral anti-diabetic drugs (HbA1c >7 % or >53 mmol/mol), to which we added a daily injection of liraglutide.
Patients were excluded from the study whether they (1) had poor compliance to treatment with liraglutide (fear of daily subcutaneous injection, elderly people unable to self-administer the drug, people suffering from senile dementia and/or neurodegenerative diseases); (2) had renal failure requiring dialysis; (3) had a history of a renal disease different from that due to diabetes or hypertension (kidney stones, metabolic diseases, glomerulonephritis, etc.); and (4) did not gave their consent to undergo a regular and periodic follow-up, as required by the Italian Agency of Drugs (AIFA).
We evaluated at baseline and after 12 months of therapy BMI, glucose, HbA1c, serum creatinine, microalbuminuria, and estimated glomerular filtrate rate (eGFR), calculated with the MDRD [10] and CKD-EPI equation [11]. All the formulas are reported in Supplemental Table 2.
Patients were subdivided in Group A and Group B according to eGFR estimated with CKD-EPI, because MDRD equation has a low accuracy for an eGFR > 90 ml/min/1.73 m2 [10, 11]. Patients were included in Group A if they had a normal renal function (eGFR > 90 ml/min) and in Group B if it was impaired (eGFR < 90 ml/min). However, nine patients were discordant for eGFR if evaluated with MDRD equation, eight in group A and 1 in group B, respectively.
Height was measured by the Harpenden stadiometer to the nearest mm with the subject head in Frankfurt plane and weight using electronic scale both taken in triplicate. Averaged BMI was calculated as body weight divided by squared height (kg/m2).
Plasma glucose levels (mg/dl; 1 mg/dl: 0,05551 mmol/l) were measured by the gluco-oxidase colorimetric method (GLUCOFIX, by Menarini Diagnostici, Florence, Italy). HbA1c levels were measured by the high-performance liquid chromatography (HPLC), using a Variant machine (Biorad, Hercules, CA); intra- and inter-assay coefficients of variation are, respectively, lower than 0.6 and 1.6 %. Linearity is excellent from 3.2 (11 mmol/mol) to 18.3 % (177 mmol/mol). Serum creatinine levels were assessed with the enzimatic method of creatinine deamidase/GLDH (Adivia Chemistry -Bayer).
Statistical analysis
All data are expressed as mean ± standard deviation (SD), absolute or delta values. A sample of 60 individuals has been estimated to be sufficient to demonstrate a difference of 10.0 ml/min in eGFR with an SD of 16 with 90 % power, a significance level of 95 % using the Student t test, and a drop out of 10 %. Distributions of continuous variables were examined for skewness and were logarithmically transformed as appropriate. Analysis of covariance was used to determine differences in subjects with and without renal impairment. Analysis of repeated measures was used to determine differences in subjects before and after the treatment with liraglutide. Covariates were sex, age, BMI, disease duration, number of drugs for hypertension, use of ACE-inhibitors, angiotensin receptor blockers (ARBs), or diuretics (yes/no). A stepwise regression was used to determine the association of changes (expressed as delta values) among each variable and delta of the others. The χ 2 test was used to determine difference in distribution. Statistical significance was assumed at p < 0.05. The statistical analysis was performed with SPSS for Windows V.17.0 (SPSS Inc., Chicago, IL, USA).
Results
A population of 243 patients was firstly selected, but 159 subjects failed to respect the inclusion criteria and were excluded. In particular, (a) 58 subjects did not gave the informed consent; (b) 41 did not performed the evaluation of creatinine at baseline or after 12 months of therapy; (c) 37 showed no compliance to therapy; (d) 20 had discrepant data between our database and that of AIFA; and (e) three drop out for gastrointestinal adverse events. Of the 84 patients (age 59.9 ± 11.1 years) who met inclusion criteria and completed the 12-month follow-up and were definitively studied, 73 of them were on therapy with 1.2 mg/day of liraglutide, ten with 1.8 mg/day, and only one with 0.6 mg/day. Liraglutide treatment was associated with metformin (42 patients), sulphonylurea (two patients), metformin plus sulphonylurea (37 patients), or repaglinide (three patients). Sixty-six patients had hypertension, of which 18 took ACE-inhibitors, 30 ARBs, and 22 diuretics (19 of them in co-administration with ACE-inhibitors or ARBs).
Complete clinical characteristics of Group A and B are listed in Table 1. Group A and B were composed by 43 and 41 patients, respectively. The liraglutide dose was 1.2 ± 0.1 and 1.2 ± 0.2 mg/day in Group A and B, respectively. In Group B, 30 patients had a mild renal impairment (eGFR 60–89 ml/min), meanwhile 11 of them had a moderate renal impairment (eGFR 31–59 ml/min).
In the whole population, 12-month therapy with liraglutide induced a reduction in fasting plasma glucose (∆ −2.39 ± 4.08 mmol/l; p < 0.01), HbA1c (∆ −1.05 ± 1.71 %; ∆ −11.20 ± 17.74 mmol/mol; p < 0.003), BMI (∆ −2.13 ± 6.39 kg/m2; p < 0.01), and systolic (∆ −13.51 ± 27.20 mmHg; p < 0.01) and diastolic blood pressure (∆ −5.42 ± 13.91 mmHg; p < 0.006). Similar results were recorded for both Group A and Group B (Table 1). The reduction of systolic and diastolic blood pressure remained significant also when weighted for changes in BMI, glucose levels, and HbA1c in the whole population and in both subgroups. The number of hypertensive patients did not change (31 and 35 patients in Group A and B, respectively); however, five out of 66 patients (all of Group B) reduced the number of drugs for the treatment of hypertension. The liraglutide dose was 1.2 mg/day in all of them.
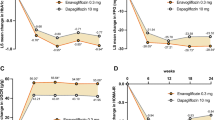
Concerning renal function, creatinine levels had a trend to decrease without reaching significance, without any differences between the two groups (Table 1). After 12 months of treatment, eGFR irrespective of the formula used did not change in Group A, while increased in Group B (p < 0.05) (Fig. 1). The variation of eGFR irrespective of the formula used was found to be independent from the concomitant changes of the other parameters (BMI, glucose, HbA1c, and blood pressure values) and by the number of the type of drugs for hypertension or renal impairment. Moreover, at the end of the study period, three patients of Group A showed a reduction in eGFR values below the normal range (<90 ml/min), while seven patients of Group B had increased eGFR levels which improved and reached the normal values (>90 ml/min). The liraglutide dose was 1.2 mg/day in all of these seven patients. All the three patients of Group A with a worsening in eGFR had basal eGFR < 100 ml/min (respectively 94, 96, and 99 ml/min), a BMI >30 kg/m2, dyslipidemia, and hypertension under specific treatment, but not albuminuria. The liraglutide dose was 1.2 mg/day in two of them and 1.8 mg/day in the last one. No complications or worsening of comorbidities occurred during the 12 months of observation in these three subjects.
At baseline, five patients had pathological microalbuminuria (three from Group A and two from Group B, respectively). At 12 months, three of them presented albuminuria in the normal range (p < 0.006) and the other two of them (both from group A) showed an improvement in microalbuminuria levels. Total microalbuminuria levels improved in both groups (∆ −2.54 ± 10.32 mg/24 h; p < 0.02) also when weighted for number of drugs for hypertension, or use of potential interfering drugs.
Discussion
Liraglutide is not predominantly eliminated by renal excretion. Some meta-analyses of Phase III studies showed that liraglutide is safe and effective in patients with type 2 diabetes at different stages of renal function [4, 12–14]. Our study confirmed the efficacy of liraglutide in improving glycemic control in patients with type 2 diabetes, by reducing fasting plasma glucose and HbA1c levels, irrespective of eGFR, but more strikingly showed that liraglutide did not deteriorate serum creatinine levels and eGFR in patients with normal renal function and improved eGFR in those with a mild or moderate renal impairment.
First of all, we confirmed data of the registration studies on the efficacy of liraglutide about the reduction of fasting glucose, HbA1c, and BMI in a single-center daily clinical practice, where the compliance to treatment is frequently poor, number of visits each year is less, and patients’ characteristics are more heterogeneous with respect to registration studies [13–15]. The reduction of parameters was comparable to Phase III studies [2, 15, 16] and also with the ABCD nationwide post-marketing study conducted in UK which included patients also with mild or moderate renal impairment [17]. Moreover, the degree of clinical improvement is irrespective of renal function at baseline. It has to note that ABCD study referred to first 6 months of treatment, meanwhile our data confirmed the efficacy of liraglutide in renal impairment over 12 months of therapy.
We also observed a reduction in both systolic and diastolic blood pressure irrespective of the renal function. Data derived by previous studies [18–21] showed positive effects of liraglutide on the reduction of diastolic and, more strongly, systolic blood pressure irrespective of the combined therapy for the treatment of diabetes, as also an improvement of the cardiac function [22]. The main hypothesized mechanism for the reduction of blood pressure was the weight decrease; however, we observed that this was independent of the changes in glucose, HbA1c, and BMI. It has to mention that GLP-1 has been demonstrated to have natriuretic and vasodilatatory effects due to inhibition of Na+ reabsorption in the proximal tubule, attenuation of angiotensin II-induced phosphorylation of extracellular signal-regulated kinase-1/2 in renal cells, and NO-dependent and independent mechanism in endothelium [5, 7, 19, 23, 24]. The fact that the magnitude of changes in blood pressure was identified also in mild or moderate renal impairment and that five patients with this alteration reduced the number of drugs for hypertension leads to hypothesize that the treatment could have a further role in this condition where lower blood pressure levels are recommended and, frequently, difficult to achieve.
Furthermore, we did not observe any significant changes in serum creatinine levels, independently by daily dosing of liraglutide, even if a trend to decrease was observed. These data are in agreement with those pooled by meta-analysis and systematic reviews of clinical trials on patients with mild-to-severe renal impairment [2, 25, 26]. This is in line with those reported in the ABCD study where significance, maybe due to the higher sample size, was reached [17]. However, a direct effect on kidney with a following improvement of nephropathy cannot be excluded, by considering that the activation of GLP-1 receptor was associated with the reversion of nephropathy in diabetic rats [27]. Accordingly, with this hypothesis and with data in animals, our observation of a reduction of percentage of patients with albuminuria is in agreement with other pilot studies with exenatide [28] or DPP-4 inhibitors [29]. Finally, we observed stable eGFR levels in those with normal renal function, and, more strikingly, an improvement in those patients with a mild or moderate renal impairment with a tendency towards normal levels in a subgroup of them. To evaluate this aspect, we used two formulas, the MDRD and the CKD-EPI equations. This was done because the Cockcroft-Gault formula for calculation of eGFR has some limitations. The MDRD equation overtakes the limits due to body weight or height in patients with chronic kidney diseases. Although helpful, poorer performance of the MDRD formula was reported at low plasma creatinine concentrations (corresponding to higher levels of renal function) and tends to underestimate renal function in those with a normal eGFR > 90 mL/min/1.73 m2 [10]. The CKD-EPI equation was validated to match the accuracy of the MDRD equation at GFR < 60 mL/min/1.73 m2 and to offer a greater accuracy at higher GFR, minimizing the over-diagnosis of chronic kidney diseases with the MDRD equation, being accurate as MDRD in the subgroup of patients with an eGFR < 60 ml/min/1.73 m2 and substantially more accurate in the subgroup of them with an eGFR > 60 ml/min/1.73 m2 [11]. Our results on eGFR were evaluated with both equations and were unrelated to which formula was used to estimate the eGFR. Since the increase in eGFR levels in patients with reduced renal function is independent of the changes in other variables considered, this could address a direct effect of liraglutide on the kidney. As discussed above, this hypothesis is supported by a few studies in animals, which showed that GLP-1 is responsible for a natriuretic effect, by reducing sodium reuptake in proximal tube and aldosterone levels in mice [23]. Moreover, the infusion of native GLP-1 activates an intracellular pathway mediated by cAMP/PKA which also causes an increase in eGFR, renal plasma flow, and bicarbonate and fractional potassium excretion [7], suggesting a direct effect on renal vasculature, most likely by decreasing the resistance of the pre-glomerular capillaries [8, 9]. This is reinforced by a recent re-evaluation of tissue GLP-1 receptor distribution which showed that in the kidney GLP-1 receptor was exclusively expressed in smooth muscle cells of arteries and arterioles [30]. Since the presence of microalbuminuria in diabetes implies either dysfunction of the glomerular filtration barrier and/or dysfunction in tubular reabsorption [29, 31], the improvement of eGFR could partially explain also the reduction in frequency and total amount of microalbuminuria we showed.
The results on creatinine and eGFR should be interpreted with caution since the study is of relatively short duration. However, the population is less homogenous of that selected for Phase III studies and better mirror conditions of unknown potential risks. However, the heterogeneity of patients included and the small number of those who had a mild deterioration in Group A and, foremost, of those who had a restoration of eGFR in Group B did not allow to phenotype these two subgroups which need further studies over the next few years in addition to the ongoing LEADER study [32]. Since our study had relatively small numbers of patients with moderate renal impairment or under the maximal daily dosing of 1.8 mg of liraglutide, there is a need for other investigations to evaluate the safety in these specific conditions. Moreover, particular attention has to be paid to extend our data to all the class of drugs acting on the GLP-1 system, by considering that other derived GLP-1 drugs are mainly eliminated through the kidney, first of all exenatide. Another limitation of our study is the unavoidable loss of data from patients being lost to follow up or with incomplete data in real-life clinical practice. This has the potential to introduce bias of results for those patients attending visits more frequently. Nevertheless, the high concordance of our data with the results on glucose, BMI, and blood pressure of published meta-analysis on registration studies [2, 20] is a reassuring finding. It should also be considered that the sample size was calculated for eGFR changes but not for microalbuminuria and that we weighted the analyses for the number or the type of potential interfering drugs, but the specific dose was not considered. Wider studies are warranted to better investigate these aspects.
Our study firstly showed that liraglutide is effective in preserving GFR in diabetic patients, increasing glomerular filtration rate in those with reduced renal function, independently by the changes in glucose levels or BMI. This effect was associated with a trend to decrease serum creatinine levels and number of patients positive to microalbuminuria. Further real-life studies are needed to confirm these data.
Abbreviations
- CKD-EPI:
-
Chronic kidney disease epidemiology collaboration
- eGFR:
-
Estimated glomerular filtration rate
- GLP-1:
-
Glucose-like peptide 1
- MDRD:
-
Modification of diet in renal disease
- NO:
-
Nitrogen monoxide
References
M. Malm-Erjefält, I. Bjørnsdottir, J. Vanggaard, H. Helleberg, U. Larsen, B. Oosterhuis, Metabolism and excretion of the once-daily human glucagon-like peptide-1 analog liraglutide in healthy male subjects and its in vitro degradation by dipeptidyl peptidase IV and neutral endopeptidase. Drug Metab. Dispos. 38(11), 1944–1953 (2010)
J.A. Davidson, J. Brett, A. Falahati, D. Scott, Mild renal impairment and the efficacy and safety of liraglutide. Endocr. Pr. 17(3), 345–355 (2011)
D. Russell-Jones, The safety and tolerability of GLP-1 receptor agonists in the treatment of type-2 diabetes. Int. J. Clin. Pract. 64(10), 1402–1414 (2010)
S. Madsbad, Liraglutide effect and action in diabetes (LEAD™) trials. Expert Rev. Endocrinol. Metab. 4(2), 119–129 (2009)
J.P. Gutzwiller, S. Tschopp, A. Bock, C.E. Zehnder, A.R. Huber, M. Kreyenbuehl et al., Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J. Clin. Endocrinol. Metab. 89(6), 3055–3061 (2004)
P. Schlatter, C. Beglinger, J. Drewe, H. Gutmann, Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul. Pept. 141(1–3), 120–128 (2007)
R.O. Crajoinas, F.T. Oricchio, T.D. Pessoa, B.P. Pacheco, L.M. Lessa, G. Malnic et al., Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am. J. Physiol. Ren. Physiol. 301(2), 355–363 (2011)
C. Moreno, M. Mistry, R.J. Roman, Renal effects of glucagon-like peptide in rats. Eur. J. Pharmacol. 434(3), 163–167 (2002)
T. Nyström, A.T. Gonon, A. Sjöholm, J. Pernow, Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul. Pept. 125(1–3), 173–177 (2005)
C.M. Florkowski, J.S. Chew-Harris, Methods of Estimating GFR - Different Equations Including CKD-EPI. Clin. Biochem. Rev. 32(2), 75–79 (2011)
A.S. Levey, L.A. Stevens, C.H. Schmid, Y. Zhang, A.F. Castro, H.I. Feldman et al., A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009)
M. Marre, J. Shaw, M. Brändle, W.M. Bebakar, N.A. Kamaruddin, J. Strand, M. Zdravkovic et al., LEAD-1 SU study group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet. Med. 26(3), 268–278 (2009)
J.J. Neumiller, T.E. Sonnett, L.D. Wood, S.M. Setter, R.K. Campbell, Pharmacology, efficacy and safety of liraglutide in the management of type 2 diabetes. Diabetes Metab. Syndr. Obes. 3, 215–226 (2010)
M. Rigato, G.P. Fadini, Comparative effectiveness of liraglutide in the treatment of type 2 diabetes. Diabetes Metab. Syndr. Obes. 7, 107–120 (2014)
G. Aimaretti, Liraglutide: a once-daily human glucagon-like peptide-1 analogue. J. Endocrinol. Invest. 32(8), 701–703 (2009)
R.R. Henry, J.B. Buse, G. Sesti, M.J. Davies, K.H. Jensen, J. Brett et al., Efficacy of antihyperglycemic therapies and the influence of baseline hemoglobin A(1C): a meta-analysis of the liraglutide development program. Endocr. Pract. 17(6), 906–913 (2011)
K.Y. Thong, C. Walton, R. Ryder, Safety and efficacy of liraglutide 1.2 mg in patients with mild and moderate renal impairment: the ABCD nationwide liraglutide audit. Prac. Diabetes J. 30(2), 71–76 (2013)
T. Vilsbøll, M. Zdravkovic, T. Le-Thi, T. Krarup, O. Schmitz, J.P. Courrèges et al., Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 30(6), 1608–1610 (2007)
B. Wang, J. Zhong, H. Lin, Z. Zhao, Z. Yan, H. He, Y. Ni et al., Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes. Metab. 15(8), 737–749 (2013)
L. E. Robinson, T. A. Holt, K. Rees, H. S. Randeva, J. P. O’Hare, Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open 3(1), e001986 (2013). doi:10.1136/bmjopen-2012-001986
M. Katout, H. Zhu, J. Rutsky, P. Shah, R.D. Brook, J. Zhong et al., Effect of GLP-1 mimetics on blood pressure and relationship to weight loss and glycemia lowering: results of a systematic meta-analysis and meta-regression. Am. J. Hypertens. 27(1), 130–139 (2014)
K. Okada, K. Kotani, H. Yagyu, A. Ando, J. Osuga, S. Ishibashi, Effects of treatment with liraglutide on oxidative stress and cardiac natriuretic peptide levels in patients with type 2 diabetes mellitus. Endocrine 47(3), 962–964 (2014)
K. Hirata, S. Kume, S. Araki, M. Sakaguchi, M. Chin-Kanasaki, K. Isshiki et al., Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem. Biophys. Res. Commun. 380(1), 44–49 (2009)
P. Anagnostis, V.G. Athyros, F. Adamidou, A. Panagiotou, M. Kita, A. Karagiannis, D.P. Mikhailidis, Glucagon-like peptide-1-based therapies and cardiovascular disease: looking beyond glycaemic control. Diabetes Obes. Metab. 13(4), 302–312 (2011)
L.V. Jacobsen, C. Hindsberger, R. Robson, M. Zdravkovic, Effect of renal impairment on the pharmacokinetics of the GLP-1 analogue liraglutide. Br. J. Clin. Pharmacol. 68(6), 898–905 (2009)
C.B. Giorda, E. Nada, B. Tartaglino, Pharmacokinetics, safety, and efficacy of DPP-4 inhibitors and GLP-1 receptor agonists in patients with type 2 diabetes mellitus and renal or hepatic impairment. A systematic review of the literature. Endocrine 46(3), 406–419 (2014)
W.J. Liu, S.H. Xie, Y.N. Liu, W. Kim, H.Y. Jin, S.K. Park et al., Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J. Pharmacol. Exp. Ther. 340(2), 248–255 (2012)
H. Zhang, X. Zhang, C. Hu, W. Lu, Exenatide reduces urinary transforming growth factor-β1 and type IV collagen excretion in patients with type 2 diabetes and microalbuminuria. Kidney Blood Press Res. 35(6), 483–488 (2012)
M. Haluzík, J. Frolík, I. Rychlík, Renal effects of DPP-4 inhibitors: a focus on microalbuminuria. Int. J. Endocrinol. (2013). doi:10.1155/2013/895102
C. Pyke, R.S. Heller, R.K. Kirk, C. Orskov, S. Reedtz-Runge, P. Kaastrup et al., GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 155(4), 1280–1290 (2014)
M.J. Lazzara, W.M. Deen, Model of albumin reabsorption in the proximal tubule. Am. J. Physiol. Renal. Physiol. 292(1), 430–439 (2007)
S.P. Marso, N.R. Poulter, S.E. Nissen, M.A. Nauck, B. Zinman, G.H. Daniels et al., Design of the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. Am. Heart J. 166(5), 823–830 (2013)
Conflict of interest
The authors declare no conflict of interest. There is not conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent
Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zavattaro, M., Caputo, M., Samà, M.T. et al. One-year treatment with liraglutide improved renal function in patients with type 2 diabetes: a pilot prospective study. Endocrine 50, 620–626 (2015). https://doi.org/10.1007/s12020-014-0519-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0519-0