Abstract
Prediabetes represents an elevation of plasma glucose above the normal range but below that of clinical diabetes. Prediabetes includes individuals with IFG, IGT, IFG with IGT and elevated HbA1c levels. Insulin resistance and β-cell dysfunction are characteristic of this disorder. The diagnosis of prediabetesis is vital as both IFG and IGT are indeed well-known risk factors for type 2 diabetes with a greater risk in the presence of combined IFG and IGT. Furthermore, as will be illustrated in this review, prediabetes is associated with associated disorders typically only considered in with established diabetes. These include cardiovascular disease, periodontal disease, cognitive dysfunction, microvascular disease, blood pressure abnormalities, obstructive sleep apnea, low testosterone, metabolic syndrome, various biomarkers, fatty liver disease, and cancer. As the vast majority of individuals with prediabetes are unaware of their diagnosis, it is therefore vital that the associated conditions are identified, particularly in the presence of mild hyperglycemia, so they may benefit from early intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prediabetes represents an elevation of plasma glucose (PG) above the normal range but below that of clinical diabetes. Prediabetes, as defined by a measure of glucose metabolism, includes individuals with IFG, IGT, and IFG with IGT. Elevated HbA1c levels which integrate PG overtime, is now validated as another indicator of this condition [1].
As far as the pathophysiology of prediabetes is concerned, insulin resistance and β-cell dysfunction are the “substrates” of this disorder. However, there is some heterogeneity with respect to the nature of the metabolic defects specifically involved in prediabetes. Thus, IGT is associated with greater, skeletal muscle (peripheral) insulin resistance than IFG which is characterized by hepatic insulin resistance and excessive endogenous glucose production. In addition, subjects with IGT have impaired first and second phase insulin secretion while IFG rather shows an isolated defect in first-phase secretion [2, 3].
The diagnosis of prediabetes, recently reviewed by Buysschaert and Bergman [1, 4, 5], is vital as both IFG and IGT are indeed well-known risk factors for type 2 diabetes with a greater risk in the presence of combined IFG and IGT [6, 7]. Furthermore, as will be illustrated in this review, prediabetes is associated with a variety of associated disorders often only considered typically in the presence of established diabetes. These include cardiovascular disease (CVD), periodontal disease, cognitive dysfunction, microvascular disease, blood pressure abnormalities, obstructive sleep apnea (OSA), low testosterone, metabolic syndrome, presence of various biomarkers, fatty liver disease, and cancer.
Prediabetes is often reflected on as “benign” with the absence of co-morbid conditions. Furthermore, as the vast majority of individuals with prediabetes are unaware of their diagnosis, it is therefore vital that these associated conditions are identified, particularly in the presence of mild hyperglycemia, so these individuals may benefit from early intervention.
Cardiovascular risk
Prediabetes is associated with microangiopathy and also with more advanced atherosclerotic vascular damage than in normoglycemia [1, 4]. In this context, compared with normoglycemic subjects, Ferrannini suggested that individuals with IFG, IGT or both, also have a “diabetic phenotype” signifying that aside from having mild hyperglycemia, they are older, have a higher BMI as well as more central fat distribution and higher waist-to-hip ratio [8]. In addition, they are dyslipidemic and tend to have higher arterial blood pressure levels. Most of those variables are components of the metabolic syndrome (accounting for the insulin resistance), therefore commonly present in those with prediabetes [9]. In other words, prediabetes clearly aggregates with other CVD risk factors—a point that should be taken into account in the further analysis of the relationship between prediabetes and the development of CV events [10].
The frequent observation of abnormal glucose metabolism in patients with coronary heart disease or stroke is consistent with the relationship between prediabetes and macroangiopathy [11]. In this context, the proportion of individuals with IGT in subjects with acute myocardial infarction (MI) was 35 % in the GAMI Study [12]. Similarly, in a cohort of patients with transient ischemic attacks or stroke, based on fasting plasma glucose (FPG), 2 h post-load and HbA1c levels, 52 % were identified as “prediabetics” [13].
Consequently, on the basis of the aforementioned considerations, and the presence of various CV risk factors in prediabetes, it is rational to estimate the magnitude of the relative risk for CVD associated with IFG and/or IGT from published studies.
Association of prediabetes and cardiovascular events
Several meta-analyses have clearly demonstrated that diabetes imparts a two to threefold increase in the risk of developing macroangiopathy [14–16]. Prediabetes has also been linked with an increased risk of major manifestations of vascular disease, suggested by a substantial number of longitudinal studies, which provide evidence favoring vascular risk with mild to moderate fasting and/or post-load hyperglycemia lower than the currently defined threshold for diabetes. Thus, in the Whitehall study, the risk for CV disease was almost doubled in subjects with IGT when compared with those with normal glucose tolerance [17]. Similarly, Balkau et al. observed that the highest quintile of a 2 h post-load blood glucose was associated with an almost double risk, 1.6 for total mortality and 1.8 for CV deaths in middle-aged non-diabetic men [18]. Coutinho et al. carried out a meta-analysis comprising more than 95,000 individuals (94 % males) followed over 12 years and found a relationship between blood glucose and CV events at glucose levels well below the diabetic threshold: compared with a glucose level of 75 mg/dl (4.2 mmol/l), fasting and 2 h post-load glucose levels of 110 mg/dl (6.1 mmol/l) and 140 mg/dl (7.8 mmol/l) were associated with relative CV event risks of 1.33 (95 % confidence interval [CI] 1.06–1.67) and 1.58 (1.19–2.10), respectively [19].
Other data extend these observations. In the 2007 Australian Diabetes, Obesity and Lifestyle Study, after a follow-up of 5.2 years, the adjusted hazard ratio [HR] in men and women for CV mortality was increased among patients with IFG (HR 1.6 [1.0–2.4]) [20]. Moreover, Hoogwerf et al. showed that the relationship between glucose and coronary heart disease risk was continuous and graded across the range of non-diabetic values, independent of traditional risk factors [21]. Finally, Ford et al. carried out an important systematic review of the relationship between prediabetes and CV risk [22].Thus, 18 reports examined IFG [110–125 mg/dl (6.1–6.9 mmol/l)] and concluded that the fixed-effects summary RR was estimated to be 1.20 (1.12–1.28). Another eight reports looked for IFG [100–125 mg/dl; 5.6–6.9 mmol/l)] and showed a RR estimate of 1.18 (1.09–1.28). In eight reports studying IGT, the summary RR estimate was 1.20 (1.07–1.34). Five studies combined IFG and IGT with a summary RR of 1.10 (0.99–1.23). Overall, there was no significant difference between estimates for men and women [22]. In addition, Barr et al. in 2009 observed that in individuals without diagnosed diabetes, FPG, 2 h post-load glucose and HbA1c were significant predictors of CVD mortality [23]. As evidenced by Selvin et al., HbA1c levels were also strongly associated with CV risk in a population of non-diabetic adults [24]. The multivariable-adjusted HR was 1.78 (1.48–2.15) and 1.95 (1.53–2.48) for HbA1c concentrations between 5.5 and 6.0 % and 6.0–6.5 %, respectively. Prediabetes was also associated with a higher future risk of stroke when compared with normoglycemic status [25]. Di Pino et al., consistent with these data, demonstrated a correlation between HbA1c and arterial intima-media thickness (IMT) in prediabetes [26].
In view of all these results, most authors emphasize the potential consequences in men and women of the association between prediabetes and increased CV morbidity and/or mortality although a few studies did not demonstrate such a relationship [27–29].
Association of “borderline” normoglycemia and cardiovascular events
Reinforcing the concept of a relationship between prediabetes and CVD, several reports converge and indicate that an elevated CV risk is strongly and independently associated with glucose levels within the normoglycemic range, prior to the development of prediabetes, as already indicated by Hoogwerf et al. in 2002 [21]. In the study of Shaye et al. in 2012, subjects with a FPG 95–99 mg/dl (5.3–5.5 mmol) had an increased CV risk when compared with levels <80 mg/dl (4.44 mmol/l) (HR 1.53 [1.22–1.91], after adjustment for conventional CV confounding risk factors [30]. In phase with these results, the Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe (DECODE) Study Group demonstrated elegantly that the relation between glycemia and CV risk started within the normal BG range with a linear relationship and no evidence of threshold effect [31].
Data using HbA1c as an independentearly predictor of CV risk confirm this premise. Thus, in the Norfolk cohort, the effect of HbA1c on CV mortality was already evident at the lower end of the population distribution and there was no apparent threshold effect: men with an HbA1c above 5 % had greater risk than those with values below 5 %, suggesting a continuous relation with CV risk [32, 33]. In agreement with the data of Selvin et al. [24], in the INTERHEART Study, after adjustment for conventional CV risk factors, higher HbA1c percentiles above the lowest fifth (<5.4 %) were associated with progressively higher odds ratio (OR) of MI with OR for the highest fifth percentile (>6.12 %) being 1.55 (1.37–1.75). When analyzed as a continuous variable, every 1 % increase in HbA1c was associated with a 19 % (14–23) higher OR for MI while every 0.5 % increase in HbA1c indicated a 9 % higher OR of MI (7–11) [34].
IFG versus IGT versus HbA1c: is there a better predictor?
Many studies suggested that the risk for CVD in prediabetes was better predicted by IGT than by IFG.
In line with Qiao et al. [35], the DECODE Study Group indicated that the dose–response effect of fasting hyperglycemia for vascular mortality might be weaker than that of post-load glucose. They showed indeed in pooling studies of European cohorts that IGT was associated in men and women with an increased risk of cardiovascular deaths, independent of FPG, although the converse was not the case [36]. Thus, a high 2 h post-load glucose level predicted all-cause and CVD mortality after adjustment for other major CV risk factors. The relationship between post-load glycemia and mortality was linear and this relationship was not observed with FPG [36]. Similar observations have been reported in the Framingham Offspring Study [37] and in the Hoorn Study in The Netherlands [38]. Similarly, IFG was not independently associated with an increased short-term risk for incident CV events in a multivariate model (HR 1.16 [0.88–1.52], when compared with normal FPG [27]. The Funagata Study also showed higher CV events in individuals with IGT versus IFG [39].
Furthermore, in a study involving Japanese non-diabetic participants, the carotid artery IMT gradually increased across the tertiles of fasting, 1, 2 h post-load and HbA1c levels. However, in a multiple linear regression analysis, 2 h PG was the only independent determinant of IMT [40]. Lee et al. observed that the risk of stroke was associated with IGT or combined IGT and IFG [25].
However, more recent analyses do not support the assertion of increased risk of IGT vis-a-vis IFG. The Australian Diabetes Obesity and Lifestyle Study found that IFG, but not IGT, was an independent predictor of CV mortality [20]. Similarly, the Emerging Risk Factor Collaboration Group meta-analysis of 102 prospective studies concluded that FPG was non-linearly associated in patients without known CVD with incident CVD, with a significant increase in multi-adjusted HR becoming evident for glucose values above 108 mg/dl (6.0 mmol/l) [16, 41]. Moreover, IFG as well as type 2 diabetes were also related to the risk of sudden cardiac death and all-cause mortality during a 19 year follow-up, even after adjustment for confounding factors [42]. The meta-analysis of Ford et al. could not show a difference in CV risk between IFG and IGT [22]. Finally, the Framingham Heart Study confirmed that IFG was associated with an increased CVD risk in women [43].
Irrespective of whether basal or post-load BG concentrations is more closely associated with the development of atherogenesis, average glucose concentrations indexed by HbA1c predicts incident coronary disease at least as well as FPG and post-load glucose, demonstrated by the Norfolk and INTERHEART studies [32–34], Selvin et al. [44] and Sarwar et al. [45]. Moreover, HbA1c in those at high-risk of diabetes was also associated with increased all-cause mortality reported recently by Skriver et al. [46].
It is also of interest to mention, as indicated by Faerch et al. [47] and others [34, 48] that the relative importance of PG levels (IFG, IGT, HbA1c) on CVD risk could decrease with age, possibly as a result of the influence of other cardiometabolic risk factors.
Mechanisms of CVD in prediabetes
Thus, as indicated in most studies, prediabetes by itself imparts a (modest) (±20 %) increase in risk for CV morbidity and mortality. However, this does not prove that a prediabetic range of glucose levels “directly” causes macroangiopathy. The epidemiological relationship between prediabetes, hyperglycemia, and CV events could indeed be confounded by a clustering of vascular risk factors within individuals, including general and central obesity with insulin resistance, high blood pressure levels, dyslipidemia, and proinflammatory and prothrombic states, as all these variables, features of the metabolic syndrome, are commonly present in (pre)diabetes. Therefore, the strength of the glycemic effect in the reported studies depends on the extent to which related vascular risk factors were taken into account in the statistical analysis. Nevertheless, the data of a direct relationship between hyperglycemia and CVD are consistent as in most studies multivariable-adjusted analysis independently demonstrated the association between hyperglycemia and CVD events. Thus, IFG, post-load glucose as well as HbA1c were all reported as robust predictors of vascular morbid and mortality.
On the other hand, even if we accept that mild hyperglycemia by itself could increase the risk for CV morbidity and mortality, non-glycemic factors, in particular the components of the metabolic syndrome, mainly insulin resistance, hypertension, and/or dyslipidemia, have also to be taken into account in the development of CV events [2, 9, 10]. Several studies, including meta-analyses, have shown that the presence of the metabolic syndrome raised the risk for CVD by approximately twofold [49, 50]. Grundy indicated in 2012 that the association between prediabetes and CVD could largely be mediated by the metabolic syndrome [9]. Hypertensionalone is 2–3 times more common in prediabetic than in normoglycemic subjects. Moreover, prediabetes and hypertension are additive risk factors for atherosclerosis and CVD [51]. With regard to atherogenic dyslipidemia, prediabetes is characterized by elevated LDL-C, non-HDL-C, small, dense LDL particles, and reduced HDL-C which are major risk factors for CVD. The role of hypertriglyceridemia as a major risk factor for vasculopathy in prediabetes remains more controversial [2].
The role of both glycemic and non-glycemic factors in the development of CVD during prediabetes is supported by the different pathophysiologic pathways leading to vasculopathy recently reviewed by Milman and Crandall [52].
As illustrated in Fig. 1, hyperglycemia contributes directly to the pathogenesis of CVD. Hyperglycemia can induce oxidative stress by overproduction of reactive oxygen species (ROS) in the vasculature. This, in conjunction with the inactivation of NO, leads to endothelial dysfunction with impaired relaxation in arterial vascular smooth muscle cells. Hyperglycemia also results in an increased production of advanced glycosylation end products, abnormal levels of cytokines and in the activation of transcription factor NF-κB with expression of adhesion molecules. Finally, hyperglycemia is associated with a prothrombotic and procoagulant state also contributing, along with the other physiopathological defects, tomacroangiopathy.
Main glycemic and non-glycemic pathogenic pathways leading to macroangiopathy in prediabetes PI3 phosphatidyl inositol-3 kinase, NO nitric oxide, ROS reactive oxygen species, FFA free fatty acid, NF nuclear factor adipocytokines mean leptin/TNFα/interleukin 6 which are increased and adiponectin which is decreased
The development of CVD is also mediated by non-glycemic mechanisms. Insulin resistance with the release of free fatty acids from adipose tissue directly impairs insulin sensitivity, blunting the vascular endothelium phosphatidylinositol 3-kinase insulin signaling pathway with the consequence of reduced NO production due to lower endothelial nitric oxide synthase (eNOS) activity and vascular endothelium dysfunction and remodeling. In this setting, compensatory hyperinsulinemia, via the MAP-kinase pathway, leads to mitogenesis and vasoconstriction that secondarily promote atherosclerosis. Visceral fat is also a source of proinflammatory cytokines (leptin, TNF-alpha, interleukin-6) which contribute to endothelial dysfunction and stimulate transcription factor NF-κB, this phenomenon being amplified by reduced circulating levels of antiatherosclerotic adiponectin.
In parallel, atherogenic dyslipidemia and high blood pressure play a direct, well known role in the development of CVD as reviewed by DeFronzo et al. and Grundy [3, 9].
Treatment considerations
Overall, if we assume a causal relationship between mild hyperglycemia and CVD in prediabetes, this could translate, in view of the high prevalence of prediabetes, into substantial numbers of individuals developing or dying from CVD. Interventions in prediabetes with lifestyle or pharmacological therapy have been shown to reduce the rate of progression to diabetes [53–55] and are associated with improvement of several CV risk factors such as blood pressure and dyslipidemia [56]. Preventing diabetes has the potential therefore to reduce CV disease, [57] but to what extent, remains uncertain. The Stop-NIDDM trial involving acarbose reported a 49 % RR reduction in major CV events during a follow-up of 3.3 years [58]. Similarly, rosiglitazone as well as insulin glargine modestly reduced the progression of arterial IMT in individuals with prediabetes [59, 60]. However, in a meta-analysis by Hopper et al., there was no difference in risk of all-cause or CV mortality in the intervention (lifestyle or pharmacological therapy) versus the control group. There was, however, a trend toward fewer fatal and non-fatal MI (RR 0.59 [0.23–1.50] while a 24 % risk reduction in fatal and non-fatal stroke was of borderline statistical significance (RR 0.76 [0.58–0.99]) [61]. Prolonged period of metabolic optimization is needed to demonstrate a benefit in the reduction of vasculopathy. This is confirmed by the recent findings of the Da Quing Diabetes Prevention Study, emphasizing the long-term clinical benefits of lifestyle intervention. The latter, during a 23-year follow-up, showed that the cumulative incidence of CV mortality was 11.9 % (8.8–15.0) in the intervention group versus 19.6 % (12.9–26.3) in the control group (HR 0.59 [0.36–0.96]. All-cause mortality was 28.1 versus 38.4 % (HR 0.71 [0.51–0.99]) [62]. These results directly emphasize the need for a prevention strategy as stressed by Bergman et al. [63].
These results suggest that lifestyle interventions for those with prediabetes are of main importance for preventing diabetes, for improvement of cardiovascular risk factors and for reducing the long-term burden of CV events.
There is a consensus, furthermore, that conventional non-glycemic CV risk factors (components of the metabolic syndrome) in prediabetes should also be treated aggressively [64, 65].
Because prediabetes and diabetes represent a continuum of dysglycemia and CV risk, the same principles that apply to the treatment of type 2 diabetes should apply to the prediabetic state [2]. Thus, according to the recent American Diabetes Association (ADA) recommendations, hypertension should be treated with a systolic blood pressure (SBP) goal of less 140 mmHg (less 130 mmHg may be appropriate for individuals if this can be achieved without undue treatment burden). Prediabetic subjects should be treated to a diastolic blood pressure lower than 80 (85) mmHg [64, 65]. Pharmacological therapy should comprise, when needed, in addition to lifestyle, a regimen that includes either an ACE inhibitor or an angiotensin receptor blocker [65].
Concerning dyslipidemia, LDL-C targeted therapy remains the preferred strategy as evidenced especially in the study of Kearney et al. [66]. This means a target LDL-C lower than 100 mg/dl (2.6 mmol/l) in those with prediabetes without associated CVD and/or any major CV risk factor and LDL-C target lower than 70 mg/dl (1.8 mmol/l) in prediabetic subjects with known CVD or without CVD but with additional major CV risk factors. It is important, however, to note that in the 2014 position statement, the ADA recommends that statin therapy should be added to lifestyle approach regardless of basal lipid levels in patients with overt CVD or without CVD over the age of 40 years and have one or more other CVD risk factors. For lower-risk subjects than statin therapy should also be considered if LDL-C remains above 100 mg/dl (2.6 mmol/l) [65]. The reduction in CVD risk with statins use out weighs the (minor) risk of incident diabetes [67]. Triglycerides levels less than 150 mg/dl (1.7 mmol/l) and appropriate levels of HDL-C [greater than 40 mg/dl (1.0 mmol/l) in men and 50 mg/dl (1.3 mmol/l) in women] are also desirable, according to ADA recommendations [65].
Finally, aspirin therapy should be considered mainly in primary prevention for those with increased CV risk as well as in secondary prevention. Smoking cessation should be recommended [65].
In conclusion, individuals with prediabetes, defined by IFG/IGT and/or HbA1c should be targeted for CVD prevention. According to the association between prediabetes and CV events, lifestyle intervention aiming at treating dysglycemia is of importance for preventing diabetes, for improvement of several CV risk factors and for reducing from a long-term perspective, the burden of cardiovascular events. Treatment of prediabetes should be multifactorial including in most cases, in addition to lifestyle, a pharmacological approach in order to optimize the various components of the associated metabolic syndrome.
Periodontal disease
Periodontitis is a common chronic inflammatory disease characterized by destruction of the supporting structures of the teeth (the periodontal ligament and alveolar bone). Epidemiological studies confirm that diabetes is a major risk factor for periodontitis increasing susceptibility by approximately threefold [68]. There is a clear relationship between degree of hyperglycemia and severity of periodontitis. Peridontal disease has been suggested as the “sixth complication of diabetes” [68, 69]. Incidences of macro-albuminuria and end-stage renal disease are increased 2- and 3-fold, respectively, in those with diabetes who also have severe periodontitis. Furthermore, the risk of cardio-renal mortality is three times higher in those with diabetes having severe periodontitis.
There is emerging evidence to support a two-way relationship between diabetes and periodontitis, with diabetes increasing the risk for periodontitis, and periodontal inflammation negatively affecting glycemic control [70]. This has been shown in the in the Zucker fatty rat [71]. Treatment of periodontitis has been associated with reduction in HbA1c levels approximating 0.4 % [68].
Although the exact mechanism through which diabetes and prediabetes promote periodontal inflammation remains unclear, it has been proposed that an interaction between advanced glycation end products (AGEs) and their receptors (RAGEs) in periodontal tissues impairs chemotactic and phagocytic function of polymorphonuclear leukocytes and produces proinflammatory cytokines, thereby leading to periodontal inflammation and bone loss [8, 9]. In addition, function of cells involved in immune-inflammatory responses is impaired with chronic hyperglycemia [69]. Manouchehr-Pour et al. [72] reported that chronic hyperglycemia may prevent breakdown of bacteria in periodontal pockets, thereby increasing periodontal degeneration [69].
Several studies have reported that individuals with impaired glucose tolerance (IGT) demonstrate severe periodontal inflammatory parameters [69, 70]. Severe periodontitis was associated with a 93 % increase in the odds of IGT after multivariable adjustment [70]. However, a trial evaluating periodontal conditions found that prediabetic and non-diabetic subjects had identical periodontal status whereas those with poorly controlled type 2 diabetes had severe periodontal conditions [73]. In contrast to these findings, Javed et al. [69] found that self-perceived oral health status, severity of periodontal parameters, and marginal bone loss (MBL) were worse in patients with prediabetes than controls. Glycemic control significantly reduced the severity of these parameters and the state of prediabetes in affected individuals [69]. The authors therefore recommended that individuals with prediabetes, particularly if fasting blood glucose (FBG) >100 mg/dl (5.6 mmol/l), and gingival bleeding should be referred to oral health care providers as they are more susceptible to periodontal breakdown compared with those maintaining lower glucose levels. Findings from this study provide evidence regarding the importance of glycemic control, oral hygiene maintenance, and regular medical and dental check-ups, which may benefit oral health in those with prediabetes. Hence, oral health should therefore be promoted as an integral component of overall diabetes management. Closer collaboration between medical and dental care providers should therefore be fostered in managing those with diabetes and periodontitis [68].
Cognitive dysfunction
Chronic hyperglycemia and microvascular disease contribute to cognitive dysfunction in both types 1 and 2 diabetes which may also make progression to dementia more likely [74, 75]. Neurocognitive deficits, mostly psychomotor impairment, were observed within days of diagnosing type 1 diabetes in children and adolescents [76]. This may serve as an early marker for broader neuropsychological deficits having implications for long-term diabetes management. The risk for dementia is increased by 50 % in those with type 2 diabetes [77] while those with prediabetes are also at increased but lesser risk. Type 2 diabetes may confer a 1.4- to 1.8-fold greater risk for minimal cognitive impairment (MCI), a 1.5- to 2-fold greater risk for Alzheimer disease (AD) and a 2- to 2.5-fold greater risk for vascular dementia. The effect of diabetes on cognitive decline might accelerate with age. Thus, 10 years of diabetes could correspond to 2.5–10 years of extra-cognitive aging [78].
Cognitive decline in diabetes affects memory, measures of attention, information processing, and executive functioning, is associated with mental and motor slowing and is affected by diabetes duration and glycemic control [74, 78–80]. Clinically relevant diabetes-related cognitive decrements occur in two crucial periods: during brain development in childhood and aging when the brain undergoes neurodegenerative changes [81].
As noted, those with pre-diabetes, when compared with a normoglycemic population, are at increased risk of cognitive decline as well. Higher glucose levels, in the absence of diabetes or IGT, have been associated with lower memory and reduced hippocampal microstructure and volume [82]. The ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET/TRANSCEND) cognitive baseline analysis showed that in approximately 20,000 individuals without diabetes there was an inverse association between higher levels of FPG levels and cognitive function assessed by the Mini-Mental State Examination (MMSE) [83].
Sanz et al. [84] demonstrated that a high, but normal HbA1c level was negatively associated with cognitive performance assessing processing speed in healthy middle-aged adults without diabetes. Tan et al. found [85] that diabetic and prediabetic states characterized by insulin resistance, hyperinsulinemia, and hyperglycemia, when present in late middle age, were related to decreased brain volume and lower cognitive performance on executive function and memory tasks. Nazaribadie et al. [86] also found significant differences in cognitive functioning in individuals with diabetes and prediabetes and suggested therefore that neuropsychological status should be monitored in addition to controlling glucose levels.
An increase in 2-h post-load glucose levels but not fasting levels was also linked to increased risk of all-cause dementia, Alzheimer’s disease and vascular dementia in a Japanese population [87]. Furthermore, in a community-based cohort study, Crane et al. [88] demonstrated that average higher glucose levels even at the lower end of the glucose spectrum during a median follow-up of 6.8 years may be a risk factor for dementia even among those without diabetes [88]. These findings suggest that higher levels of glucose may have deleterious effects on the aging brain. This is compounded by the observation that older adults with prediabetes also have limitations in physical function [89]. However, in contrast to the aforementioned observations, cognitive decline in those with newly diagnosed diabetes and prediabetes was not different from that in normoglycemic individuals in the Whitehall cohort study [80].
In a study assessing hyperglycemia determined by HbA1c concentrations did not add predictive power beyond diabetes status for cognitive decline [90]. This observation though was based on a single baseline HbA1c value, limited cognitive battery, dependence on self-reported diagnoses and lack of information on highly relevant clinical factors such as occurrence of severe hypoglycemia [91]. In addition, large changes in cognition did not occur during the 6-year study period.
Findings associated with cognitive impairment and dementia and a higher prevalence of brain atrophy in types 1 and 2 diabetes are characterized by neural slowing, increased cortical atrophy, microstructural abnormalities in white matter, infarctions and changes in brain neurometabolites [74, 92, 93]. Elderly individuals with diabetes without dementia have progression of brain atrophy and cognitive decline when compared to those without diabetes [92, 94]. The increased risk appears to include Alzeheimer’s disease as well as vascular dementia [94]. Bryan and colleagues using MRI scans found that brain volume loss, particularly in the gray matter, was associated with duration of disease. Gray matter includes areas involved in muscle control, vision and hearing, memory, emotions, speech, decision-making, and self-control [95]. For every 10 years with diabetes, the brain was about 2 years older than that of an individual without diabetes.
Mechanistic studies suggest that vascular disease and alterations in glucose, insulin, and amyloid metabolism define the underlying pathophysiology [94]. Disruption of insulin action, a key homeostatic factor in the brain, leads to impairment of neuronal function which can contribute to the development of mood disorders and neurodegenerative disease [96]. Through direct neuronal effects, regulation of cholesterol synthesis, mitochondrial function, tau phosphorylation, and aggregated amyloid-β (Aβ) processing, defects in insulin signaling, provide a link between diabetes and CNS disorders. Insulin action has been shown to play a role in the progressive pathogenesis of AD. Aggregated Aβ fibrils and hyperphosphorylation of tau proteins causing amyloid plaques and helical neurofibrillary tangles are hallmarks of AD which can be ameliorated with insulin signaling [96].
As both prediabetes and diabetes are associated with increased cardiovascular risk, these may be associated with poor motor performance independent of their impact on cognition suggesting that better control of vascular risk factors in midlife may prevent physical impairment and disability in the elderly [97].
Although diabetes is associated with cognitive decline and late-onset dementia, intensive glucose treatment over a 40-months period does not seem to benefit cognition [81] in older individuals (mean age 62 years) with long-standing type 2 diabetes and high-risk of CVD [98]. This combined with the non-significant effects on other outcome measures and increased mortality with intensive treatment does not support the use of intensive therapy to reduce the adverse effects of diabetes on the brain. Early prevention strategies to reduce the risk of cognitive impairment are needed especially as longevity of individuals with diabetes increases achieving an age when cognitive disorders become apparent. [98]. It should also be noted that no overall reduction in cognitive decline was observed through intensive blood pressure therapy or adding a fibrate to well-controlled LDL-c levels [99].
Given the increasing public health burden associated with the diabetes epidemic and aging, prevention, and control of diabetes and hypertension may prevent or delay ischemic injury and neurodegeneration and the onset of cognitive impairment [93].
Microvascular disease
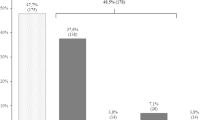
Microvascular and macrovascular complications may be present in prediabetes [100]. As many as 25 % of individuals with newly diagnosed diabetes have diabetic retinopathy or microalbuminuria suggesting that there is on the average a 7-year gap between the actual onset and diagnosis of diabetes. Early detection is therefore critical to identify the presence of these complications.
Retinopathy
Microvascular complications may occur in those with prediabetes including retinopathy observed in 8–12 % of individuals [101]. This is typically background retinopathy although sight-threatening retinopathy has been described [101]. In a study relating FPG with retinopathy did not find a uniform glycemic threshold with a substantial proportion of retinopathy occurring in those without diabetes [FPG < 7.0 mmol/l (126 mg/dl)] [102]. The prevalence of retinopathy increased gradually with FPG strongly suggesting a continuous relationship. Retinopathy was present in 7–13 % at concentrations below a FPG of 5.6 mmol/l (100.8 mg/dl). When examining the association of HbA1c and FPG with retinopathy prevalence in a US population ≥40 years, retinopathy began to rise steeply when HbA1c exceeded 5.5 % and FPG exceeded 5.8 mmol/l (104.4 mg/dl) [103].
The Diabetes Prevention Program (DPP) demonstrated the benefit of lifestyle intervention or metformin in preventing diabetes in individuals with elevated FPG levels and IGT and provided the opportunity to examine whether retinopathy occurs in prediabetes. Retinal lesions were found before the onset of diabetes and increased in prevalence early in the course of diabetes. Retinopathy was detected in 12.6 % of diabetic and 7.9 % of non-diabetic participants. Documentation of retinopathy in prediabetes suggests that retinopathy occurs over a wider continuum of glycemia than is provided for by established diagnostic criteria. Hence, diagnostic criteria for diabetes based predominantly on associated risk for retinopathy may need to be reconsidered. However, as the characteristics of lesions are benign in prediabetes, routine screening may not be justifiable [104]. These observations are supported by studies suggesting that the effects of glucose and blood pressure on the retinal microvasculature, using techniques that quantify retinal vascular caliber, are graded and continuous and therefore current definitions of diabetic and hypertensive retinopathy are arbitrary and do not capture early disease [105]. Finally, changes in retinal vascular caliber may predict the onset of type 2 diabetes and IFG and have been linked to CVDs.
Neuropathy
Neuropathy is more prevalent than expected in newly diagnosed diabetes with 18–25 % demonstrating abnormalities in nerve conduction studies or electromyography suggesting that neuropathy developed during the prediabetic period. Between 25 and 62 % of those with idiopathic peripheral neuropathy have prediabetes. Among those with prediabetes, 11–25 % may have peripheral neuropathy and 13–21 % have neuropathic pain [106]. An oral glucose tolerance test (OGTT) should therefore be performed in those with idiopathic neuropathy to determine if a glucose abnormality underlies this condition. Population studies suggest the presence of a gradient for the prevalence of neuropathy being highest in diabetes, followed by IGT, then IFG and least in those with normoglycemia. Pathogenesis of neuropathy involves hyperglycemia, microvascular abnormalities, dyslipidemia, and the metabolic syndrome.
There is a high prevalence of unawareness of distal sensorimotor polyneuropathy in those with prediabetes and diabetes. Neuropathy in those with IGT and appears to be milder than that found in with diabetes involving predominantly small rather than large fibers [107]. Neuropathy is typically painful and predominantly sensory with motor involvement being rare [106, 107]. Little is known about the efficacious treatment of neuropathy in prediabetes. Diet and exercise are beneficial for those with IGT-associated neuropathy and may require symptomatic treatment [106, 107] as well as treating other features of the metabolic syndrome. As the frequency of foot examinations is insufficient and diabetic foot prevention practiced inadequate [108], these aspects clearly need to be improved upon.
Nephropathy
The prevalence of chronic kidney disease (CKD), characterized by albuminuria or reduced kidney function, is high among those with diagnosed diabetes and prediabetes [109, 110]. Population-based studies have demonstrated a stepwise increase in the prevalence of CKD as glucose tolerance decreases [110]. In a study of US adults without diabetes, the prevalence of CKD (GFR < 60 ml/min/1.73 m2) increased from 1.2 % with FPG levels of 89–95 mg/dl (4.94–5.27 mmol/l) to 4 % with FPG of at least 102 mg/dl (5.66 mmol/l) [110]. A strong association has also been found between the metabolic syndrome and CKD.
The National Health and Nutrition Examination Survey (NHANES) (1999–2006) found that 39.6 % of those with diagnosed and 41.7 % with undiagnosed diabetes had CKD whereas 17.7 % had prediabetes. The rate of CKD was 10.6 % in those without prediabetes or diabetes [109]. CKD prevalence in individuals with prediabetes and no self-reported hypertension was approximately 10 %. Thus, many individuals with prediabetes and without diagnosed hypertension, a risk factor for CKD screening as is diabetes, are at risk for CKD therefore suggesting that those with prediabetes might be screened as well. Although more than half of those with prediabetes and evidence of CKD had reduced kidney function, only approximately 20 % of those individuals had micro- or macro-albuminuria. Thus, both urinary protein and kidney functional measurements would need to be performed to determine the presence of CKD in adults with prediabetes as recommended for those with diabetes.
Campaigns promoting awareness of kidney damage and decline in kidney function at this early stage may be beneficial. Furthermore, screening for prediabetes in those with hypertension would be important given the additive effect in increasing CKD risk. Hence, those with prediabetes warrant earlier detection and management efforts to prevent development, progression, and complications of both diabetes and CKD associated with diabetes [109–111]. Pharmacologic intervention with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers should be considered [111].
Circadian blood pressure
It is well recognized that diabetes is a CVD risk equivalent [112]. Prediabetes is also associated with early carotid atherosclerosis [113], coronary artery calcification [114], and increased CVD risk [20]. One marker to measure CVD risk is circadian blood pressure abnormalities. The circadian rhythm of blood pressure has a normal daily variation characterized by high blood pressures during the day, reduction during the night, a nocturnal dip, and a morning surge upon awakening [115]. A 10–20 % fall in SBP during the course of the night is thought to be a normal daily variation [116]. Patients in whom the nighttime drop in blood pressure is absent or decreased (<10 % fall in nocturnal SBP) are referred to as “non-dippers”, and these patients appear to have greater target-organ involvement and morbidity [117]. A small proportion of patients can also have a nocturnal increase in blood pressure, known as “reverse dippers”, and these patients are at increased risk for left ventricular hypertrophy, cerebrovascular disease, and MI [118]. Other abnormal circadian rhythms include decreased heart rate variability, excessive pulse pressure, circadian-hyper-amplitude-tension (CHAT), and impaired vascular compliance. These abnormalities have also been shown to be independent risk factors for heart disease and stroke [119].
Decreased circadian blood pressure variability, especially a blunted nocturnal decrease in blood pressure, has been observed in type 1 and type 2 diabetes and is associated with autonomic neuropathy and nephropathy [120]. The pathophysiology of non-dipping is not fully understood, but it is largely felt to be secondary to autonomic neuropathy [121]. Studies have shown that the non-dipping phenomenon in diabetes can, in fact, be considered a marker of autonomic neuropathy [122]. Abnormal circadian rhythm is also felt to have prognostic value—in individuals with diabetes having reverse circadian blood pressure have been found to be at high-risk for both fatal and nonfatal vascular events [123]. One research group found that adults with abnormal circadian blood pressure variability also had a higher incidence of prediabetes and endothelial dysfunction, the initial abnormality in the process of atherosclerosis. They hypothesized that endothelial dysfunction leads to an increased risk of CVD [124].
Autonomic dysfunction is also seen in individuals with IGT [125] and research suggests that prediabetes is associated with abnormal circadian blood pressure variability. Kumarasamy et al. [126] investigated the relationship between prediabetes and abnormal circadian rhythms in an animal model. They found a cause and effect relationship between caloric excess, systemic inflammation, prediabetes, prehypertension, and abnormal circadian rhythm. The hypothesis was that caloric excess results in adipose tissue deposition, leading to an inflammatory state and altered glycemia, which thereby potentiates the renin–angiotensin–aldosterone system resulting in a loss of blood pressure control.
A small study by Gupta et al. [116] compared the circadian blood pressure variability of healthy overweight adults with prediabetes to healthy adults with normoglycemia using ambulatory blood pressure monitoring over the period of 7 days. Glycemic status was assessed using an OGTT and an ambulatory blood pressure monitor (ABPM) measured blood pressure and heart rate every 30 min during the day and every 60 min during the night. Of the twelve subjects enrolled in the study, six had prediabetes and six had normal glucose tolerance. The investigators found that while none of the normoglycemic subjects had abnormal circadian rhythm, four of the six subjects with prediabetes had abnormalities in circadian blood pressure including excessive pulse pressure and CHAT. While the study size was small, it did provide preliminary data that prediabetes is associated with circadian blood pressure abnormalities.
A later study further investigated the relationship between prediabetes and circadian blood pressure [127]. Individuals with impaired fasting glucose (IFG) and normal blood pressure were matched with healthy volunteers and 24-h ambulatory blood pressure and heart rate variability were evaluated. Results of the study were notable for significantly higher mean 24-h systolic and diastolic blood pressures in the group with IGT [126 ± 12 (mean ± SD) vs. 117 ± 10, 75 ± 7 vs. 71 ± 6 mmHg, both P < 0.05]. The IGT group also had significantly lower systolic and diastolic diurnal indices and frequency of dipping, all markers of circadian rhythm dysfunction.
Patients with hypertension and diabetes are at increased risk for CVD, and abnormalities in circadian blood pressure likely increase the risk for cardiovascular adverse events in prediabetic patients. Early recognition of this risk is highly desirable. Ambulatory blood pressure monitoring could be a potential target to assess cardiovascular risk in those with prediabetes and aid in risk modification before the onset of clinical disease.
Obstructive sleep apnea
Sleep-disordered breathing (SDB) is characterized by breathing difficulties while sleeping and encompasses occurrences of apnea (complete cessation of airflow during sleep) and hypopnea (decrease in airflow). The apnea–hypopnea index (AHI) is the number of disordered breathing events per hour of sleep, and is the standard by which severity of disease is measured. SDB has been associated with a number of adverse metabolic outcomes including hypertension, CVD, obesity, insulin resistance, glucose intolerance, and type 2 diabetes [128].
OSA is the most common of the SDB and an association between type 2 diabetes and OSA has long been recognized [129, 130]. The Sleep Heart Health Study, a cross-sectional analysis of over 2,000 patients without known diabetes, showed that the prevalence of type 2 diabetes was twofold higher among those with OSA compared with those without [131]. A prospective study from Japan spanning 5 years examined nocturnal oximetry in over 4,000 patients and found that in subjects with moderate to severe nocturnal intermittent hypoxia the HR for developing type 2 diabetes was 1.69 (1.04–2.76, 95 % CI) [132]. An observational cohort study of Veterans Affairs patients with OSA found an independent association between sleep apnea and incident diabetes (HR per quartile 1.43, CI 1.10–1.86) after adjusting for age, sex, baseline glucose, and BMI [133]. A more recent study evaluated whether risk of incident diabetes was related to the severity of OSA in a large cohort of patients followed for more than a decade [134]. The investigators found that an AHI >30 had a 30 % higher hazard of developing diabetes than those with an AHI <5 and concluded that OSA severity predicted subsequent risk for incident diabetes.
OSA is believed to impair glucose tolerance (IGT) and lead to insulin resistance. Over the past few decades a number of studies have demonstrated an independent association between OSA and prediabetes. Overall, the prevalence of prediabetes has been estimated to range from 20 to 67 % in patients with OSA [135]. A study by Punjabi et al. examined SDB in 2,656 subjects and found that those with moderate to severe SDB (AHI >15) had an OR of 1.46 (95 % CI 1.09–1.97) for IGT [130]. This was independent of the confounding influences of age, gender, weight, and body fat distribution. The relationship between SDB and prediabetes was further investigated by Alshaarawy et al. who evaluated 5,685 participants without diabetes gathered from the NHANES [136]. The relationship between markers of SDB (such as sleep duration less than 6 h, snoring, snorting, and daytime sleepiness) and prediabetes was studied. Compared to those without any sleep disturbance, the multivariable OR of prediabetes among those with three or more SDB markers was 1.69 (1.28–2.22). Though the causal mechanism for the association between SDB and prediabetes is not known, hypoxia [137], immune activation [138], and sympathetic hyperreactivity [139] are felt to play roles in the development of metabolic dysfunction.
In diabetes, the prevalence of OSA has been shown to range between 54 and 94 % [140, 141], and this relationship is believed to be independent of obesity [130]. One study assessing polysomnography and glycosylated hemoglobin (HbA1c) in diabetic patients demonstrated that increasing OSA severity is associated with poorer glucose control [142]. Compared with patients without OSA, the adjusted mean HbA1c was increased by 1.49 % (P = 0.0028) in patients with mild OSA, 1.93 % (P = 0.0033) in patients with moderate OSA, and 3.69 % (P = 0.0001) in patients with severe OSA. Evidence suggests that diabetes might increase predisposition for OSA through development of autonomic neuropathy and upper airway muscle dysfunction [143].
Limited data exists as to whether prediabetes increases the risk of developing OSA. A recent cross-sectional study evaluated 137 morbidly obese individuals who underwent a sleep study and 2-h OGTT [144]. The investigators found after adjustment for several variables (including, age, gender, BMI, etc.), that those with prediabetes had a higher OR of OSA, 3.18 (95 % CI 1.00–10.07, P = 0.049), compared to their counterparts with normal glucose tolerance. A clear mechanism for this relationship has not yet been elucidated, though one suggested mechanism is that the inflammation associated with hyperinsulinemia, insulin resistance, and visceral adiposity lead to narrowing of the upper airways, and thus increase upper airway obstruction [145].
Continuous positive airway pressure (CPAP) is an effective therapy for OSA, and in light of evidence that OSA could lead to insulin resistance, prediabetes, and diabetes, it could be inferred that CPAP therapy improves those parameters. However, uncontrolled and randomized studies that have explored whether CPAP therapy in diabetes leads to improvement in glucose control have had mixed results. Some have shown an improvement in insulin sensitivity [146] and reduction in HbA1c [147, 148], while others have reported no change [149]. The same has been true for numerous studies that have examined the effects of CPAP treatment on prediabetes [135, 150]. A large confounding factor in many of these studies was CPAP non-compliance, a problem addressed in a study by Pamidi [151]. In this study, those with prediabetes and OSA were treated with CPAP versus placebo for 2 weeks, with supervisors ensuring CPAP compliance. Compared with placebo, those treated with CPAP showed significantly decreased 2-h glucose levels and area under the glucose curve during the OGTT. Data from this preliminary study suggest that treating OSA with optimal CPAP therapy could significantly improve insulin sensitivity in prediabetes, though large scale, randomized controlled trials will be required to fully determine the effects of CPAP on glucose metabolism. An assessment for sleep apnea could potentially play an important role in risk stratification in individuals with prediabetes.
Testosterone
There is a recognized bidirectional association between type 2 diabetes and male hypogonadism. Cross-sectional studies have shown that between 25 and 40 % of men with diabetes have low testosterone levels [152–154], with about 3 nmol/l lower levels compared to those without diabetes [155]. Low levels of testosterone are manifested by erectile dysfunction, reduced sexual desire, and loss of morning erections. There is increasing evidence that low serum testosterone is associated with other comorbidities such as hypertension, CVD, and all-cause mortality [156]. The preponderance of testosterone is bound tightly to sex hormone-binding globulin (SHBG), with a smaller portion bound weakly to albumin, and a tiny fraction represents unbound or free testosterone. Insulin is important in regulating testosterone secretion, and inhibition of insulin secretion has been shown to decrease total and free testosterone and increase SHBG levels [157]. Guidelines recommend checking testosterone levels routinely in patients with type 2 diabetes, along with other high-risk groups such as those with metabolic syndrome and osteoporosis [158].
Reduced levels of testosterone have also been associated with insulin resistance [153] and studies have shown that men with low testosterone are at increased risk for developing diabetes [159]. A longitudinal population-based study followed a random sample of men for 7–10 years and used testosterone and SHBG levels to predict new cases of diabetes [159]. Lower baseline levels of free testosterone and SHBG predicted diabetes at follow-up. The OR for developing diabetes was 1.58 for 1 standard deviation (SD) decrease in testosterone, and 1.89 for 1 SD decrease in SHBG. Another study examined 221 middle-aged, men without diabetes, and found an inverse association between testosterone, fasting glucose, and insulin resistance, independent of body fat or abdominal fat [160]. Interestingly, administering testosterone to obese men has been found to improve insulin sensitivity [161].
The relationship between insulin resistance and testosterone levels naturally raises the question as to whether there is a link between prediabetes and low testosterone. A rabbit model of prediabetes demonstrated that mild hyperglycemia and glucose intolerance were associated with reduced gonadotropin-releasing hormone neurons in the hypothalamus, decreased gonadotropin and testosterone levels, and hypotrophy of prostate and seminal vesicles [162]. Few studies have investigated the risk of testosterone deficiency and low sex hormone levels in men with prediabetes, though the literature revealed several positive studies demonstrating an association between prediabetes and testosterone deficiency. A community-based cross-sectional study by Goodman et al. [163] evaluated the association between androgen levels and glucose tolerance status in men over the age of 55 and found that men with IFG or IGT had significantly lower total testosterone levels compared with those with normal glucose tolerance, even after adjustment for age and BMI.
Another cross-sectional and longitudinal studies investigated the impact of IFG in men with sexual dysfunction [164]. 3,541 men were studied, of whom 19.1 % had IFG [between 100 and 125 mg/dl (5.6–6.9 mmol/l)]. All patients had SHBG, free and bioavailable testosterone, and penile color Doppler ultrasound measured. The study found that those with IFG had erectile dysfunction, reduced penile blood flow, and biochemical evidence of hypogonadism compared to their euglycemic counterparts. There was a progressive increase in the prevalence of hypogonadism (HR 1.139; P = 0.001 for trend), and a progressive decrease in SHBG-bound and free testosterone levels as a function of glucose impairment.
A recently published cross-sectional study evaluated the risk of testosterone deficiency in men with prediabetes [165]. It included 1,306 men whose sex hormones were measured during a routine medical examination (excluding patients on testosterone therapy or androgen deprivation therapy). Total testosterone and SHBG were measured in conjunction with fasting glucose (IFG), postprandial glucose (IPG), and glycated hemoglobin (HbA1c). In those with prediabetes, the OR for decreased total testosterone levels compared to individuals with normoglycemia was 1.87 (95 % CI 1.38–2.54) and 2.38 (95 % CI 1.57–3.6) in those with diabetes. After adjustment for the metabolic syndrome, the OR in men with prediabetes was equal to that of diabetic men (1.49 vs. 1.50). All measures of prediabetes, including IFG, IPG, and HbA1c were associated with an increased risk of testosterone deficiency. The study found that HbA1c appeared to be a stronger predictor of low total testosterone, even across all multivariate analyses, with or without impaired fasting or postprandial glycemia. IFG was also a reliable predictor, although IPG was only weakly associated with testosterone deficiency.
Current guidelines recommend testosterone replacement therapy only in those men with both testosterone deficiency and clinical symptoms of hypogonadism [158]. Numerous small studies have demonstrated that testosterone replacement therapy in androgen-deficient men, with or without diabetes, improves insulin resistance [161], HbA1c, waist circumference, cholesterol, and fasting glucose levels [166–168]. One study was multicenter, prospective, randomized, double-blind, placebo-controlled that examined the effects of testosterone replacement therapy in hypogonadal men with type 2 diabetes over a 1-year period [168]. Testosterone replacement therapy reduced homeostasis model of insulin resistance (HOMA-IR) by 16.4 % at 12 months (P = 0.006). Additionally, HbA1c was significantly better in the testosterone therapy group than the placebo group (treatment difference −0.446 %; P = 0.035). Whether testosterone replacement has similar effects in hypogonadal men with prediabetes would be of interest, especially with regard to the prevention of overt diabetes, requiring specific clinical trials in this population.
Metabolic syndrome
Metabolic syndrome is characterized by several interrelated risk factors that increase the risk of CVD, type 2 diabetes, and all-cause mortality [169]. The metabolic syndrome is a significant public health issue and its prevalence in the United States in 2010 was estimated to be around 23 % [170]. The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) defines metabolic syndrome as the presence of three of five clinical measures: increased weight circumference (abdominal obesity), elevated triglycerides, decreased high-density lipoprotein (HDL) cholesterol, hypertension, and dysglycemia [171]. Elevated PG can fall in the category of prediabetes (fasting glucose ≥100 mg/dl; (5.6 mmol/l)] or diabetes.
The pathogenesis of metabolic syndrome is multifactorial and a consequence of complex interaction between environmental, genetic, physiological, and biochemical factors [172, 173]. Obesity and insulin resistance are two important risk factors for the development of metabolic syndrome [169], and many investigators believe that insulin resistance is the major mechanism that leads to its development [174]. Insulin resistance is also associated with high-risk for the development of prediabetes, type 2 diabetes, and obesity [175].
There is an overlap in the presence of prediabetes and metabolic syndrome in the general population, and the two seem to be strongly interrelated. One study found that among non-diabetic patients above age 50, about twice as many have IFG plus metabolic syndrome as have IFG alone [176]. Another study compared patients with metabolic syndrome and prediabetes in terms of cardiovascular risk factors, target-organ dysfunction, and insulin resistance [177]. It included 524 obese and overweight non-diabetic patients, and screened these patients for metabolic syndrome, prediabetes, and insulin resistance. Investigators found that only about one-third of those with metabolic syndrome had prediabetes, whereas over half of patients with prediabetes had metabolic syndrome. Metabolic syndrome had a more pronounced effect on early renal dysfunction and increased inflammation, while prediabetes was associated with early carotid structural changes and increased insulin resistance. They concluded that metabolic syndrome and prediabetes represented overlapping but not identical patient populations.
Individuals with metabolic syndrome have about a fivefold increase in diabetes risk [178, 179], and epidemiologic studies have shown that the adverse effects of metabolic syndrome begin in childhood [180, 181]. This is evident from the Bogalusa Heart Study, a long-term study that followed a population of school kids into adulthood and collected data about heart disease risk factors, and the incidence of obesity and CVD. One examined the effects of various metabolic syndrome components on the development of prediabetes and type 2 diabetes [180]. Subjects were first surveyed as children (ages 4–11 years, excluding those with diabetes or elevated fasting glucose) and then followed serially for cardiovascular risk factors for about 21 years. The investigators also found that individuals with prediabetes (compared to those with normoglycemia) had higher LDL-cholesterol, insulin, and homeostasis model assessment of insulin resistance (HOMA-IR) beginning in adolescence. Starting from childhood, diabetic subjects had significantly higher levels of BMI, triglycerides, glucose, insulin, and HOMA-IR, and lower levels of HDL cholesterol.
Whether metabolic syndrome in those with prediabetes predicts the future development of diabetes has been less well studied. A group of researchers recently published a study investigating this very question. About 3,234 participants with IGT were assessed for the presence of metabolic syndrome and followed over an average of 3.2 years [182]. Development of diabetes was determined by annual OGTT and semiannual fasting PG tests. The presence of the metabolic syndrome at baseline in prediabetic individuals was associated with a 70–100 % increased risk of diabetes. Baseline fasting PG was the strongest component linking metabolic syndrome with incident diabetes, followed by baseline hypertriglyceridemia and changes in waist circumference. They concluded that the baseline presence of metabolic syndrome in patients with IGT was indeed associated with an increased risk of developing diabetes.
Identifying and targeting the cardiometabolic components of metabolic syndrome may be beneficial in preventing the long-term development of diabetes. In patients with prediabetes and metabolic syndrome effective preventive approaches include lifestyle changes such as weight loss, diet, and exercise [55, 169]. If these are ineffective, pharmacologic treatment should be considered, and is usually directed at individual components of the metabolic syndrome. Targets of therapy to prevent macrovascular disease include hyperlipidemia, hypertension, and prothrombotic factors [183].
Whether to treat prediabetes to prevent metabolic syndrome and its associated comorbidities is less well studied and controversial. The DPP Research Group investigated the effects of lifestyle interventions and metformin therapy on the incidence of metabolic syndrome and found a 17 % reduction in the metformin-treated group (driven mainly by improvements in waist circumference and fasting glucose) and a 59 % reduction in the lifestyle intervention group [184]. In the Study to Prevent Non-Insulin Dependent Diabetes Mellitus (STOP-NIDDM) trial, acarbose therapy in prediabetes showed a reduction in risk of diabetes, as well as a 34 % relative risk reduction in hypertension [185]. However, 24 % of study participants dropped out of the study due to poor tolerability of acarbose. A study investigating pioglitazone in type 2 diabetics showed a reduction in multiple components of metabolic syndrome such as high blood pressure, hyperglycemia, and hypertriglyceridemia [186], though how this may translate to prediabetic patients is not clear. Thus, except for treatment with acarbose, there is little clinical evidence to show that oral hypoglycemic agents will lessen macrovascular disease in those with metabolic syndrome and prediabetes.
Biomarkers
A biomarker can be defined as a measurable indicator of the risk or presence of disease. In the context of prediabetes, there is ongoing interest in the identification of biomarkers measurable in the serum that may be associated with a higher risk of developing overt type 2 diabetes. Metabolomics refers to the simultaneous measurement of multiple biomarkers or metabolites in order to create a biochemical metabolic profile of an individual patient; these techniques could potentially be used to identify those with a high-risk of developing type 2 diabetes. Current methods of diagnosing prediabetes and diabetes—namely HbA1c, fasting glucose, or OGTT—can be limited and may be inadequate in detecting high-risk individuals. For example, HbA1c measurements may be altered in the setting of anemia or renal failure; HbA1c may also remain in the normal range in some patients with mild dysglycemia. Biomarkers and metabolomics profiles could provide alternative methods for risk stratifying patients with abnormal glucose metabolism, thus allowing for earlier intervention with aggressive lifestyle changes and reducing risk of progression to overt diabetes and its attendant complications.
Adiponectin
Adiponectin is a protein hormone produced by adipocytes that has insulin-sensitizing and anti-inflammatory effects, and measurement of its serum concentration has potential utility as a marker of type 2 diabetes risk. Its concentrations are decreased in obesity, and low concentrations have been associated with increased risk of type 2 diabetes. A 2009 meta-analysis of 13 prospective cohort studies found that a consistent inverse association between adiponectin levels and risk of incident type 2 diabetes was present across multiple diverse populations [187].
A nested case–control study within the Whitehall II prospective cohort analyzed change in adiponectin levels over time prior to diagnosis of type 2 diabetes [188]. Subjects with incident diabetes had lower adiponectin levels compared to non-diabetic control subjects throughout the 13-year follow-up period. Subjects with early-onset diabetes (diagnosed at younger than 52 years of age) had a steeper decline in adiponectin levels over time compared to controls. Female subjects with late-onset DM also demonstrated steeper decline in adiponectin levels than control cases, while adiponectin levels in male subjects with late-onset diabetes declined at the same rate as controls. These results suggest that adiponectin levels could potentially be used to risk stratify patients years before the onset of diabetes, though sex differences need to be considered.
A recent prospective study followed 5,085 subjects with IFG over 4.4 years [189]. Among subjects with fasting glucose 110–125 mg/dl (6.11–6.94 mmol/l), those with the lowest adiponectin levels had a significantly higher risk of incident diabetes as compared to those with the highest levels (HRs 1.78 in men and 2.17 in women). Low adiponectin levels were also associated with increased diabetes risk in women with less severe IFG (100–109 mg/dl; 5.56–6.05 mmol/l) but not in men. Among all subjects with fasting glucose 110–125 mg/dl (6.11–6.94 mmol/l), 45 % of those in the lowest adiponectin tertile were diagnosed with diabetes, compared to 27 % of those in the highest tertile. This study provides further evidence of the potential utility of adiponectin as a biomarker of diabetes risk.
Interleukin-1-receptor antagonist
Interleukin-1-receptor antagonist (IL-1Ra) is produced by adipocytes and acts as a competitive inhibitor of interleukin-1β binding to its receptor. Interleukin-1β is proinflammatory, inhibits pancreatic β-cell function, and promotes β-cell apoptosis [190]. The physiologic impact of blocking interleukin-1β was demonstrated by a trial in 70 patients with type 2 diabetes comparing the effect of anakinra (an IL-1Ra) to placebo; treatment with anakinra was associated with improved glycemic control, increased C-peptide secretion, and reduction in inflammatory markers [191]. A nested case–control study within the Whitehall II cohort found that IL-1Ra levels were significantly higher in subjects who developed incident type 2 diabetes compared to controls up to 13 years prior to diagnosis; IL-1Ra levels also began to increase in diabetes case subjects more steeply starting 6 years prior to diagnosis. This was postulated to be due to a compensatory anti-inflammatory response as prediabetes deteriorates into overt diabetes [192]. IL-1Ra has potential to be useful as both a biomarker of diabetes risk and as a therapeutic target.
Metabolomics
Multiple studies have utilized a metabolomics approach to identify novel biomarkers for prediabetes. This approach aims to simultaneously quantify the small molecules that are the products of multiple different metabolic pathways. In the context of diabetes risk prediction, this is useful in that it may allow detection of earlier, more subtle dysfunction of glucose metabolism than is possible with markers currently used in clinical practice. In turn, identification of metabolites that are altered in the setting of prediabetes and diabetes may help elucidate pathways that contribute to the pathogenesis of diabetes.
A nested case–control study in the Framingham Offspring Study profiled 61 metabolites using liquid chromatography–tandem mass spectrometry in 189 incident cases of diabetes and 189 controls and found higher fasting concentrations of three branched-chain amino acids (leucine, isoleucine, and valine) and two aromatic amino acids (phenylalanine and tyrosine) up to 12 years prior to the onset of diabetes. Subjects in the top quartile of individual amino acid levels had 2- to 3.5-fold higher odds of developing diabetes compared to those with levels in the lowest quartile [193].
Another group measured 140 metabolites in a population of 1,300 subjects from the KORA cohort and identified glycine, lysophosphatidylcholine (LPC) 18:2, and acetylcarnitine as significantly altered in subjects with IGT as compared to those with normal glucose tolerance. Further prospective analysis showed that low levels of glycine and LPC were predictive for developing IGT and overt type 2 diabetes [194].
A case–control study within EPIC-Potsdam profiled 163 metabolites in 800 incident cases of type 2 diabetes compared to a random subcohort of nondiabetic controls. Diabetes risk was associated with higher levels of serum hexoses, phenylalanine, and four species of diacyl-phophatidylcholines. Low levels of glycine and LPC 18:2 as well as of sphingomyelin C16:1 and five species of acyl–alkyl phosphatidylcholines were associated with increased T2DM risk [195]. The results with regards to phenylalanine, glycine, and LPC 18:2 are consistent with those from the two studies cited above.
The RISC study group used mass spectrometry to profile a cohort of 399 nondiabetic subjects and identified α-hydroxybutyrate (α-HB) and linoleoyl-glycerophosphocholine (L-GPC) as the two top biomarkers to distinguish insulin-resistant from insulin-sensitive subjects, determined by the hyperinsulinemic euglycemic clamp and OGTT [196]. In a subsequent analysis, measurements of α-HB and L-GPC were obtained in two nondiabetic cohorts—one from the RISC study with 3 years of follow-up, and another from the Botnia Prospective study, with 9.5 years of follow-up. α-HB was positively correlated with type 2 diabetes risk, while L-GPC was inversely correlated. In vitro studies with INS-1e cells demonstrated decreased glucose-induced insulin release when cells were treated with α-HB and increased insulin release with L-GPC treatment, providing a physiological correlate consistent with the observed population data [197].
Fructosamine and glycated albumin
Fructosamine is the measurement of glycated total serum proteins, of which albumin is predominant. Fructosamine and glycated albumin are markers of short-term (2–4 weeks) glycemic control, compared to HbA1c, which reflects longer term (2–3 months) glycemic control. Fructosamine currently has some utility in the assessment of glycemia in conditions in which HbA1c may be altered (e.g., any state in which red blood cell turnover is affected); it also has potential to be useful in monitoring changes in glycemic control on a short-term basis. However, its use is currently limited due to lack of evidence associating measurements to clinical outcomes. A recent prospective analysis of the ARIC study cohort analyzed associations between fructosamine and glycated albumin levels with incident diabetes, retinopathy, and CKD over 17 years of follow-up. Baseline levels of fructosamine and glycated albumin elevated above the 95th percentile were strongly associated with higher risk of incident diabetes—HRs were 2.61 and 3.27, respectively. High levels were also predictive of prevalent retinopathy and incident CKD [198]. These results suggest that fructosamine could be used as a predictive marker for diabetes as well as its complications.
Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) represents the accumulation of fat in the liver determined by imaging or histology in the absence of any other cause of fatty liver (e.g., alcohol, medications, hereditary diseases, hepatitis C). Nonalcoholic steatohepatitis (NASH) is hepatic inflammation and fibrosis that is indistinguishable from alcoholic steatohepatitis. NAFLD is the most common cause of chronic liver disease in developed countries with prevalence in the range of 20–46 % [199]. Approximately 30 % of NAFLD cases will progress to NASH; 20 % of NASH cases will progress to cirrhosis, of which 40 % will develop decompensated liver failure.
Pathogenesis
Insulin resistance is the common underlying pathogenetic factor between NAFLD and prediabetes. A vicious cycle between insulin resistance and hepatic steatosis exists—insulin resistance leads to increased levels of free fatty acids, leading to increased levels of triglycerides, and hepatic steatosis; in turn, hepatic steatosis leads to increased hepatic gluconeogenesis, thereby exacerbating hyperglycemia and insulin resistance. A “two-hit” hypothesis has been proposed to describe the progression of NAFLD to NASH: (1) hepatic steatosis develops due to insulin resistance, followed by (2) oxidative stress related to excess triglycerides stored in hepatocytes [200]. More recently, a “multiple hits” model has been proposed, which suggests that inflammation induced by gut- or adipose-derived signals may precede steatosis [201].
NAFLD as a marker of prediabetes
A question of interest regarding the relationship between NAFLD and type 2 diabetes is whether NAFLD is a manifestation of the prediabetic state. Multiple studies have evaluated the results of OGTT in subjects with NAFLD. In a 2003 study, Sargin et al. found that among non-diabetic patients with elevated ALT and “bright liver” on ultrasound, 44 % had impaired or diabetic glucose tolerance [202]. Subjects with an abnormal OGTT had higher fasting insulin levels and insulin resistance calculated by the homeostasis model assessment method compared to the group with normal glucose tolerance. Wong et al. found that in a cohort of non-diabetic subjects with biopsy-proven NAFLD, 29 % had IGT and 33 % had diabetes by OGTT, compared to 14 and 7 % of controls, respectively [203]. Yun et al. performed an OGTT in a group of young non-diabetic men (ages 19–30) with elevated ALT and sonographic changes consistent with fatty liver; they found that 32 % had IGT and 16 % had diabetes [204]. More recently, Ortiz-Lopez et al. compared subjects with NAFLD diagnosed by MR spectroscopy with overweight/obese control subjects; they found that 75 % of NAFLD subjects were prediabetic and 14 % were diabetic, compared to 25 and 5 % of controls, respectively [205]. Taken together these results show that NAFLD, whether diagnosed by lab findings, imaging, or histology, is well correlated with abnormal OGTT results across a variety of populations, including young, otherwise healthy subjects.
The correlation between NAFLD and prediabetes/type 2 diabetes was recently evaluated prospectively by Zelber-Sagi et al. A cohort of subjects without prediabetes or diabetes who were diagnosed with NAFLD by ultrasound findings were evaluated at baseline and at 7 years follow-up. 74 % of subjects with baseline NAFLD developed prediabetes or type 2 diabetes, compared to 48 % of controls without baseline NAFLD [206]. NAFLD was found to be an independent predictor for the development of prediabetes.
NAFLD as a predictor of type 2 diabetes
NAFLD has been evaluated systematically as a predictor of type 2 diabetes. Multiple prospective studies have shown a correlation between elevated liver enzymes and increased risk of type 2 diabetes [207–212]. These studies have variably shown that elevated aspartate aminotransferase, alanine aminotransferase, gamma-glutamyltransferase (GGT), and/or alkaline phosphatase levels are independent predictors of type 2 diabetes.
Two groups have prospectively evaluated a more specific diagnostic marker of NAFLD, the fatty liver index (FLI). The FLI is a scoring index that was developed to aid in the diagnosis of NAFLD in lieu of liver biopsy and incorporates GGT, triglycerides, body mass index, and waist circumference [213]. Balkau et al. calculated a baseline FLI in a cohort of 3,811 non-diabetic patients and found that the highest FLI scores were associated with ORs of 3.43 for men and 11.05 for women for incident type 2 diabetes over 9 years of follow-up [214]. Rückert et al. studied a cohort of 3,009 subjects from the KORA F4 study population [215]. All non-diabetic subjects underwent OGTT and received a FLI score. The diagnosis of NAFLD based on FLI was associated with ORs of 3.1 for IGT, 6.7 for both IFG and IGT, and 8.5 for diabetes. The results of these studies demonstrate that the diagnosis of NAFLD is strongly predictive of type 2 diabetes.
Fetuin-A
Fetuin-A is a glycoprotein secreted by the liver that acts as an inhibitor of the insulin receptor in the liver and skeletal muscle and impairs insulin signaling in animal models. Serum fetuin-A levels have been investigated as a marker both of insulin resistance and of NAFLD. Stefan et al. found that in a cohort of non-diabetic subjects, higher fetuin-A levels were associated with IGT, greater insulin resistance determined by the euglycemic-hyperinsulinemic clamp, and increased liver fat determined by MR spectroscopy [216]. Ou et al. evaluated 510 age- and sex-matched nondiabetic subjects with sn OGTT, liver ultrasound and serum fetuin-A concentrations [217]. Subjects with IGT, with or without NAFLD, had significantly higher fetuin A levels compared to those with NGT. Subjects with NAFLD had significantly higher fetuin A levels compared to those with comparable glycemic status but without NAFLD. The clinical implications of these results remain unclear, but they further reinforce the link between prediabetes and NAFLD and provide preliminary evidence that fetuin-A could potentially be used as a marker of either or both conditions.
Risk of cancer
An association between cancer and diabetes has been previously described [218]. Huang et al. [219] conducted a meta-analysis to evaluate the risk of cancer in association with IFG and IGT. Data from 891,426 participants were derived from 16 prospective cohort studies. Prediabetes was associated with a 15 %increased risk of cancer overall (RR 1.15; 95 % CI 1.06, 1.23). Prediabetes was significantly associated with increased risks of cancer of the stomach/colorectum, liver, pancreas, breast, and endometrium, but not associated with cancer of the bronchus/lung, prostate, ovary, kidney, or bladder. The risks of site-specific cancer were significantly different and were highest for liver, endometrial, and stomach/colorectal cancer. The results were consistent across cancer endpoint, age, duration of follow-up, and ethnicity. The risks were increased when a lower FPG value of 5.6–6.9 mmol/l (100–124 mg/dl) as well as in participants with IGT. There was no significant difference for the risk of cancer with different definitions of prediabetes.
Mechanisms postulated to be involved in this association include inflammatory cytokines, chronic oxidative stress, accumulation of advanced glycation end-products, increased production of ROS, and greater oxidative damage to DNA [218, 219]. Furthermore, insulin resistance with compensatory hyperinsulinemia and increased levels of IFG-1 may promote the proliferation of cancer cells. Genetic factors may play an important role as well. A deficiency in a tumor suppressor gene (nuclear receptor coactivator 5) may increase risk of both glucose intolerance and hepatocellular cancer [220]. Although metformin may reduce the risk of cancer by 30 % in individuals with diabetes, this has not been established in prediabetes [221]. Long-term studies of metformin in high-risk individuals are therefore required [219].
As the risk for cancer increased with a FPG as low as 5.6 mmol/l (100 mg/dl) in conjunction with the observed increased global prevalence of prediabetes support the importance of screening for prediabetes using the ADA criteria for IFG, with a view to cancer prevention [219]. The public health implications of the current observations are therefore considerable.
Conclusions
The considerable comorbid conditions associated with prediabetes may often go unrecognized. This is particularly important as dysglycemic states as well are largely undetected since current diagnostic criteria apply absolute threshold values to a process that is continuous [222]. The preponderance of individuals with prediabetes therefore remains undiagnosed and untreated.
Type 2 diabetes and other non-communicable diseases are a growing public health challenge globally. An estimated 285 million people, corresponding to 6.4 % of the world’s adult population has diabetes. This is expected to reach 552 million by 2030, 7.8 % of the adult population, with the African region expected to experience the greatest increase. An even larger segment of the world’s population has prediabetes [223]. Given the global shift from communicable to chronic, non-communicable diseases including obesity, prediabetes, and type 2 diabetes mellitus, the increasing prevalence of the latter creates a considerable challenge to the clinician and public health infrastructure [224].
Despite the substantial research efforts in the last 10–15 years highlighting the considerable benefit of lifestyle modification in thwarting the insidious progression to diabetes and its complications, many individuals will ineluctably progress even when initially responsive. Therefore, the responsibilities of the medical and public health communities involve identifying new methods for screening and identifying those at risk as well as refining therapeutic approaches availing as many high-risk individuals as possible to novel treatment modalities [224]. As complications frequently associated with prediabetes discussed in the present review are often unrecognized, these merit attention and should be screened for routinely.
References
M. Buysschaert, M. Bergman, Definition of prediabetes. Med. Clin. N. Am. 95(2), 289–297 (2011)
K. Færch, K. Borch-Johnsen, J.J. Holst, A. Vaag, Pathophysiology and aetiology of impaired fasting glycemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia 52, 1714–1723 (2009)
R.A. DeFronzo, M.A. Ghani, Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am. J. Cardiol. 108(Suppl), 3B–24B (2011)
M. Buysschaert, M. Bergman, Diagnosis of prediabetes and diabetes prevention, in Prevention of Diabetes, 1st edn., ed. by P. Schwarz, P. Reddy (Wiley, New York, 2013)
M. Buysschaert, V. Preumont, J.L. Medina, M. Bergman, Global Health Perspectives in Prediabetes and Diabetes (World Scientific Publishing, Hackensack, 2014)
H. Gerstein, P. Santaguida, P. Raina, K. Morrison, C. Balion, D. Hunt, H. Yazdi et al., Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic review and meta-analysis of prospective studies. Diabetes Res. Clin. Pract. 78, 305–312 (2007)
A.G. Tabák, C. Herder, W. Rathmann, E.J. Brunner, M. Kivimäki, Prediabetes: a high-risk state for diabetes development. Lancet 379, 2279–2290 (2012)
E. Ferrannini: Definition of intervention points in prediabetes. Lancet (2014). doi:10.1016/s2213-8587(13)70175-x
S.M. Grundy, Pre-diabetes, metabolic syndrome and cardiovascular risk. JACC 59, 635–643 (2012)
J.L. Chiasson, S. Bernard, Reducing cardiovascular risk factors in patients with prediabetes. Diabetes Manag. 1(4), 423–438 (2011)
L.G. Mellbin, M. Anselmino, R. Lars, Diabetes, prediabetes and cardiovascular risk. Eur. J. Cardiovasc. Prev. Rehabil. 17, S9–S14 (2010)
A. Norhammar, A. Tenerz, G. Nilsson, A. Hamsten, S. Effendic, L. Ryden, G. Malmber, Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 359, 2140–2144 (2002)
S. Fonville, A.A.M. Zandbergen, S.E. Vermeer, D.W.J. Dippel, P.J. Koudstaal, H.M. den Hertog, Prevalence of prediabetes and newly diagnosed diabetes in patients with a transient ischemic attack or stroke. Cerebrovasc. Dis. 36, 283–289 (2013)
W.L. Lee, A.M. Cheung, D. Cape, B. Zinman, Impact of diabetes on coronary artery disease in women and men: a meta-analysis of prospective studies. Diabetes Care 23(7), 962–968 (2000)
R. Huxley, F. Barzi, M. Woodward, Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 332(7533), 73–78 (2006)
Emerging Risk Factors Collaboration, N. Sarwar, P. Gao, S.R. Seshasai, R. Gobin, S. Kaptoge, E. Di Angelantonio et al., Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375, 2215–2222 (2010)
E.J. Brunner, M.J. Shipley, D.R. Witte, J.H. Fuller, M.G. Marmot, Relation between blood glucose and coronary mortality over 33 years in the Whitehall Study. Diabetes Care 29(1), 26–31 (2006)
B. Balkau, M. Shipley, R.J. Jarrett, K. Pyörälä, M. Pyörälä, A. Forhan, E. Eschwège, High blood glucose concentration is a risk factor mortality in middle-aged nondiabetic men. 20-year follow-up in the Whitehall Study. The Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care 21(3), 360–367 (1998)
M. Coutinho, H.C. Gerstein, Y. Wang, S. Yusuf, The relationship between glucose and incident cardiovascular events. Diabetes Care 22, 233–240 (1999)
E.L. Barr, P.Z. Zimmet, T.A. Welborn, D. Jolley, D.J. Magliano, D.W. Dunstan et al., Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity and lifestyle Study (AusDiab). Circulation 116(2), 151–157 (2007)
B. Hoogwerf, D. Spreche, G. Pearce, M. Alevedo, J.P. Frolkis, J.M. Foody et al., Blood glucose concentration ≤125 mg/dl and coronary heart disease risk. Am. J. Cardiol. 89, 556–559 (2002)
E.S. Ford, G. Zhao, C. Li, Pre-diabetes and the risk for cardiovascular disease. A systematic review of the evidence. J. Am. Coll. Cardiol. 55, 1310–1317 (2010)
E.L.M. Barr, E.J. Boyko, P.Z. Zimmet, R. Wolfe, A.M. Tonkin, J.E. Shaw, Continuous relationship between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and lifestyle (AusDiab) study. Diabetologia 52, 415–424 (2009)
E. Selvin, M.W. Steffes, H. Zhu, K. Matsushita, L. Wagenknecht, J. Pankow et al., Glycated hemoglobin, diabetes and cardiovascular risk in non diabetic adults. N. Engl. J. Med. 362(9), 800–811 (2010)
M. Lee, J.L. Saver, K.S. Hong, S. Song, K.H. Chang, B. Ovbiagele, Effect of pre-diabetes on future risk of stroke: meta-analysis. BMJ 344, e3564 (2012). doi:10.1136/bmj.e3564
A. Di Pino, R. Scicali, S. Calanna, F. Urbano, C. Mantegna, A.M. Rabuazzo et al., Cardiovascular risk profile in subjects with prediabetes and new-onset type 2 diabetes identified by HbA1c according to American Diabetes Association criteria. Diabetes Care 37, 1447–1453 (2014)
J. Yeboah, A.G. Bertoni, D.M. Herrington, W.S. Post, G.L. Burke, Impaired fasting glucose and the risk of incident diabetes mellitus and cardiovascular events in an adult population. J. Am. Coll. Cardiol. 58, 140–146 (2001)
B. Kowall, W. Rathmann, M. Heier, G. Giani, A. Peters, B. Thorand et al., Categories of glucose tolerance and continuous glycemic measures and mortality. Eur. J. Epidemiol. 26(8), 637–645 (2011)
P. Deedwania, K. Patel, G.C. Fonarow, R.V. Desai, Y. Zhang, M.A. Feller et al., Prediabetes is not an independent risk factor for incident heart failure, other cardiovascular events or mortality in older adults: findings from a population-based cohort study. Int. J. Cardiol. 168(4), 3616–3622 (2013)
K. Shaye, T. Amir, S. Shlomo, S. Yechezkel, Fasting glucose levels within the high normal range predict cardiovascular outcome. Am. Heart J. 164, 111–116 (2012)
For the DECODE Study Group, F. Ning, J. Tuomilehto, K. Pyörälä, A. Onat, S. Söderberg, Q. Qiao, Cardiovascular disease mortality in Europeans in relation to fasting and 2-h plasma glucose levels within a normoglycemic range. Diabetes Care 33, 2211–2216 (2010)
K.T. Khaw, N. Wareham, R. Luben, S. Bingham, S. Oakes, A. Welch, N. Day, Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European Prospective Investigation of Cancer and Nutrition (EPIC-Norfolk). BMJ 322, 1–6 (2001)
K.T. Khaw, N. Wareham, S. Bingham, R. Luben, A. Welch, N. Day, Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann. Intern. Med. 141(6), 413–420 (2004)
H.C. Gerstein, S. Islam, S. Anand, W. Almahmeed, A. Damasceno, A. Dans et al., Dysglycaemia and the risk of acute myocardial infarction in multiple ethnic groups: an analysis of 15,780 patients from the INTERHEART study. Diabetologia 53, 2509–2517 (2010)
Q. Qiao, K. Pyörälä, M. Pyörälä, A. Nissinen, J. Lindström, R. Tilvis, J. Tuomilehto, Two-hour glucose is better risk predictor for incident coronary heart disease and cardiovascular mortality than fasting glucose. Eur. Heart J. 23(16), 1267–1275 (2002)
The DECODE Study Group, On behalf of the European Diabetes Epidemiology Group, Glucose tolerance and cardiovascular mortality. Comparison of fasting and 2-hour diagnostic criteria. Arch. Intern. Med. 161, 397–404 (2001)
S.B. Meigs, D.M. Nathan, R.B. D’Agostino Sr, The Framingham Offspring Study. Fasting and postchallenge glycemic and cardiovascular disease risk. Diabetes Care. 25, 1845–1850 (2002)
F. De Vegt, J.M. Dekker, H.G. Ruhé, C.D. Stehouwer, G. Nijpels, L.M. Bouter, R.J. Heine, Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia 42(8), 926–931 (1999)
M. Tominaga, H. Eguchi, H. Manaka, K. Igarashi, T. Kato, A. Sekikawa, Impaired glucose tolerance is a risk factor for cardiovascular disease but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 22(6), 920–924 (1999)
K. Kato, T. Otsuka, N. Kobayashi, Y. Kon, T. Kawada, Two-hour post-load plasma glucose levels are associated with carotid intima-media thickness in subjects with normal glucose tolerance. Diabet. Med. 31(1), 76–83 (2014)
Emerging Risk Factors Collaboration, S.R. Seshasai, S. Kaptoge, A. Thompson, E. Di Angelantonio, P. Gao, N. Sarwar et al., Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 364(9), 829–841 (2011)
J.A. Laukkanen, T.H. Makikallio, K. Ronkainen, J. Karppi, S. Kurl, Impaired fasting plasma glucose and type 2 diabetes are related to the risk of out-of-hospital sudden cardiac death and all-cause mortality. Diabetes Care 36, 1166–1171 (2012). doi:10.2337/dc12-0110
Y.S. Levitzky, M.J. Pencina, R.B. D’Agostino, J.B. Meigs, J.M. Murabito, R.S. Vasan, C.S. Fox, Impact of impaired fasting glucose on cardiovascular disease. JACC 51, 264–270 (2008)
E. Selvin, J. Coresh, S.H. Golden, F.L. Brancati, A.R. Folsom, M.W. Steffes, Glycemic control and coronary heart disease risk in persons with and without diabetes: the atherosclerosis risk in communities study. Arch. Intern. Med. 165(16), 1910–1916 (2005)
N. Sarwar, T. Aspelund, G. Eiriksdottir, R. Gobin, S.R. Seshasai, N.G. Forouhi et al., Markers of dysglycaemia and risk of coronary heart disease in people without diabetes: Reykjavik prospective study and systematic review. PLoS Med. 7(5), e1000278 (2010). doi:10.1371/journal.pmed.1000278
M.V. Skriver, K. Borch-Johnsen, T. Lauritzen, HbA1c as predictor of all-cause mortality in individuals at high risk of diabetes with normal glucose tolerance, identified by screening: a follow-up study of the Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen-Detected Diabetes in Primary Care (ADDITION), Denmark. Diabetologia 53(11), 2328–2333 (2010)
K. Faerch, D. Vistisen, N. Johansen Borup, M.E. Jorgensen, Cardiovascular risk stratifications and management in prediabetes. Curr. Diab. Rep. 14, 493 (2014)
S. Kodama, K. Saito, S. Tanaka et al., Fasting and post-challenge glucose as quantitative cardiovascular risk factors: a meta-analysis. J. Atheroscler. Thromb. 19, 385–396 (2012)
A.S. Gami, B.J. Witt, D.E. Howard et al., Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol. 49, 403–414 (2007)
S. Mottillo, K.B. Filion, J. Genest et al., The metabolic syndrome and cardiovascular risk : a systematic review and meta-analysis. J. Am. Coll. Cardiol. 56, 1113–1132 (2010)
Y. Zhang, E.T. Lee, R.B. Devereux, J. Yeh, L.G. Best, R.R. Fabsitz, B.V. Howard, Prehypertension diabetes and cardiovascular disease risk in a population-based sample: the Strong Heart Study. Hypertension 47, 410–414 (2006)
S. Milman, J.P. Crandall, Mechanisms of vascular complications in prediabetes. Med. Clin. N. Am. 95, 309–325 (2011)
J. Tuomilehto, J. Lindström, J.G. Eriksson, T.T. Valle, H. Hämäläinen, P. Ilanne-Parikka et al., Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 344(18), 1343–1350 (2001)
R. Ratner, R. Goldberg, S. Haffner, S. Marcovina, T. Orchard, S. Fowler, Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care 28(4), 888–894 (2005)
W.C. Knowler, E. Barrett-Connor, S.E. Fowler, R.F. Hammam, J.M. Lachin, E.A. Walker et al., Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346(6), 393–403 (2002)
T. Orchard, M. Temprosa, E. Barrett-Connor et al., Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet. Med. 30, 46–55 (2013)
F.B. Hu, M.J. Stampfer, S.M. Haffner, C.G. Solomon, W.C. Willett, J.E. Manson, Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care 25(7), 1129–1134 (2002)
J.L. Chiasson, R.G. Josse, R. Gomis, M. Hanefeld, A. Karasik, M. Laakso, Acarbose treatment and risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance. The STOP-NIDDM trial. JAMA. 290, 486–494 (2003)
E. Lonn, H. Gerstein, P. Sheridan, For the DREAM and STARR Investigations et al., Effect of ramipril and of rosiglitazone on carotid intima-media thickness in people with impaired glucose tolerance or impaired fasting glucose: STARR (Study of Atherosclerosis with Ramipril and Rosiglitazone). J. Am. Coll. Cardiol. 53, 2028–2035 (2009)
E.M. Lonn, J. Bosch, R. Diaz, P. Lopez-Jaramillo, A. Ramachandran, N. Hâncu et al., Effect of insulin glargine and n-3FA on carotid intima-media thickness in people with dysglycemia at high risk for cardiovascular events : the glucose reduction and atherosclerosis continuing evaluation study (ORIGIN-GRACE). Diabetes Care 36(9), 2466–2474 (2013)
I. Hopper, B. Billah, M. Skiba, H. Krum, Prevention of diabetes and reduction in major cardiovascular events in studies of subjects with prediabetes: meta-analysis of randomised controlled clinical trials. Eur. J. Cardiovasc. Prev. Rehabil. 18(6), 813–823 (2011)
G. Li, P. Zhang, J. Wang, Y. An, Q. Gong, E.W. Gregg et al., Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet 28, 88–136 (2014). doi:10.1016/s2213-8587(14)70057-9
M. Bergman, M. Buysschaert, P.E.H. Schwarz, A. Albright, V. Narayan, D. Yach, Diabetes prevention: global health policy and perspectives from the ground. Diabetes Manag. 2(4), 309–321 (2012)
L. Rydén et al., Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD): ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with EASD. Eur. Heart J. 34, 3035–3087 (2013)
American Diabetes Association, Standards of medical care in diabetes—2014. Diabetes Care 37(Suppl. 1), s14 (2014)
P.M. Kearney, L. Blackwell, R. Collins, A. Keech, J. Simes, R. Peto et al., Efficacy of cholesterol-lowering therapy in 18.686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 371, 117–125 (2008)
P.M. Ridker, E. Danielson, F.A. Fonseca et al., JUPITER Study Group: rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359, 2195–2207 (2008)
P.M. Preshaw, A.L. Alba, D. Herrera, S. Jepsen, A. Konstantinidis, K. Makrilakis, R. Taylor, Periodontitis and diabetes: a two-way relationship. Diabetelogia 55, 21–31 (2012)
F. Javed, A.S.T. Alghamdi, T. Mikami, A. Mehmood, H.B. Ahemed, L.P. Samaranayake, H.C. Tenenbaum, Effect of glycemic control on self-perceived oral health, periodontal parameters, and alveolar bone loss among patients with prediabetes. J. Periodontol. 85, 234–241 (2014)
N. Arora, P.N. Papapanou, M. Rosenbaum, D.R. Jacobs Jr, M. Desvarieux, R.T. Demmer, Periodontal infection, impaired fasting glucose and impaired glucose tolerance: results from The Continuous National Health and Nutrition Examination Survey 2009–2010. J. Clin. Periodontol. 41, 643–652 (2014)
C.C.P. Andersen, A. Flyvbjerg, K. Buschard, P. Holmstrup, Periodontitis is associated with aggravation of prediabetes in Zucker fatty rats. J. Periodontol. 71, 1625–1631 (2007)
M. Manouchehr-Pour, P.J. Spagnuolo, H.M. Rodman, N.F. Bissada, Comparison of neutrophil chemotactic response in diabetic patients with mild and severe periodontal disease. J. Periodontol. 52, 410–415 (1981)
M. Altamash, S. Arledal, B. Klinge, P.-E. Engström, Pre-diabetes and diabetes: medical risk factors and periodontal conditions. Acta Odontol. Scand. 71, 1625–1631 (2013)
R. McCrimmon, C.M. Ryan, M. Frier, Diabetes and cognitive dysfunction. Lancet 379, 2291–2299 (2012)
T. Cukierman-Yaffe, Diabetes, dysglycemia & cognitive dysfunction. Diabetes Metab. Res. Rev. 30(5), 341–345 (2014)
D.D. Schwartz, M.E. Axelrad, B.J. Andersen, Neurocognitive function in children and adolescents at the time of type 1 diabetes diagnosis: associations with glycemic control 1 year after diagnosis. Diabetes Care 37(9), 2475–2482 (2014)
C. Dufouil, C. Brayne, The continuing challenge of turning promising observational evidence about risk for dementia to evidence supporting prevention. JAMA Intern. Med. 174, 333–335 (2014)
P.J.J. Spauwen, C.D.A. Stehouwer, Cognitive decline in type 2 diabetes. Lancet Diabetes Endocrinol. 2, 188–189 (2013). doi:10.1016/S2213-8587(13)70167-0
J.S. Roriz-Filho, T.M. Sá-Roriz, I. Rosset, A.L. Camozzato, A.C. Santos, M.L.F. Chaves, J.C. Moriguti, M. Roriz-Cruz, (Pre)diabetes, brain aging, and cognition. Biochim. Biophys. Acta 1792, 432–443 (2009)
R.H. Tuligenga, A. Dugravot, A.G. Tabák, A. Elbaz, E.J. Brunner, M. Kivimäki, A. Singh-Manoux, Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post hoc analysis of the Whitehall II cohort study. Lancet (2013). doi:10.1016/S2213-8587(13)70192-X
G.J. Biessels, Intensive glucose lowering and cognition in type 2 diabetes. Lancet Neurol. 10, 949–950 (2011)
L. Kerti, A.V. Witte, A. Winkler, U. Grittner, D. Rujescu, A. Flöel, Higher glucose levels associated with lower memory and reduced hippocampal microstructure. Neurology 81, 1746–1753 (2013)
C. Anderson et al., Glucose intolerance and diabetes as risk factors for cognitive impairment in people at high cardiovascular risk: results from the ONTARGET/TRANSCEND Research Programme. Diabetes Res. Clin. Pract. 83(3), 387–393 (2009)
C.M. Sanz, J.-B. Ruidavets, V. Bongard, J.-C. Marquié, H. Hanaire, J. Ferrières, S. Andrieu, Relationship between markers of insulin resistance, markers of adiposity, HbA1c, and cognitive functions in a middle-aged population-based sample: the MONA LISA Study. Diabetes Care 36(6), 1512–1521 (2013)
Z.S. Tan, A.S. Beiser, C.S. Fox et al., Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: the Framingham Offspring Study. Diabetes Care 34, 1766–1770 (2011)
M. Nazaribadie, K. Asgari, M. Amini, M. Ahmadpanah, M. Nazarbadie, S. Jamlipaghale, Cognitive processes and functions in patients with type 2 diabetes in comparison to pre-diabetic patients. JRHS 13(2), 208–213 (2013)
T. Ohara, Y. Doi, T. Ninomiya et al., Glucose tolerance status and risk off dementia in the community: the Hisayama Study. Neurology 77, 1126–1134 (2011)
P.K. Crane, R. Walker, R.A. Hubbard, G. Li, D.M. Nathan, H. Zheng, S. Haneuse, S. Craft, T.J. Montine, S.E. Kahn, W. McCormick, S.M. McCurry, J.D. Bowen, E.B. Larson, Glucose levels and risk of dementia. N. Engl. J. Med. 369, 540–548 (2013)
P.G. Lee, C.T. Cigolle, J. Ha, L. Min, S.L. Murphy, C.S. Blaum, W.H. Herman, Physical function limitations among middle-aged and older adults with prediabetes. Diabetes Care 36(10), 3076–3083 (2013)
A.L. Christman, K. Matsushita, R.F. Gottesman, T. Mosley, A. Alonso, J. Coresh, F. Hill-Briggs, A.R. Sharrett, E. Selvin, Glycated haemoglobin and cognitive decline: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia 54, 1645–1652 (2011)
A.M. Jacobson, Diabetes and cognitive performance: a story that is still unfolding. Diabetologia 54, 1593–1595 (2011)
S.G.C. van Elderen, A. deRoos, A.J.M. de Craen et al., Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology 75, 997–1002 (2010)
R.O. Roberts, D.S. Knopman, S.A. Przybelski et al., Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology (2014). doi:10.1212/WNL.0000000000000269
G.J. Biessels, S. Staekenborg, E. Brunner, C. Brayne, P. Scheltens, Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 5, 64–74 (2006)
R.N. Bryan, M. Bilello, C. Davatzikos, R.M. Lazar, A. Murray, K. Horowitz, J. Lovato, M.E. Miller, J. Williamson, L.J. Launer, Effect of diabetes on brain structure: the action to control cardiovascular risk in diabetes MR imaging baseline data. Radiology 271(1), 210–216 (2014)
A. Kleinridders, H.A. Ferris, W. Cai, C.R. Kahn, Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63(7), 2232–2243 (2014)
A. Elbaz, M.J. Shipley, H. Nabi, E.J. Brunner, M. Kivimäki, A. Singh-Manoux, Trajectories of the Framingham general cardiovascular risk profile in midlife and poor motor function later in life: The Whitehall II study. Int. J. Cardiol. 172, 96–102 (2014). doi:10.1016/j.ijcard.2013.12.051
L.J. Launer, M.E. Miller, J.D. Williamson, R.M. Lazar, H.C. Gertsein, A.M. Murray, M. Sullivan, K.R. Horowitz, J. Ding, S. Marcovina, L.C. Lovato, J. Lovato, K.L. Margolis, P. O’Connor, E.E. Lipkin, J. Hirsch, L. Coker, J. Maldjian, J.L. Sunshine, C. Truwit, C. Davatzikos, R.N. Bryan, For the ACCORD MIND investigators, Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND):a randomized open-label substudy. Lancet Neurol. 10, 969–977 (2011)
J.D. Williamson, L.J. Launer, R.N. Bryan, L.H. Coker, R.M. Lazar, H.C. Gerstein, A.M. Murray, M.D. Sullivan, K.R. Horowitz, S. Marcovina, L.C. Lovato, J. Lovato, K.L. Margolis, C. Davatzikos, J. Barzilay, H.N. Ginsberg, P.E. Linz, M.E. Miller, For the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Memory in Diabetes (MIND) investigators, Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels. JAMA Intern. Med. 174(3), 24–333 (2014)
C.D. Saudek, W.H. Herman, D.B. Sacks, R.M. Bergenstal, D. Edelman, M.B. Davidson, A new look at screening and diagnosing diabetes mellitus. J. Clin. Endocrinol. Metab. 93(7), 2447–2453 (2008)
S. Ghosh, A. Collier, T. Elhadd, I. Malik, Retinopathy in diabetes. Br. J. Diabetes Vasc. Dis. 10(3), 155–156 (2010)
T.Y. Wong, G. Liew, R.J. Tapp, M.I. Schmidt, J.J. Wang, P. Mitchell, R. Klein, B.E. Klein, P. Zimmet, J. Shaw, Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population-based cross-sectional studies. Lancet 371, 736–743 (2008)
Y.J. Cheng, E.W. Gregg, L.S. Geiss, G. Imperatore, D.E. Williams, X. Zhang, A.L. Albright, C.C. Cowie, R. Klein, J.B. Saaddine, Association of A1c and fating plasma glucose levels with diabetic retinopathy prevalence in the U.S. population. Diabetes Care 32(11), 2027–2032 (2009)
Diabetes Prevention Program Research Group, The prevalence of retinopathy un impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet. Med. 24, 137–144 (2007)
T.T. Nguyen, J.J. Wang, T.Y. Wong, Reinal vascular changes in pre-diabetes and prehypertension. Diabetes Care 30(10), 2708–2715 (2007)
N. Papanas, A.I. Vinik, D. Ziegler, Neuropathy in prediabetes: does the clovk start ticking early? Nat. Rev. Endocrinol. 7, 682–690 (2011)
C.J. Sumner, S. Sheth, J.W. Griffin, D.R. Cornblath, M. Polydefkis, The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 60, 108–111 (2003)
B.W.C. Bongaerts, W. Rathmann, M. Heier, B. Kowall, C. Herder, D. Stöckl, C. Meisinger, D. Ziegler, Older subjects with diabetes and prediabetes are frequently unaware of having distal sensorimotor polyneuropathy. Diabetes Care 36(5), 1141–1146 (2013)
L.C. Plantinga, D.C. Crews, J. Coresh, E.R. Miller, R. Saran, J. Yee, E. Hedgeman, M. Pavkov, M.S. Eberhardt, D.E. Williams, N.R. Powe, For the CDC CKD Surveillance team, Prevalence of chronic kidney disease in U.S. adults with undiagnosed diabetes or prediabetes. Clin. J. Am. Soc. Nephrol. 5, 673–682 (2010)
H. Kramer, Screening for kidney disease in adults with diabetes and prediabetes. Curr. Opin. Nephrol. Hypertens. 14, 249–252 (2005)
G.C. Curhan, Prediabetes, prehypertension…Is it time for pre-CKD? Clin. J. Am. Soc. Nephrol. 5, 557–559 (2010)
O. Schnell, E. Standl, Impaired glucose tolerance, diabetes, and cardiovascular disease. Endocr. Pract. 12(1), 16–19 (2006)
E.J. Diamantopoulos, E.A. Andreadis, G.I. Tsourous, P.M. Katsanou, D.X. Georgiopoulos, N.C. Nestora, S.A. Raptis, Early vascular lesions in subjects with metabolic syndrome and prediabetes. Int. Angiol. 25(2), 179–183 (2006)
J.B. Meigs, M.G. Larson, R.B. D’Agostino, D. Levy, M.E. Clouse, D.M. Nathan, P.W. Wilson, C.J. O’Donnell, Coronary artery calcification in type 2 diabetes and insulin resistance: the Framingham offspring study. Diabetes Care 25(8), 1313–1319 (2002)
M.W. Millar-Craig, C.N. Bishop, E.B. Raftery, Circadian variation of blood-pressure. Lancet 1(8075), 795–797 (1978)
A.K. Gupta, F.L. Greenway, G. Cornelissen, W. Pan, F. Halberg, Prediabetes is associated with abnormal circadian blood pressure variability. J. Hum. Hypertens. 22(9), 627–633 (2008)
W.B. White, Relevance of blood pressure variation in the circadian onset of cardiovascular events. J. Hypertens. 21(6), S9–S15 (2003)
R.C. Hermida, D.E. Ayala, F. Portaluppi, Circadian variation of blood pressure: the basis for the chronotherapy of hypertension. Adv. Drug Deliv. Rev. 59(9–10), 904–922 (2007)
W.B. White, How well does ambulatory blood pressure predict target-organ disease and clinical outcome in patients with hypertension? Blood Press. Monit. 4(2), S17–S21 (1999)
O. Torffvit, C.D. Agardh, Day and night variation in ambulatory blood pressure in type 1 diabetes mellitus with nephropathy and autonomic neuropathy. J. Intern. Med. 233(2), 131–137 (1993)
F.S. Nielsen, P. Rossing, L.E. Bang, T.L. Svendsen, M.A. Gall, U.M. Smidt, H.H. Parving, On the mechanisms of blunted nocturnal decline in arterial blood pressure in NIDDM patients with diabetic nephropathy. Diabetes 44(7), 783–789 (1995)
V. Spallone, M.R. Maiello, R. Morganti, S. Mandica, G. Frajese, Usefulness of ambulatory blood pressure monitoring in predicting the presence of autonomic neuropathy in type I diabetic patients. J. Hum. Hyperten. 21(7), 381–386 (2007)
S. Nakano, M. Fukuda, F. Hotta, T. Ito, T. Ishii, M. Kitazawa, M. Nishizawa, T. Kigoshi, K. Uchida, Reversed circadian blood pressure rhythm is associated with occurrence of both fatal and nonfatal vascular events in NIDDM subjects. Diabetes 47(9), 1501–1506 (1998)
A.K. Gupta, G. Cornelissen, F.L. Greenway, V. Dhoopati, F. Halberg, W.D. Johnson, Abnormalities in circadian blood pressure variability and endothelial function: pragmatic markers for adverse cardiometabolic profiles in asymptomatic obese adults. Cardiovasc. Diabetol. (2010). doi:10.1186/1475-2840-9-58
B. Isak, B. Oflazoglu, T. Tanridag, I. Yitmen, O. Us, Evaluation of peripheral and autonomic neuropathy among patients with newly diagnosed impaired glucose tolerance. Diabetes Metab. Res. Rev. 24(7), 563–569 (2008)
S. Kumarasamy, K. Gopalakrishnan, D.H. Kim, N.G. Abraham, W.D. Johnson, B. Joe, A.K. Gupta, Dysglyceia induces abnormal circadian blood pressure variability. Cardiovasc. Diabetol. (2011). doi:10.1186/1475-2840-10-104
Z. Putz, N. Németh, I. Istenes, T. Martos, R.A. Gandhi, A.E. Körei, Z. Hermányi, M. Szathmári, G. Jermendy, S. Tesfaye, Á.G. Tabák, P. Kempler, Autonomic dysfunction and circadian blood pressure variations in people with impaired glucose tolerance. Diabet. Med. 30(3), 358–362 (2013)
J.E. Shaw, N.M. Punjabi, J.P. Wilding, K.G. Alberti, P.Z. Zimmet, Sleep-disordered breathing and type 2 diabetes: a report from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Diabetes Res. Clin. Pract. 81(1), 2–12 (2008)
S.R. Coughlin, L. Mawdsley, J.A. Mugarza, P.M. Calverley, J.P. Wilding, Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur. Heart J. 25(9), 735–741 (2004)
N.M. Punjabi, E. Shahar, S. Redline, D.J. Gottlieb, R. Givelber, H.E. Resnick, Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am. J. Epidemiol. 160(6), 521–530 (2004)
S. Seicean, H.L. Kirchner, D.J. Gottlieb, N.M. Punjabi, H. Resnick, M. Sanders, R. Budhiraja, M. Singer, S. Redline, Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care 31(5), 1001–1006 (2008)
I. Muraki, T. Tanigawa, K. Yamagishi, S. Sakurai, T. Ohira, H. Imano, A. Kitamura, M. Kiyama, S. Sato, T. Shimamoto, M. Konishi, H. Iso, Nocturnal intermittent hypoxia and the development of type 2 diabetes: the Circulatory Risk in Communities Study (CIRCS). Diabetologia 53(3), 481–488 (2010)
N. Botros, J. Concato, V. Mohsenin, B. Selim, K. Doctor, H.K. Yaggi, Obstructive sleep apnea as a risk factor for type 2 diabetes. Am. J. Med. 122(12), 1122–1127 (2009)
T. Kendzerska, A.S. Gershon, G. Hawker, G. Tomlinson, R.S. Leung, Obstructive sleep apnea and incident diabetes. A historical cohort study. Am. J. Respir. Crit. Care Med. 190(2), 218–225 (2014)
S. Pamidi, E. Tasali, Obstructive sleep apnea and type 2 diabetes: is there a link? Front. Neurol. 3, 126 (2012). doi:10.3389/fneur.2012.00126
O. Alshaarawy, S. Teppala, A. Shankar, Markers of sleep-disordered breathing and prediabetes in US adults. Int. J. Endocrinol. (2012). doi:10.1155/2012/902324
N. Cheng, W. Cai, M. Jiang, S. Wu, Effect of hypoxia on blood glucose, hormones, and insulin receptor functions in newborn calves. Pediatr. Res. 41(6), 852–856 (1997)
J.P. Chaput, J.P. Despres, C. Bouchard, A. Tremblay, Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Quebec family study. Obesity 15(1), 253–261 (2007)
N. Peled, A. Greenberg, G. Pillar, O. Zinder, N. Levi, P. Lavie, Contributions of hypoxia and respiratory disturbance index to sympathetic activation and blood pressure in obstructive sleep apnea syndrome. Am. J. Hypertens. 11(11), 1284–1289 (1998)
D. Einhorn, D.A. Stewart, M.K. Erman, N. Gordon, A. Philis-Tsimikas, E. Casal, Prevalence of sleep apnea in a population of adults with type 2 diabetes mellitus. Endocr. Pract. 13(4), 355–362 (2007)
G.D. Foster, M.H. Sanders, R. Millman, G. Zammitt, K.E. Borradaile, A.B. Newman, T.A. Wadden, D. Kelley, R.R. Wing, F.X. Sunyer, V. Darcey, S.T. Kuna, Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 32(6), 1017–1019 (2009)
R.S. Aronsohn, H. Whitmore, E. Van Cauter, E. Tasali, Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am. J. Respir. Crit. Care Med. 181(5), 507–513 (2010)
R.N. Aurora, N.M. Punjabi, Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir. Med. 1(4), 329–338 (2013)
J.M. Fredheim, J. Rollheim, T. Omland, D. Hofsø, J. Røislien, K. Vegsgaard, J. Hjelmesæth, Type 2 diabetes and pre-diabetes are associated with obstructive sleep apnea in extremely obese subjects: a cross-sectional study. Cardiovasc. Diabetol. (2011). doi:10.1186/1475-2840-10-84
P. Levy, M.R. Bonsignore, J. Eckel, Sleep, sleep-disordered breathing and metabolic consequences. Eur. Respir. J. 34(1), 243–260 (2009)
I.A. Harsch, S.P. Schahin, K. Brückner, M. Radespiel-Tröger, F.S. Fuchs, E.G. Hahn, P.C. Konturek, T. Lohmann, J.H. Ficker, The effect of continuous positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration 71(3), 252–259 (2004)
H.A. Hassaballa, A. Tulaimat, J.J. Herdegen, B. Mokhlesi, The effect of continuous positive airway pressure on glucose control in diabetic patients with severe obstructive sleep apnea. Sleep Breath. 9(4), 176–180 (2005)
A.R. Babu, J. Herdegen, L. Fogelfeld, S. Shott, T. Mazzone, Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch. Intern. Med. 165(4), 447–452 (2005)
S.D. West, D.J. Nicoll, T.M. Wallace, D.R. Matthews, J.R. Stradling, Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 62(11), 969–974 (2007)
S. Surani, S. Subramanian, Effect of continuous positive airway pressure therapy on glucose control. World J. Diabetes 3(4), 65–70 (2012)
S. Pamidi, Abstract #39588. American Thoracic Society 2013 International Conference, Philadelphia, May 17–22, 2013
D. Kapoor, H. Aldred, S. Clark, K.S. Channer, T.H. Jones, Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 30(4), 911–917 (2007)
M. Grossmann, M.C. Thomas, S. Panagiotopoulos, K. Sharpe, R.J. Macisaac, Low testosterone levels are common and associated with insulin resistance in men with diabetes. J. Clin. Endocrinol. Metab. 93(5), 1834–1840 (2008)
A.A. Al Hayek, Y.S. Khader, S. Jafal, N. Khawaja, A.A. Robert, K. Ajilouni, Prevalence of low testosterone levels in men with type 2 diabetes mellitus: a cross-sectional study. J. Fam. Community Med. 20(3), 179–186 (2013)
E.L. Ding, Y. Song, V.S. Malik, S. Liu, Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 295(11), 1288–1299 (2006)
G. Hackett, M. Kirby, A.J. Sinclair, Testosterone deficiency, cardiac health, and older men. Int. J. Endocrinol. (2014). doi:10.1155/2014/143763
R. Pasquali, C. Macor, V. Vicennati, F.R. Delasio, P. Mesini, S. Boschi, F. Casimirri, R. Vettor, Effects of acute hyperinsulinemia on testosterone serum concentrations in adult obese and normal-weight men. Metabolism 46(5), 526–529 (1997)
S. Bhasin, G.R. Cunningham, F.J. Hayes, A.M. Matsumoto, P.J. Snyder, R.S. Swerdloff, V.M. Montori, Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 95(6), 2536–2559 (2010)
R.K. Stellato, H.A. Feldman, O. Hamdy, E.S. Horton, J.B. McKinlay, Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care 23(4), 490–494 (2000)
E.C. Tsai, A.M. Matsumoto, W.Y. Fujimoto, E.J. Boyko, Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care 27(4), 861–868 (2004)
P. Marin, S. Homang, L. Jonsson, L. Sjostrom, H. Kvist, G. Holm, G. Lindstedt, P. Bjorntorp, The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int. J. Obes. Relat. Metab. Disord. 16(12), 991–997 (1992)
S. Filippi, L. Vignozzi, A. Morelli, A.K. Chavalmane, E. Sarchielli, B. Fibbi, F. Saad, P. Sandner, P. Ruggiano, G.B. Vannelli, E. Mannucci, M. Maggi, Testosterone partially ameliorates metabolic profile and erectile responsiveness to PDE5 inhibitors in an animal model of male metabolic syndrome. J. Sex Med. 6(12), 3274–3288 (2009)
D. Goodman-Gruen, E. Barrett-Connor, Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care 23(7), 912–918 (2000)
G. Corona, G. Rastrelli, G. Balercia, F. Lotti, A. Sforza, M. Monami, G. Forti, E. Mannucci, M. Maggi, Hormonal association and sexual dysfunction in patients with impaired fasting glucose: a cross-sectional and longitudinal study. J. Sex Med. 9(6), 1669–1680 (2012)
C.H. Ho, H.J. Yu, C.Y. Wang, F.S. Jaw, J.T. Hsieh, W.C. Liao, Y.S. Pu, S.P. Liu, Prediabetes is associated with an increased risk of testosterone deficiency, independent of obesity and metabolic syndrome. PLoS ONE (2013). doi:10.1371/journal.pone.0074173
D. Kapoor, E. Goodwin, K.S. Channer, T.H. Jones, Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur. J. Endocrinol. 154(6), 899–906 (2006)
M.I. Naharci, M. Pinar, E. Bolu, A. Olgun, Effect of testosterone on insulin sensitivity in men with idiopathic hypogonadotropic hypogonadism. Endocr. Pract. 13(6), 629–635 (2007)
T.H. Jones, S. Arver, H.M. Behre, J. Buvat, E. Meuleman, I. Moncada, A.M. Morales, M. Volterrani, A. Yellowlees, J.D. Howell, K.S. Channer, Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 34(4), 828–837 (2011)
J. Kaur, A comprehensive review on metabolic syndrome. Cardiol Res Pract. (2014). doi:10.1155/2014/943162
H. Beltran-Sanchez, M.O. Harhay, M.M. Harhay, S. McElligott, Prevalance and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J. Am. Coll. Cardiol. 62(8), 697–703 (2013)
Expert Panel on Detection, Evaluation and treatment of high cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel detection, evaluation and treatment of high cholesterol in adults (Adult Treatment Panel III). JAMA 285(19), 2486–2497 (2001)
S.M. Grundy, J.I. Cleeman, S.R. Daniels, K.A. Donato, R.H. Eckel, B.A. Franklin, D.J. Gordon, R.M. Krauss, P.J. Savage, S.C. Smith Jr, J.A. Spertus, F. Costa, Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol. Rev. 13(6), 322–327 (2005)
K.G. Alberti, P. Zimmet, J. Shaw, The metabolic syndrome—a new worldwide definition. Lancet 366(9491), 1059–1062 (2005)
G.M. Reaven, The insulin resistance syndrome: definition and dietary approaches to treatment. Annu. Rev. Nutr. 25, 391–406 (2005)
R.A. DeFronzo, E. Ferrannini, Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia and atherosclerotic cardiovascular disease. Diabetes Care 14(3), 173–194 (1991)
C.M. Alexander, P.B. Landsman, S.M. Grundy, Metabolic syndrome and hyperglycemia: congruence and divergence. Am. J. Cardiol. 98(7), 982–985 (2006)
E.J. Diamantopoulos, E.A. Andreadis, G.I. Tsourous, G.K. Ifanti, P.M. Katsanou, D.X. Georgiopoulos, C.V. Vassilopoulos, G. Dimitriadis, S.A. Raptis, Metabolic syndrome and prediabetes identify overlapping but not identical populations. Exp. Clin. Endocrinol. Diabetes 144(7), 377–383 (2006)
C. Lorenzo, K. Williams, K.J. Hunt, S.M. Haffner, The National Cholesterol Education Program—Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care 30(1), 8–13 (2007)
C. Lorenzo, M. Okoloise, K. Williams, M.P. Stern, S.M. Haffner, The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care 26(11), 3153–3159 (2003)
Q.M. Nguyen, S.R. Srinivasan, J.H. Xu, W. Chen, G.S. Berenson, Changes in risk variables of metabolic syndrome since childhood in pre-diabetic and type 2 diabetic subjects: the Bogalusa Heart Study. Diabetes Care 31(10), 2044–2049 (2008)
J.A. Morrison, L.A. Friedman, P. Wang, C.J. Glueck, Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J. Pediatr. 152(2), 201–206 (2008)
H. Florez, M.G. Temprosa, T.J. Orchard, K.J. Mather, S.M. Marcovina, E. Barrett-Connor, E. Horton, C. Saudek, X.F. Pi-Sunyer, R.E. Ratner, R.B. Goldberg, Metabolic syndrome components and their response to lifestyle and metformin interventions are associated with differences in diabetes risk in persons with impaired glucose tolerance. Diabetes Obese Metab. 16(4), 325–333 (2014)
S.M. Grundy, Pre-diabetes, metabolic syndrome, and cardiovascular risk. J. Am. Coll. Cardiol. 59(7), 635–643 (2012)
T.J. Orchard, M. Temprosa, R. Goldberg, S. Haffner, R. Ratner, S. Marcovina, S. Fowler, The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann. Intern. Med. 142(8), 611–619 (2005)
J.L. Chiasson, R.G. Josse, R. Gomis, M. Hanefeld, A. Karasik, M. Laakso, Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 290(4), 486–494 (2003)
R. Rajagopalan, S. Iyer, M. Khan, Effect of pioglitazone on metabolic syndrome risk factors: results of double-blind, multicenter, randomized clinical trials. Curr. Med. Res. Opin. 21(1), 163–172 (2005)
S. Li, H.J. Shin, E.L. Ding, R.M. van Dam, Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 302(2), 179–188 (2009)
A.G. Tabak, M. Cartensen, D.R. Witte, E.J. Brunner, M.J. Shipley, M. Jokela, M. Roden, M. Kivimaki, C. Herder, Adiponectin trajectories before type 2 diabetes diagnosis. Diabetes Care 35(12), 2540–2547 (2012)
H. Kim, J. Jo, J.E. Lim, Y.D. Yun, S.J. Baek, T. Lee, K.B. Huh, S.H. Jee, Adiponectin as predictor for diabetes among pre-diabetic groups. Endocrine 44(2), 411–418 (2013)
M.Y. Donath, D.M. Schumann, M. Faulenbach, H. Ellingsgaard, A. Perren, J.A. Ehses, Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care 31(Suppl 2), S161–S164 (2008)
C.M. Larsen, M. Faulenbach, A. Vaag, A. Volund, J.A. Ehses, B. Seifert, T. Mandrup-Poulsen, M.Y. Donath, Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356(15), 1517–1526 (2007)
M. Carstensen, C. Herder, M. Kivimaki, M. Jokela, M. Roden, M.J. Shipley, D.R. Witte, E.J. Brunner, A.G. Tabak, Accelereated increase in serum interleukin-1 receptor antagonist starts 6 years before diagnosis of type 2 diabetes: Whitehall II prospective cohort study. Diabetes 59(5), 1222–1227 (2010)
T.J. Wang, M.G. Larson, R.S. Vasan, S. Cheng, E.P. Rhee, E. McCabe, G.D. Lewis, C.S. Fox, P.F. Jacques, C. Fernandez, C.J. O’Donnell, S.A. Carr, V.K. Mootha, J.C. Florez, A. Souza, O. Melander, C.B. Clish, R.E. Gerszten, Metabolite profiles and the risk of developing diabetes. Nat. Med. 17(4), 448–453 (2011)
R. Wang-Sattler, Z. Yu, C. Herder, A.C. Messias, A. Floegel, Y. He, K. Heim, M. Campillos, C. Holzapfel, B. Thorand, H. Grallert, T. Xu, E. Bader, C. Huth, K. Mittelstrass, A. Doring, C. Meisinger, C. Gieger, C. Prehn, W. Roemisch-Margl, M. Carstensen, L. Xie, H. Yamanaka-Okumura, G. Xing, U. Ceglarek, J. Thiery, G. Giani, H. Lickert, X. Lin, Y. Li, H. Boeing, H. Joost, M. Hrabé de Angelis, W. Rathmann, K. Suhre, H. Prokisch, A. Peters, T. Meitinger, M. Roden, H. Wichmann, T. Pischon, J. Adamski, T. Illig, Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 8, 615 (2012)
A. Floegel, N. Stefan, Z. Yu, K. Mühlenbruch, D. Drogan, H. Joost, A. Fritsche, H. Häring, M. Hrabe de Angelis, A. Peters, M. Roden, C. Prehn, R. Wang-Sattler, T. Illig, M.B. Schulze, J. Adamski, H. Boeing, T. Pischon, Identifcation of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomics approach. Diabetes 62(2), 639–648 (2013)
W.E. Gall, K. Beebe, K.A. Lawton, K. Adam, M.W. Mitchell, P.J. Nakhle, J.A. Ryals, M.V. Milburn, M. Nannipieri, S. Camastra, A. Natali, E. Ferrannini, RISC Study Group, α-Hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 5(5), e10883 (2010)
E. Ferranini, A. Natali, S. Camastra, M. Nannipieri, A. Mari, K. Adam, M.V. Milburn, G. Kastenmüller, J. Adamski, T. Tuomi, V. Lyssenko, L. Groop, W.E. Gall, Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes 62(5), 1730–1737 (2013)
E. Selvin, A.M. Rawlings, M. Grams, R. Klein, A.R. Sharrett, M. Steffes, J. Coresh, Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2(4), 279–288 (2014)
J.M. Pappachan, F.A. Antonio, M.E. Edavalath, A. Mukherjee, Non-alcoholic fatty liver disease: a diabetologist’s perspective. Endocrine 45(3), 334–353 (2014)
C.P. Day, O.F. James, Steatohepatitis: a tale of two “hits”? Gastroenterology 114(4), 842–845 (1998)
H. Tilg, A.R. Moschen, Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52(5), 1836–1846 (2010)
M. Sargin, O. Uygur-Bayramiçli, H. Sargin, E. Orbay, A. Yayla, Association of nonalcoholic fatty liver disease with insulin resistance: is OGTT indicated in nonalcoholic fatty liver disease? J. Clin. Gastroenterol. 37(5), 399–402 (2003)
V.W.S. Wong, A.Y. Hui, S.W.C. Tsang, J.L.Y. Chan, G.L.H. Wong, A.W.H. Chan, W.Y. So, A.Y.S. Cheng, P.C.Y. Tong, F.K.L. Chan, J.J.Y. Sung, H.L.Y. Chan, Prevalence of undiagnosed diabetes and postchallenge hyperglycaemia in Chinese patients with non-aloholic fatty liver disease. Aliment. Pharmacol. Ther. 24(8), 1215–1222 (2006)
J.W. Yun, Y.K. Cho, J.H. Park, H.J. Kim, D.I. Park, C.I. Sohn, W.K. Jeon, B.I. Kim, Abnormal glucose tolerance in young male patients with nonalcoholic fatty liver disease. Liver Int. 29(4), 525–529 (2009)
C. Ortiz-Lopez, R. Lomonaco, B. Orsak, J. Finch, Z. Chang, V.G. Kochunov, J. Hardies, K. Cusi, Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care 35(4), 873–878 (2012)
S. Zelber-Sagi, R. Lotan, O. Shibolet, M. Webb, A. Buch, D. Nitzan-Kaluski, Z. Halpern, E. Santo, R. Oren, Non-alcoholic fatty liver disease independently predicts prediabetes during a 7-year prospective follow-up. Liver Int. 33(9), 1406–1412 (2013)
B. Vozarova, N. Stefan, R.S. Lindsay, A. Saremi, R.E. Pratley, C. Bogardus, P.A. Tataranni, High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 51(6), 1889–1895 (2002)
A.J. Hanley, K. Williams, A. Festa, L.E. Wagenknecht, R.B. D’Agostino Jr, J. Kempf, B. Zinman, S.M. Haffner, Insulin resistance atherosclerosis study, elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 53(10), 2623–2632 (2004)
M. Nannipieri, C. Gonzales, S. Baldi, R. Posadas, K. Williams, S.M. Haffner, M.P. Stern, E. Ferrannini, Mexico City Diabetes Study, liver enzymes, the metabolic syndrome, and incident diabetes: the Mexico City diabetes study. Diabetes Care 28(7), 1757–1762 (2005)
Y. Doi, M. Kubo, K. Yonemoto, T. Ninomiya, M. Iwase, Y. Tanizaki, K. Shikata, M. Iida, Y. Kiyohara, Liver enzymes as predictor for incident diabetes in a Japanese population: the Hisayama study. Obesity (Silver Spring) 15(7), 1841–1850 (2007)
E.S. Ford, M.B. Schulze, M.M. Bergmann, C. Thamer, H.G. Joost, H. Boeing, Liver enzymes and incident diabetes: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes Care 31(6), 1138–1143 (2008)
A. Fraser, R. Harris, N. Sattar, S. Ebrahim, G.D. Smith, D.A. Lawlor, Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women’s Heart and Health Study and meta-analysis. Diabetes Care 32(4), 741–750 (2009)
G. Bedogni, S. Bellentani, L. Miglioli, F. Masutti, M. Passalacqua, A. Castiglione, C. Tiribelli, The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 33 (2006)
B. Balkau, C. Lange, S. Vol, F. Fumeron, F. Bonnet, Group Study D.E.S.I.R., Nine-year incident diabetes is predicted by fatty liver indices: the French D.E.S.I.R. study. BMC Gastroenterol. 10, 56 (2010)
I. Rückert, M. Heier, W. Rathmann, S.E. Baumeister, A. Döring, C. Meisinger, Association between markers of fatty liver disease and impaired glucose regulation in men and women from the general population: the KORA-F4-Study. PLoS ONE 6(8), e22932 (2011)
N. Stefan, A.M. Hennige, H. Staiger, J. Machann, F. Schick, S.M. Kröber, F. Machicao, A. Fritsche, H. Häring, α2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 29(4), 853–857 (2006)
H. Ou, Y. Yang, H. Wu, J. Wu, F. Lu, C. Chang, Increased fetuin-A concentrations in impaired glucose tolerance with or without nonalcoholic fatty liver disease, but not impaired fasting glucose. J. Clin. Endocrinol. Metab. 97(12), 4717–4723 (2012)
E. Giovannucci, D.M. Harlan, M.C. Archer, R.M. Bergnestal, S.M. Gapstur, L.A. Habel, M. Pollak, J.G. Regensteiner, D. Yee, Diabetes and Cancer. A consensus report. Diabetes Care 33(7), 1674–1685 (2010)
Y. Huang, X. Cai, M. Qiu, P. Chen, H. Tang, Y. Hu, Y. Huang, Prediabetes and the risk of cancer: a meta-analysis. Diabetologia (2014). doi:10.1007/s00125-014-3361-2
S. Gao, A. Li, F. Liu et al., NCOA5 haploinsufficency results in glucose intolerance and subsequent heaptocellualr carcinoma. Cancer Cell 24, 725–737 (2013)
A. Leone, E. DiGennaro, F. Bruzzese, A. Avallone, A. Budillon, New perspective for an old antidiabteic drug: metformin as anticancer agent. Cancer Treat Res 159, 355–376 (2014)
M. Bergman, R. Dankner, J. Roth, K.M. Venkat Narayan, Are current diagnostic guidelines delaying early detection of dysglycemic states? Time for new approaches. Endocrine 44(1), 66–69 (2013)
M. Bergman, Pathophysiology of prediabetes and treatment implications for the prevention of type 2 diabetes mellitus. Endocrine 43(3), 504–513 (2013)
M. Bergman, Inadequacies of current approaches to prediabetes and diabetes prevention. Endocrine 44(3), 623–633 (2013)
Conflict of interest
The authors have no conflicts to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buysschaert, M., Medina, J.L., Bergman, M. et al. Prediabetes and associated disorders. Endocrine 48, 371–393 (2015). https://doi.org/10.1007/s12020-014-0436-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0436-2