Abstract
Interleukin 15 (IL-15) has recently been proposed as a myokine involved in regulating lipid metabolism. We investigated the effect of exercise training on IL-15 content in skeletal muscle and expression of IL-15 receptor (IL-15R) in adipose tissue of obese rats. After 12 weeks of a high-fat diet, obese rats underwent treadmill running at 26 m/min (60 min each, 5 days/week for 8 weeks). High-fat diet induced obesity, with increased body weight, body fat, and lipid profile. The level of IL-15 immunoreactivity (IL-15-ir) in plasma and gastrocnemius muscle was lower in obese than control rats, and the mRNA level of IL-15 in gastrocnemius muscle was markedly decreased. The mRNA and protein levels of IL-15R in adipose tissue were markedly lower in obese rats. Compared with sedentary obese rats, treadmill running showed decreased body weight and elevated mRNA expression of IL-15 in muscle and elevated IL-15-ir level in plasma and muscle. The mRNA and protein level of IL-15R were increased in adipose tissue in treadmill running obese rats. Our results showed that exercise training improve obesity and reversed the downregulation of the IL-15 in muscle and IL-15R in adipose tissue induced by high-fat diet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interleukin-15 (IL-15), a recently discovered cytokine that belongs to the four α-helix bundle family of cytokines, is widely expressed among tissue and cell types. In addition to lymphoid tissues, IL-15 mRNA is also expressed in cardiac muscle, lung, liver, kidney, brain, pancreas, placenta, and particularly highly expression in skeletal muscle [1]. IL-15 receptor (IL-15R) expresses in various tissue, and is characterized as trimeric in structure, composed of IL-2 receptor β (IL-2Rβ), the common gamma chain (γc), and a specific IL-15Rα chain [2, 3]. IL-15 is a pleiotropic cytokine that involved in the regulation of energy homeostasis [4–6]. IL-15−/− mice experienced significant weight gain without altering appetite [7]. Systemically administered IL-15 reduces fat deposition in normal and obese rodents by inhibiting lipogenesis in liver and adipose tissue [8–11]. In vitro studies demonstrated that IL-15 can directly inhibit preadipocyte differentiation and lipogenesis, thus stimulating lipolysis [12–15]. Furthermore, Nielsen AR et al. reported that plasma IL-15 and muscle IL-15 mRNA were negatively correlated with obesity parameters in obese human [16]. Theses findings suggested that IL-15 could be a novel endogenous anti-obesity factor and that IL-15 could be markedly depressed in obesity.
A lot of population-based studies showed that regular physical activity plays an important, perhaps even a dominant role, in obesity [17–20]; however, the specific mechanism of the effects of exercise on weight loss have not been well elucidated. Recently, skeletal muscle has been identified as a secretory organ, and cytokines or peptides that are expressed and released by muscle cell have been termed as myokines, which could be novel mediators of the multiple health benefits of exercise [21]. In obesity, myokines, such as IL-6, tumor necrosis factor-α (TNF-α), myostatin, and brain-derived neurotrophic factor, were disturbed in skeletal muscle [22]. Contracting skeletal muscle during exercise can regulate the expression and secretion of myokines, such as IL-6 and myostatin, which involve in the weight loss in response to exercise [22]. Several lines of evidence demonstrated that IL-15 is highly expressed in skeletal muscle and functioned as a myokine [23], by muscle-to-fat axes [23], reducing adipose tissue weight [8]. However, the effect of exercise training on IL-15 expression in skeletal muscle and IL-15Rα expression in adipose tissue has not been adequately investigated in obese rats. We hypothesized that exercise training can restore the depression of skeletal muscle IL-15 expression and adipose tissue IL-15Rα expression in obesity. In this study, we wanted to observe the alteration in IL-15 expression in skeletal muscle, and IL-15R expression in adipose tissue during treadmill running in obese rats induced by a high-fat diet.
Materials and methods
Animals and reagents
All animal care and experimental protocols complied with the Animal Management Rules (document No. 55, 2001) of the Ministry of Health of the People’s Republic of China and the guide for the Care and Use of the Laboratory Animals of Peking University Health Science Center. The 5-week-old male Sprague–Dawley (SD) rats were provided by the Animal Department, Peking University Health Science Center. The animals were housed in pairs, on a 12:12 h light–dark cycle in a temperature-controlled room at 22 ±2 °C.
Cholesterol and aprotinin were from Sigma (St. Louis, MO, USA). IL-15 radioimmunoassay kits were from Beijing Sino-UK Institute of Biological Technology. Assay kits for total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were from Biosino Bio-technology and Science (Beijing). Antibody against IL-15Rα (sc-9172), β-actin (sc-47778), and all secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Nitrocellulose membrane was from Hybond-C (Amersham Life Science, England), and the enhanced chemiluminescence kit was from Beijing Applygen Technologies. Other chemicals and reagents were of analytical grade.
Preparation of animal model
We randomly assigned 5-week-old male SD rats to 4 groups (n = 8 each) for treatment: (1) control, normal diet (normal diet: 14,610 kJ/kg, energy content in %: carbohydrate: 66.50; fat: 10.21; protein: 23.29); (2) control + treadmill running, normal diet and treadmill running progressively increased to reach 60 min at 26 m/min (5 days/week for 8 weeks) [24], and all training sessions took place during the afternoon (3:00–6:00 p.m.); (3) high-fat diet (HFD; 19,315 kJ/kg), 200 g fat/kg (170 g lard + 30 g corn oil to provide essential fatty acids) and 1 % cholesterol by weight plus normal drinking water [25] (The HFD was formulated to provide 40 % of total energy from fat by replacing carbohydrate energy with lard and corn oil and had the same amount of vitamins and minerals per kilojoule as the normal diet); and (4) HFD + treadmill running, training beginning 12 weeks after introducing the HFD.
At the end of the dietary and exercise protocol, rats fasted overnight but had free access to water and were anesthetized with use of urethane (1 g/kg, intraperitoneally). Blood was drawn from the abdominal aorta into syringes pretreated with EDTA (15 %), then centrifuged (3,000 rpm for 8 min, 4 °C), and plasma was kept for further analysis. Soleus and gastrocnemius muscle and visceral and subcutaneous fat were excised and weighed. All tissue samples were frozen in liquid nitrogen. Subcutaneous adipose tissue was removed from the inguinal region and visceral adipose tissue from the perirenal and epididymal fat depots. Plasma TC, TG, HDL-C, and LDL-C were measured by colorimetric enzymatic assays.
Radioimmunoassay of IL-15 level in muscle
Excised soleus and white gastrocnemius muscle were immediately acidified with 1.0 mol/L acetic acid and then heated at 100 °C for 10 min to inactivate proteases. The plasma samples were pre-treated with aprotinin (500 KIU/mL). Plasma or the supernatant extracted from skeletal muscle subjected to radioimmunoassay. The range of the standard curve was 1.02–16.3 pg/mL, in which a double-logarithmic scale showed a linear relationship with r2 = 0.999. All samples were within the range of the standard curve. The detection limit was calculated to be 2.14 pg/mL. The intra- and interassay coefficients of variation were validated within our work and were 6.1 and 10.2 %, respectively. There is no cross-reactivity found with rat IL-2, IL-6, or tumor necrosis factor α (TNF-α).
Real-time PCR analysis
Total RNA from soleus and white gastrocnemius muscle and epididymal adipose tissue was isolated and reverse transcribed by use of a reverse-transcription system (Promega, Madison, WI, USA). In total, 20 μL of the reaction mixture underwent real-time PCR and then evaluated by SYBR Green I fluorescence. The rat primers were for IL-15: forward, 5′-CTT CTT AAC TGA GGC TGG-3′ and reverse, 5′-GCA ACT GGG ATG AAA GTC-3′; IL-15Rα: forward, 5′-GAA AGC AGG CAC ATC CAC-3′ and reverse, 3′-GAG GCT CTC TGG TTG TGA G-5′; and β-actin: forward, 5′-GAG ACC TTC AAC ACC CCA GCC-3′and reverse, 5′-TCG GGG CAT CGG AAC CGC TCA-3′. All amplification reactions involved use of the M×3000 Multiplex Quantitative PCR System (Stratagene, La Jolla, CA, USA). After being denatured at 95 °C for 7 min, the solution underwent PCR for IL-15 at 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 40 s for 43 cycles and for IL-15R at 95 °C for 30 s, 54 °C for 30 s, and 72 °C for 40 s for 45 cycles.
Western blot analysis of adipose tissue
Epididymal adipose tissue was homogenized in lysis buffer [0.1 mol/L NaCl, 0.01 mol/L Tris–HCl (pH 7.5), 1 mmol/L EDTA, and 500 KIU/mL aprotinin]. Equal amounts of protein samples were loaded on 10 or 12 % SDS-PAGE and then transferred to a nitrocellulose membrane, for blocking with 5 % nonfat dried milk for 1 h, and then incubated with the primary antibodies anti-IL-15Rα (1:600) or anti-β-actin (1:2,000) overnight at 4 °C, then secondary antibody (anti-goat or anti-rabbit IgG conjugated to horseradish peroxidase) for 1 h [26]. Protein expression was analyzed by use of NIH image software (Bethesda, Maryland, USA) and normalized to β-actin expression. All experiments were repeated at least three times.
Statistical analysis
Data are shown as mean ± SEM. A repeated measures ANOVA model was used to examine the effects of diet, exercise, time, and their interaction on body weight during 12–20 weeks. Significance of dietary fat content and exercise was determined by two-way ANOVA and Bonferroni post-tests. The differences between groups were considered significant when P < 0.05.
Results
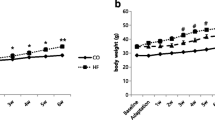
Treadmill running exercise attenuates obesity induced by a HFD
Treadmill running significantly decreased body weight (P < 0.01) (Fig. 1) and the ratios of visceral fat to body weight of normal diet rats compared to the control rats (P < 0.01) (Fig. 2a, b). No significant difference was observed in subcutaneous fat to body weight and lipid profile between control and exercise alone groups (P > 0.05) (Fig. 2c–f). Body weight was significantly heavier for rats fed a HFD than control rats (P < 0.01) (Fig. 1). Twelve weeks after introducing the HFD, rats underwent 8 weeks of treadmill running, which significantly decreased the body weight of the HFD rats by 13 % (P < 0.01) (Fig. 1) and ratios of visceral and subcutaneous fat to body weight, by 40 and 44 % (all P < 0.01), respectively (Fig. 2a, b), compared to rats fed a HFD alone. Plasma TC, TG, and LDL-C concentrations were greater by 21 % (P < 0.05), 29 % (P < 0.01), and 73 % (P < 0.01), respectively, and HDL-C concentration was lower by 50 % (P < 0.01) in rats fed a HFD alone than control rats (Fig. 2c–f). Exercise lowered the plasma TC and LDL-C level, by 20 and 28 %, respectively, and increased HDL-C level by 28 % (all P < 0.05) but had no effect on plasma TG concentration (P > 0.05; Fig. 2c–f). Overall, exercise training prevented the effects of the HFD on body weight, body total fat, and lipid profile.
Treadmill running improves serum lipid profile in high-fat diet (HFD) rats. a–b Ratio of visceral fat to body weight and subcutaneous fat to body weight. c–f Serum levels of cholesterol (TC), TG, HDL-C, and LDL-C. Data are mean ± SEM, n = 8. Different letters indicate groups differed significantly (P < 0.05) by two-way ANOVA. EX, exercise; SED, sedentary
Treadmill running increased IL-15 immunoreactivity (IL-15-ir) and IL-15 mRNA level
Compared with the control group, treadmill running alone increased IL-15-ir and IL-15 mRNA level in soleus and gastrocnemius muscle by 27 % (P < 0.01), 26 % (P < 0.05) and 59 %, 65 % (all P < 0.01), respectively, with no significant changes in plasma IL-15-ir (Fig. 3a–e). HFD decreased IL-15-ir in plasma and gastrocnemius muscle significantly, by 15 % (P < 0.01) and 26 % (P < 0.05), respectively, and reduced IL-15 mRNA level in gastrocnemius muscle by 40 % (P < 0.05), with no difference in soleus muscle (Fig. 3a–e). Compared with HFD treatment alone, treadmill running markedly elevated IL-15-ir in plasma and soleus and gastrocnemius muscle by 16 % (P < 0.05), 26 % (P < 0.01), and 47 % (P < 0.01), respectively, and increased IL-15 mRNA level in soleus and gastrocnemius muscle by 56 % (P < 0.05) and 138 % (P < 0.01), respectively (Fig. 3a-e).
Treadmill running increased IL-15 immunoreactivity (IL-15-ir) and IL-15 mRNA level. a–c IL-15-ir content in plasma and soleus and gastrocnemius muscle. Data are mean ± SEM, n = 6. d–e Real-time PCR analysis of mRNA level of IL-15 in soleus and gastrocnemius muscle. Data are mean ± SEM, n = 3. Different letters indicate groups which differed significantly (P < 0.05) by two-way ANOVA. HFD, high-fat diet; EX, exercise; SED, sedentary
Treadmill running increased adipose tissue IL-15Rα mRNA and protein expression
Transcripts for IL-15Rα have wide tissue distribution, and adipose tissue contains an abundance of IL-15Rα mRNA [9]. Compared with control rats, normal rats running the treadmill showed increased mRNA and protein levels of IL-15Rα by 28 % (P < 0.05) and 45 % (P < 0.01), respectively, in adipose tissue, which was decreased in HFD rats by 58 and 56 % (all P < 0.01) (Fig. 4a–c), respectively. Treadmill running ameliorated the reduced mRNA and protein levels of IL-15Rα in HFD rats by 148 % (P < 0.01) and 70 % (P < 0.05) (Fig. 3a–c), respectively.
Treadmill running increased IL-15 receptor α (IL-15Rα) mRNA and protein expression in adipose tissue. a Real-time PCR analysis of mRNA level of IL-15Rα. b Western blot analysis of protein level of IL-15Rα and c quantification of IL-15Rα. Data are mean ± SEM, n = 3. Different letters indicate groups differed significantly (P < 0.05) by two-way ANOVA. HFD, high-fat diet; EX, exercise; SED, sedentary
Discussion
This study was undertaken to determine if exercise stimulated the skeletal muscle IL-15 expression and adipose tissue IL-15Rα expression in obesity. In this study, plasma IL-15 content and gastrocnemius IL-15 mRNA expression and content were lower in HFD rats, however, no difference in soleus. Exercise training increased soleus and gastrocnemius IL-15 mRNA expression and content not only in HFD rats, but also in normal diet rats. Additionally, treadmill running restored the adipose tissue IL-15 Rα mRNA and protein expression in HFD rats. Therefore, our data indicated that exercise improved obesity and reversed the downregulation of the IL-15 in muscle and IL-15R in adipose tissue induced by HFD.
Skeletal muscle behaving as an endocrine organ, express several myokines and has paracrine or endocrine effects, which involved in metabolism regulation. IL-15 was the most highly expressed of all cytokines measured at the mRNA level in human skeletal muscle [27]. Biologically active IL-15 has been detected in primary human myoblast and cultures supernatant [28], IL-15 has been regarded as a myokine [23]. In obese human, plasma IL-15 and vastus lateralis muscle IL-15 mRNA were found negatively related to obesity parameters [16]. In this work, we found that plasma and muscle IL-15 content in gastrocnemius decreased in HFD rats, however, no difference in soleus. Furthermore, we also found that muscle IL-15 mRNA was downregulated in obese rats and no significant difference was observed in soleus. These data indicated that IL-15 expression in rats is regulated in a muscle fiber type-dependent manner.
There are inconsistent findings from previous studies as to the effects of exercise on IL-15 expression in skeletal muscle and plasma IL-15 content [29]. However, most subjects of previous studies were normal individuals, and changes in IL-15 level in obese subjects after exercise were unclear. In the current study, exercise training markedly increased IL-15-ir content and mRNA expression in soleus and gastrocnemius muscle in chow-fed and HFD rats. Furthermore, two-way ANOVA tests demonstrated that there was no significant interaction between exercise and diet, which indicated that exercise itself can significantly affect the skeletal muscle IL-15 expression independently of weight loss. In contrast, Christiansen et al. reported that exercise alone had no effect on muscle IL-15 expression in obese subjects who undertook a program of exercise, very low energy diet, or the combination of diet and exercise [30]. The difference of diet regimen and exercise regimen may result in the discrepancy. Additionally, we found that treadmill running increased plasma IL-15 level in HFD rats, but no change in normal diet rats. Studies of Tamura et al. [31] and Richman et al. [32] reported that serum IL-15 content was increased immediately following acute exercise. In our work, the plasma was sampled at the next day morning of the end of exercise protocol, rather than immediately after exercise, which hinted that chronic exercise may not affect the circulating IL-15 level. Alternatively, IL-15 forms functional complexes with soluble high affinity IL-15Rα in serum, which will interfere with the detection of IL-15 [33]. Therefore, the exact role of exercise on circulating IL-15 content still awaits further research.
IL-15 receptor (IL-15R) is a heterotrimeric receptor comprising IL-15Rα plus IL-2Rβ and IL-2Rγ, and all three subunits were expressed in adipose tissue [9]. However, little or no IL-15 mRNA has been detected in undifferentiated preadipocytes or differentiated adipocytes [23]. Quinn et al. reported that IL-15 functions in a muscle-to-fat endocrine axis and has powerful actions in the regulation of fat–lean body composition [34]. However, the effect of HFD and exercise training on adipose tissue IL-15R expression is yet unknown. So we detected the IL-15R expression in adipose tissue, rather than other tissue. Whether other tissues contribute to adipose tissue reduction need to be further investigated. In our work, we found that the expression of IL-15Rα in adipose tissue decreased in obese rats. IL-15Rα binding with its unique high affinity ligand IL-15, functions primarily to confer high affinity binding to the receptor β and γ c subunits, which are responsible for signal transduction [29, 35]. Therefore, the decreased protein level of IL-15Rα may blunt the sensitivity of adipose tissue to IL-15. Treadmill running stimulated the IL-15Rα expression in normal diet rats and restored the IL-15Rα expression in HFD rats; however, the regulation of IL-15Rα was still unknown. Our data showed that both exercise and diet significantly affected the adipose tissue IL-15Rα expression, which indicated that exercise itself and exercise-induced adipose tissue reducing involved in the regulation of the adipose tissue IL-15Rα expression.
In summary, we showed that long-term treadmill running improved obesity and reversed the downregulation of the IL-15 in muscle and IL-15R in adipose tissue induced by high-fat diet.
References
K.H. Grabstein, J. Eisenman, K. Shanebeck, C. Rauch, S. Srinivasan, V. Fung, C. Beers, J. Richardson, M.A. Schoenborn, M. Ahdieh et al., Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science 264, 965–968 (1994)
J.G. Giri, S. Kumaki, M. Ahdieh, D.J. Friend, A. Loomis, K. Shanebeck, R. DuBose, D. Cosman, L.S. Park, D.M. Anderson, Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 14, 3654–3663 (1995)
J.G. Giri, M. Ahdieh, J. Eisenman, K. Shanebeck, K. Grabstein, S. Kumaki, A. Namen, L.S. Park, D. Cosman, D. Anderson, Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 13, 2822–2830 (1994)
B.K. Pedersen, F. Edward, Adolph distinguished lecture: muscle as an endocrine organ: IL-6 and other myokines. J. Appl. Physiol. 107, 1006–1014 (2009)
B.K. Pedersen, T.C. Akerström, A.R. Nielsen, C.P. Fischer, Role of myokines in exercise and metabolism. J. Appl. Physiol. 103, 1093–1098 (2007)
E. González-Reimers, C.M. Fernández-Rodríguez, F. Santolaria-Fernández, M.J. de la Vega-Prieto, C. Martín-González, M.Á. Gómez-Rodríguez, M.R. Alemán-Valls, M. Rodríguez-Gaspar, Interleukin-15 and other myokines in chronic alcoholics. Alcohol Alcohol 46, 529–533 (2011)
N.G. Barra, S. Reid, R. MacKenzie, G. Werstuck, B.L. Trigatti, C. Richards, A.C. Holloway, A.A. Ashkar, Interleukin-15 contributes to the regulation of murine adipose tissue and human adipocytes. Obesity (Silver Spring) 18, 1601–1607 (2010)
L.S. Quinn, B.G. Anderson, L. Strait-Bodey, A.M. Stroud, J.M. Argilés, Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am. J. Physiol. Endocrinol. Metab. 296, E191–E202 (2009)
B. Alvarez, N. Carbó, J. López-Soriano, R.H. Drivdahl, S. Busquets, F.J. López-Soriano, J.M. Argilés, L.S. Quinn, Effects of interleukin-15 (IL-15) on adipose tissue mass in rodent obesity models: evidence for direct IL-15 action on adipose tissue. Biochim. Biophys. Acta 1570, 33–37 (2002)
J. López-Soriano, N. Carbó, V. Almendro, M. Figueras, V. Ribas, S. Busquets, F.J. López-Soriano, J.M. Argilés, Rat liver lipogenesis is modulated by interleukin-15. Int. J. Mol. Med. 13, 817–819 (2004)
V. Almendro, S. Busquets, E. Ametller, N. Carbó, M. Figueras, G. Fuster, J.M. Argilés, F.J. López-Soriano, Effects of interleukin-15 on lipid oxidation: disposal of an oral [(14)C]-triolein load. Biochim. Biophys. Acta 1761, 37–42 (2006)
V. Almendro, G. Fuster, E. Ametller, P. Costelli, F. Pilla, S. Busquets, M. Figueras, J.M. Argilés, F.J. López-Soriano, Interleukin-15 increases calcineurin expression in 3T3-L1 cells: possible involvement on in vivo adipocyte differentiation. Int. J. Mol. Med. 24, 453–458 (2009)
G. Fuster, V. Almendro, C.C. Fontes-Oliveira, M. Toledo, P. Costelli, S. Busquets, F.J. López-Soriano, J.M. Argilés, Interleukin-15 affects differentiation and apoptosis in adipocytes: implications in obesity. Lipids 46, 1033–1042 (2011)
K.M. Ajuwon, M.E. Spurlock, Direct regulation of lipolysis by interleukin-15 in primary pig adipocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R608–R611 (2004)
K.M. Ajuwon, S.K. Jacobi, J.L. Kuske, M.E. Spurlock, Interleukin-6 and interleukin-15 are selectively regulated by lipopolysaccharide and interferon-gamma in primary pig adipocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R547–R553 (2004)
A.R. Nielsen, P. Hojman, C. Erikstrup, C.P. Fischer, P. Plomgaard, R. Mounier, O.H. Mortensen, C. Broholm, S. Taudorf, R. Krogh-Madsen, B. Lindegaard, A.M. Petersen, J. Gehl, B.K. Pedersen, Association between interleukin-15 and obesity: interleukin-15 as a potential regulator of fat mass. J. Clin. Endocrinol. Metab. 93, 4486–4493 (2008)
A.M. Prentice, S.A. Jebb, Obesity in Britain: gluttony or sloth? BMJ 311, 437–439 (1995)
R.L. Weinsier, G.R. Hunter, A.F. Heini, M.I. Goran, S.M. Sell, The etiology of obesity: relative contribution of metabolic factors, diet, and physical activity. Am. J. Med. 105, 145–150 (1998)
A.F. Heini, R.L. Weinsier, Divergent trends in obesity and fat intake patterns: the American paradox. Am. J. Med. 102, 259–264 (1997)
I. Castan-Laurell, C. Dray, C. Attané, T. Duparc, C. Knauf, P. Valet, Apelin, diabetes, and obesity. Endocrine 40, 1–9 (2011)
B.K. Pedersen, The diseasome of physical inactivity and the role of myokines in muscle–fat cross talk. J. Physiol. 587, 5559–5568 (2009)
B.K. Pedersen, M.A. Febbraio, Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012)
L.S. Quinn, L. Strait-Bodey, B.G. Anderson, J.M. Argilés, P.J. Havel, Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: evidence for a skeletal muscle-to-fat signaling pathway. Cell Biol. Int. 29, 449–457 (2005)
M.S. Gauthier, K. Couturier, A. Charbonneau, J.M. Lavoie, Effects of introducing physical training in the course of a 16-week high-fat diet regimen on hepatic steatosis, adipose tissue fat accumulation, and plasma lipid profile. Int. J. Obes. Relat. Metab. Disord. 28, 1064–1071 (2004)
Y.J. Kim, T. Park, Genes are differentially expressed in the epididymal fat of rats rendered obese by a high-fat diet. Nutr. Res. 28, 414–422 (2008)
J. Zhang, W. Wu, D. Li, Y. Guo, H. Ding, Overactivation of NF-κB impairs insulin sensitivity and mediates palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Endocrine 37, 157–166 (2010)
D.C. Nieman, J.M. Davis, D.A. Henson, J. Walberg-Rankin, M. Shute, C.L. Dumke, A.C. Utter, D.M. Vinci, J.A. Carson, A. Brown, W.J. Lee, S.R. McAnulty, L.S. McAnulty, Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3 h run. J. Appl. Physiol. 94, 1917–1925 (2003)
T. Sugiura, M. Harigai, Y. Kawaguchi, K. Takagi, C. Fukasawa, S. Ohsako-Higami, S. Ohta, M. Tanaka, M. Hara, N. Kamatani, Increased IL-15 production of muscle cells in polymyositis and dermatomyositis. Int. Immunol. 14, 917–924 (2002)
L.S. Quinn, B.G. Anderson, Interleukin-15, IL-15 receptor-alpha, and obesity: concordance of laboratory animal and human genetic studies. J. Obes. 2011, 456347 (2011)
T. Christiansen, S.K. Paulsen, J.M. Bruun, S.B. Pedersen, B. Richelsen, Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: a 12-week randomized intervention study. Am. J. Physiol. Endocrinol. Metab. 298, E824–E831 (2010)
Y. Tamura, K. Watanabe, T. Kantani, J. Hayashi, N. Ishida, M. Kaneki, Upregulation of circulating IL-15 by treadmill running in healthy individuals: is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr. J. 58, 211–215 (2011)
S.E. Riechman, G. Balasekaran, S.M. Roth, R.E. Ferrell, Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J. Appl. Physiol. 97, 2214–2219 (2004)
E. Bulanova, V. Budagian, E. Duitman, Z. Orinska, H. Krause, R. Rückert, N. Reiling, S. Bulfone-Paus, Soluble Interleukin IL-15Ralpha is generated by alternative splicing or proteolytic cleavage and forms functional complexes with IL-15. J. Biol. Chem. 282, 13167–13179 (2007)
L.S. Quinn, Interleukin-15: a muscle-derived cytokine regulating fat-to-lean body composition. J. Anim. Sci. 86, E75–E83 (2008)
J. Eisenman, M. Ahdieh, C. Beers, K. Brasel, M.K. Kennedy, T. Le, T.P. Bonnert, R.J. Paxton, L.S. Park, Interleukin-15 interactions with interleukin-15 receptor complexes: characterization and species specificity. Cytokine 20, 121–129 (2002)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 81270921), and Beijing Natural Science Foundation (Effects and mechanisms of aerobic exercise on active-peptide secreted by skeletal muscle of rat with insulin resistance), and the Fundamental Research Funds for the Central Universities.
Ethical standards
All animal care and experimental protocols complied with the Animal Management Rules (document No. 55, 2001) of the Ministry of Health of the People’s Republic of China and the guide for the Care and Use of the Laboratory Animals of Peking University Health Science Center.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, H., Chang, J., Chen, W. et al. Treadmill exercise promotes interleukin 15 expression in skeletal muscle and interleukin 15 receptor alpha expression in adipose tissue of high-fat diet rats. Endocrine 43, 579–585 (2013). https://doi.org/10.1007/s12020-012-9809-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9809-6