Abstract
Acromegaly secondary to extra-pituitary tumors secreting growth hormone releasing hormone (GHRH) is rarely encountered. We review the literature on ectopic acromegaly and present the index report of ectopic acromegaly secondary to GHRH secretion from a mediastinal paraganglioma. Clinical and pathological manifestations and therapeutic management of 99 patients with ectopic acromegaly are reviewed. Acromegaly secondary to ectopic GHRH secretion is usually caused by a neuroendocrine tumor in the lung and pancreas. We report an additional cause of ectopic acromegaly from a mediastinal paraganglioma. Diagnostic criteria of ectopic GHRH syndrome include biochemical and pathologic tumoral confirmation of GHRH secretion and expression. Management of ectopic acromegaly consists of surgical resection of the primary tumor and biochemical normalization, with possible adjuvant use of somatostatin analogs. The review demonstrates that there are several tumor types, including paragangliomas which may secrete GHRH, leading to acromegaly. Clinical and laboratory manifestations of the syndrome and challenges in diagnosis and management of these rarely encountered patients require early diagnosis and appropriate treatment to prevent long-term morbidity and mortality with ectopic acromegaly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acromegaly is usually due to hypersecretion of growth hormone (GH) from a benign pituitary adenoma and is characterized by discordant tissue, skeletal, and organ growth. The incidence of acromegaly is approximately 3 cases per one million persons per year, and the prevalence is about 60 cases per million. In less than 1 % of cases acromegaly may develop because of ectopic secretion of growth hormone releasing hormone (GHRH) [1–6] or more rarely GH, from a nonpituitary origin, mostly from a neuroendocrine tumor (NET) [7–9]. In 1959, Altman and Schutz reported an acromegaly patient who did not respond to pituitary irradiation but improved markedly after surgical resection of a lung carcinoid tumor [2]. Subsequent reports confirmed the association of acromegaly with carcinoid tumors, and ectopic GHRH secretion was definitively identified as the cause through isolation and extraction of GHRH from pancreatic tumor tissue in patients with acromegaly [10, 11]. Most cases of ectopic acromegaly result from GHRH derived from lung carcinoid tumors, leading to pituitary hyperplasia, and GH hypersecretion [12–51]. Other reported cases include pancreatic cell tumors [28, 41, 44, 51–69], gastrointestinal tract [51, 70–75], thymus [76, 77], adrenal pheochromocytomas [78, 79], lung (adenoid cystic carcinoma) [80] and pituitary [81].
Here, we comprehensively review all the published cases of ectopic acromegaly and also report a new cause i.e., acromegaly secondary to a GHRH secreting mediastinal paraganglioma with ectopic GHRH secretion.
Ectopic acromegaly
Acromegaly secondary to ectopic GHRH secretion is rare. Since the initial reports of the association of bronchial carcinoid and acromegaly due to ectopic GHRH syndrome, 98 cases have been reported, of whom 2/3 were female. Mean age at diagnosis was 41 years, similar to pituitary acromegaly. Time from onset of symptoms to diagnosis, was between 1 and 22 years (8.3 ± 5.8 years). Mean duration of the disease to time of diagnosis, approximately 8 years, was also similar to the usual course of acromegaly, emphasizing the delay that often occurs in diagnosis due to gradual development of symptoms and small size of the corresponding tumors which can easily escape detection [18, 82].
Published causes of ectopic acromegaly
Ectopic acromegaly is almost always secondary to an NET originating in the lung and pancreas [44]. Association of acromegaly and pheochromocytoma has been reported in some patients (Table 1). Some of the cases were published before 1980 prior to isolation of GHRH and were labeled as part of MEN syndrome; hence, their association with GHRH secretion cannot be proven. In a recent case, a 23-year-old man who had ectopic acromegaly secondary to an incidentally discovered pheochromocytoma demonstrated improved symptoms after tumor resection, and positivity of the tumor tissue with GHRH immunostaining [79]. Rarely, ectopic acromegaly may be secondary to secretion of GH, as reported in a pancreatic islet cell tumor, a bronchial carcinoid tumor and lymphoma [7–9].
An additional cause—mediastinal paraganglioma
This cause was encountered by the authors on evaluation of a 56-year-old woman for facial coarsening over the past 6 years. She complained of facial puffiness, increasing jaw under-bite, widening of facial creases, and enlargement of nose, hands, and feet. She complained of low back and bilateral knee pain but did not elicit galactorrhea. Past medical history was significant for menopause at age 52, lung surgery for bronchiectasis, hypertension, prediabetes, and a benign colon polyp. Physical examination was remarkable for the presence of multiple neck and chest skin tags. Her upper incisors were spread apart, soft tissue swelling of hands and feet and deep facial creases and coarse features were noted.
Endocrine testing demonstrated elevated serum IGF-1 levels of 780, 525, and 616 ng/ml (normal range 94–284 ng/ml). The diagnosis of acromegaly was confirmed after a 75 g oral glucose tolerance test (OGTT) with nadir GH level of 2.2 ng/ml. Plasma GHRH levels were unable to be obtained. FSH and LH levels were in the menopausal range and thyroid function tests, morning cortisol and serum prolactin were within normal limits. Fasting blood glucose was 105 and 126 mg/dl, serum calcium was 9.6 mg/dl and 24-h urine calcium was 304 mg and routine laboratory tests were within normal limits.
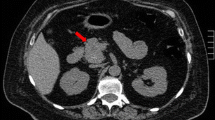
Pituitary MRI was performed twice and no pituitary adenoma or pituitary hyperplasia was visualized (Fig. 1). Computed tomography (CT) scan of the chest revealed a 4.5 by 3.5 cm lobulated soft tissue mass in the subcarinal region, posterior to the left atrium (Fig. 2). Abdominal CT scan showed a round lesion in the right lobe of the liver consistent with either a metastasis or a hemangioma (Fig. 2). Whole body Octreotide scan showed uptake concordant with the mediastinal mass but not with the liver lesion (Fig. 3).
An 18 g mediastinal tumor was resected. Pathological analysis in Teheran University of Medical Sciences demonstrated the tumor to be highly positive for synaptophysin and chromogranin and scattered positivity for EMA, GH, and S100 and negative for cytokeratin. Tumor Ki-67 was estimated at 7 %. Subsequent pathological analysis at Cedars-Sinai Medical Center in Los Angeles was obtained and showed a monomorphous population of tumor cells arranged in nests and solid sheets. Tumor cells had moderate amounts of pale eosinophilic cytoplasm and uniform round nuclei with salt and pepper-like nuclear chromatin. Nucleoli were inconspicuous. Rare mitotic figures were noted (1/10 HPF), and there was no evidence of necrosis. Rare sustentacular cells were highlighted by S100 stain. Immunostaining confirmed that the tumor was immunopositive for GHRH (Fig. 4). Immunostaining for TTf-1 and for GH were both negative. There was strong and diffuse immunoreactivity for chromogranin and no staining for keratin (AE1/AE3). Final pathologic diagnosis was that of paraganglioma.
Despite postoperative symptom improvement, subsequent biochemical evaluation showed elevated basal serum GH and IGF-1 levels, 2.9 and 506 ng/ml, respectively. The patient underwent a CT-guided biopsy of the hepatic lesion, and histopathologic evaluation revealed that the tissue biopsy had morphologic characteristics similar to the original tumor. The patient was then started on monthly injection of long-acting octreotide 20 mg/month which resulted in normalization of serum IGF-1 (132 ng/ml at 3 months and 196 ng/ml at 1 year) and lowering of basal GH levels to 0.7 and 0.9 ng/ml, at 3 and 12 months, respectively.
Paragangliomas
The above case is the first published report of acromegaly caused by ectopic GHRH secretion secondary to a mediastinal paraganglioma.
Paragangliomas originate from extra-adrenal chromaffin cells located outside adrenal glands and comprise 10–18 % of all catecholamine secreting tumors of the body. The tumors are located along the sympathetic chain from neck to the pelvis with the majority in the abdominal and pelvic region. While parasympathetic paragangliomas are primarily localized to neck or base of skull, sympathetic paragangliomas are mainly in the abdominal and pelvic region. Paragangliomas may manifest with symptoms of catecholamine hypersecretion, mass effect or may be incidentally discovered during imaging. Mediastinal paragangliomas are very rare and comprise only 2 % of extra-adrenal catecholamine-secreting tumors. Secretion of ectopic hormones from these tumors is very unusual. Most reported tumors secrete norepinephrine presenting with hypertension. The presence and bioactivity of GHRH has been demonstrated in pheochromocytomas, but not in paragangliomas [60, 83, 84]. In one series of 14 cases of mediastinal paragangliomas, all patients had hypertension, and one case was a nonfunctional paraganglioma [85–87]. Approximately, one-fourth to one-third of paragangliomas are hereditary and can be seen in the settings of familial paragangliomas, neurofibromatosis type 1, Von Hippel-Lindau disease, Carney triad, and rarely MEN 2. Mutations in succinate dehydrogenase (SDH) gene or mitochondrial complex 2 gene are responsible for most cases of hereditary paragangliomas.
Evaluation of patients for paragangliomas includes assessment of functional status through measurement of plasma or urinary fractionated metanephrines. Computed tomographic scanning (CT) can detect extra-adrenal paragangliomas larger than 2 cm with a sensitivity of 95 % and a specificity of 70 %. Many tumors harbor extensive areas of necrosis. MRI has a sensitivity of 90 % and a specificity of 70 % in extra-adrenal paragangliomas with brightness in T2-weighted images—a characteristic finding of pheochromocytoma and paragangliomas [82]. Somatostatin receptor scintigraphy (SRS) has also been used in localization of paraganglioma though In-Octreotide may be more sensitive and specific in detecting head and neck paragangliomas [88].
Metaiodo-benzylguanidine (MIBG) using iodine-123 has an overall sensitivity of 83–100 % and specificity of 95–100 % [89]. However, in metastatic paragangliomas and head and neck paragangliomas, the sensitivity decreases to as low as 65 % [90, 91]. In addition, Gallium-68 somatostatin receptor PET or PET/CT have a 93 % sensitivity and 91 % specificity for thoracic and gastroenteropancreatic NETs [92] and may eventually become first line tests in localization [93].
On light microscopy, paragangliomas are composed of cells arranged in nests or trabecular (zellballen pattern), separated with a thin vascular stroma. On electron microscopy, large dense core catecholamine-secreting granules measuring 150–250 nm in diameter can be detected [82].
In the current case, there were no specific symptoms of catecholamine excess as hypertension was mild and well controlled. Indeed, the patient did not present with features of a paraganglioma but rather presented functionally with acromegaly.
Clinical presentation of ectopic acromegaly
Signs and symptoms of ectopic acromegaly are similar to pituitary-dependent acromegaly. In some cases, patients may present with symptoms specific to the corresponding tumor such as jaundice, cough, unilateral wheezing, or other endocrinopathies [57, 67]. There may be an association of ectopic acromegaly with MEN1 as reported in 19 patients and presumptively in five additional cases [28, 40, 44, 51, 54, 55, 57–63, 66, 68, 69, 77]. Accordingly, common genetic pathways may underlie the pathogenesis of the two disorders. Secretory ability of the tumor can change over time, as in a case of a GHRH producing tumor, which changed to an insulin producing tumor [73]. Several cases of co-secretion of GHRH with other hormones such as ACTH have been reported [51, 76, 80].
Diagnostic evaluation of ectopic acromegaly
Distinction between pituitary and ectopic acromegaly is essential as pituitary hyperplasia secondary to GHRH production in ectopic acromegaly may be misdiagnosed as pituitary tumor leading to unnecessary pituitary surgery. Appropriate diagnosis of the source of acromegaly is of utmost significance as treatment strategies differ. In most cases in the literature, the preliminary diagnosis was pituitary acromegaly and diagnosis of ectopic acromegaly was confirmed either during the diagnostic work-up or after unsuccessful pituitary surgery. There was a delay of 2–18 years between pituitary surgery and establishment of correct diagnosis [29, 45, 66]. Most patients had pituitary hyperplasia visible on imaging with some characteristics indistinguishable from pituitary adenomas, leading often to unnecessary transsphenoidal surgery (TSS) [14, 21, 22, 28, 29, 34, 39–41, 43, 44, 51, 53, 56, 62, 63, 66, 67, 69, 70, 74, 78, 79, 81, 94, 95]. Of the 98 reported cases, 30 patients underwent transsphenoidal resection for presumed GH-secreting pituitary adenoma. Key findings leading to correct diagnosis of ectopic acromegaly are summarized in Table 2. Pituitary surgery was indicated in one case where pituitary macro-adenoma was the origin of ectopic GHRH secretion [81] and in other cases where the tumor had metastasized to the pituitary gland [95, 96].
In most patients, corresponding tumors were detected by CT or MRI. The size of the tumors ranged from 1 to 25 cm (6.3 ± 4.0, mean ± SD). Pituitary enlargement was present in about 75 % of cases and 1/3 were diagnosed with a pituitary adenoma. In some cases, normal appearance of the pituitary gland prompted workup for ectopic acromegaly (Table 4). Positive Octreotide scan in 19 out of 21 cases shows a high sensitivity for this modality in the diagnosis of GHRH-secreting tumors.
Serum levels of GH and IGF-1 are invariably elevated in patients with ectopic acromegaly, with nadir GH on OGTT > 1 mcg/l and IGF-1 levels above the age and gender matched normal range [97]. More sensitive assays lower the nadir GH cutoff to >0.3 mcg/l [98]. Mean values of GH after OGTT may show a paradoxical increase. Elevation of serum GH after thyrotropin releasing hormone (TRH) stimulation has been shown in some cases [53], but other laboratory tests are not useful in distinguishing pituitary from ectopic acromegaly. Measurement of plasma GHRH is the simplest and most accurate for diagnosis provided the laboratory facilities are available. The values for GHRH which are usually undetectable in pituitary acromegaly are elevated in patients with ectopic acromegaly reaching values hundreds or thousands-fold normal values [36, 44, 67]. Laboratory results including basal and post GTT values, serum IGF-1, and plasma GHRH for reported cases are summarized in Table 3. Thyrotropin releasing hormone (TRH) stimulation test was performed in 31 cases.
Diagnostic criteria for ectopic acromegaly include elevated plasma GHRH, complete recovery after resection of the corresponding tumor, positive GHRH immunostaining, positive bioassay (the ability of cultured rat pituitary cells to produce GH in response to the tumor extract) [15, 53, 70], arterio-venous gradient of GHRH across the tumor [31], detection of GHRH mRNA [64], or GHRH extraction from the tumor tissue [22, 31, 53, 56].
Pathologic evaluation of GHRH secreting tumors
Since 1985, immunohistochemistry (IHC) has been used to pathologically characterize GHRH secreting tumors. Positive immunoexpression for GHRH is seen in most tumors with ectopic acromegaly, though GHRH immunopositivity may not translate to biochemical and clinical manifestations of acromegaly [99]. The discordance between immunoexpression and clinical manifestations has been attributed to low concentration of GHRH in the vesicles, intravesicular degradation of GHRH, or defects in secretory capacity of GHRH [99].
Histopathologic evaluation of reported GHRH secreting tumors confirmed that all except three were of neuroendocrine origin. One of these was an adenoid cystic carcinoma of the lung [80] and the other was a pituitary adenoma secreting both GH and GHRH [81]; the third one was a case of acromegaly with elevated GHRH and normal pituitary MRI in whom no tumor was identified despite extensive work-up [51]. Eighty-four were bronchial carcinoid or endocrine pancreas tumors (Table 1). In 55 tumors, GHRH immunostaining was positive. In one case, tissue GHRH was measured using HPLC [22]. Bioassay was employed as confirmation of GHRH bioactivity in 3 cases [15, 53, 70].
General neuroendocrine markers such as chromogranin A, synaptophysin, and NSE were positive by IHC in most cases. Results of pituitary histopathology from 29 cases are shown in Table 4.
In this report, plasma GHRH measurement was unavailable. However, negative pituitary imaging, positive IHC expression for GHRH, and persistently elevated IGF-1 levels in the presence of hepatic metastasis are suggestive of a diagnosis of ectopic acromegaly secondary to GHRH secretion.
Management of ectopic acromegaly
Management of acromegaly from ectopic GHRH secretion consists of surgical resection of the primary tumor and biochemical normalization. Surgery is the treatment of choice and may be curative. In patients with extensive metastases or with unresectable tumor, somatostatin analogs may be used [22, 45, 47, 51]. Treatment modalities of the reported cases consisted of tumor resection in 55 cases, somatostatin analog therapy in 13 patients, and adjuvant chemotherapy and radiotherapy in 2 patients. Of 90 cases, cure was documented in 44 %, partial improvement in 31 %, no improvement in 5 %, and deaths in 10 %. Lymph node or distant metastases were documented in 39 cases [15, 19, 21–24, 28–30, 32, 33, 43–45, 51, 55, 57, 60, 65, 70, 72–76, 80]. Outcome data was ascertained in 90 cases with mean follow-up of 5.3 ± 5.5 years. Although there is limited follow-up of patients, GHRH-producing NETs have a favorable prognosis if diagnosed early and surgically resected, as other forms of NET, even in the presence of metastases [100–102]. Anti-angiogenic medications and tyrosine kinase inhibitors may potentially have a role in inhibiting tumor growth of NETs [103, 104].
In the index case reported here, a mediastinal tumor was completely resected, but biochemical acromegaly persisted postoperatively due to a functional liver metastasis. Surgical resection of the liver metastasis would be the preferred treatment but the patient opted for therapy with monthly injections of Octreotide LAR which has controlled GH levels.
Conclusion
We report acromegaly secondary to ectopic GHRH secretion from a mediastinal paraganglioma in the context of ectopic acromegaly. Criteria consistent with this diagnosis include: clinical signs and symptoms of acromegaly, biochemically confirmed acromegaly, normal pituitary imaging, a pathologically confirmed mediastinal paraganglioma, and positive immunostaining for GHRH. The case highlights that paragangliomas may be functional and though very rare, can lead to ectopic GHRH secretion and consequent acromegaly.
Methods
Hormonal analysis
Plasma GH, IGF1, FSH, LH, TSH, T4, T3, cortisol, and prolactin concentrations were measured by commercial kits.
Histopathologic examination
Resected specimens were fixed in formalin and embedded in paraffin. Paraffin sections were used for Hematoxylin-Eosin staining. Immunocytochemistry was performed using 0.03 % H2O2 (DAKO solution). Immunohistochemical staining was performed with antibodies against chromogranin, synaptophysin, EMA, GH, S 100, and cytokeratin. Ki-67 nuclear antigen immunostaining was also performed. Rabbit anti-GHRH antibody was used at dilution 1:1,000 and kindly provided by Dr. Lawrence Frohman (University of Illinois at Chicago).
Imaging studies
High resolution computerized tomography (HRCT) scanning was used for chest imaging and T1-weighted sagittal and coronal pituitary MRI views were used. Whole body Somatostatin receptor scintigraphy was performed using 99mTc-pentetreotide. Also 99mTc-pentetreotide single photon emission computerized tomography (SPECT) was utilized for enhanced anatomic localization.
Ectopic acromegaly cases in the literature
A comprehensive search of the English literature revealed that 98 cases of acromegaly secondary to ectopic secretion of GHRH were published between 1974 and 2011. Most were single case reports, and there were limited reviews on the topic. The results were prepared and analyzed using SPSS version 16. Data of the present case is also included in the analysis.
References
M.O. Thorner, L.A. Frohman, D.A. Leong, J. Thominet, T. Downs, P. Hellmann, J. Chitwood, J.M. Vaughan, W. Vale, Extrahypothalamic growth-hormone-releasing factor (GRF) secretion is a rare cause of acromegaly: plasma GRF levels in 177 acromegalic patients. J. Clin. Endocrinol. Metab. 59, 846–849 (1984)
G. Faglia, M. Arosio, N. Bazzoni, Ectopic acromegaly. Endocrinol. Metab. Clin. North Am. 21, 575–595 (1992)
M. Losa, K. von Werder, Pathophysiology and clinical aspects of the ectopic GH-releasing hormone syndrome. Clin. Endocrinol. (Oxf.) 47, 123–135 (1997)
M. Doga, S. Bonadonna, A. Burattin, A. Giustina, Ectopic secretion of growth hormone-releasing hormone (GHRH) in neuroendocrine tumors: relevant clinical aspects. Ann. Oncol. 12(Suppl 2), S89–S94 (2001)
M. Gola, M. Doga, S. Bonadonna, G. Mazziotti, P.P. Vescovi, A. Giustina, Neuroendocrine tumors secreting growth hormone-releasing hormone: pathophysiological and clinical aspects. Pituitary 9, 221–229 (2006)
S. Melmed, Medical progress: acromegaly. N. Engl. J. Med. 355, 2558–2573 (2006)
S. Melmed, C. Ezrin, K. Kovacs, R.S. Goodman, L.A. Frohman, Acromegaly due to secretion of growth hormone by an ectopic pancreatic islet-cell tumor. N. Engl. J. Med. 312, 9–17 (1985)
F. Beuschlein, C.J. Strasburger, V. Siegerstetter, D. Moradpour, P. Lichter, M. Bidlingmaier, H.E. Blum, M. Reincke, Acromegaly caused by secretion of growth hormone by a non-Hodgkin’s lymphoma. N. Engl. J. Med. 342, 1871–1876 (2000)
S. Biswal, B. Srinivasan, P. Dutta, P. Ranjan, K. Vaiphei, R.S. Singh, S.S. Thingnam, Acromegaly caused by ectopic growth hormone: a rare manifestation of a bronchial carcinoid. Ann. Thorac. Surg. 85, 330–332 (2008)
J. Rivier, J. Spiess, M. Thorner, W. Vale, Characterization of a growth hormone-releasing factor from a human pancreatic islet tumour. Nature 300, 276–278 (1982)
R. Guillemin, P. Brazeau, P. Bohlen, F. Esch, N. Ling, W.B. Wehrenberg, Growth hormone-releasing factor from a human pancreatic tumor that caused acromegaly. Science 218, 585–587 (1982)
F.T. Dabek, Bronchial carcinoid tumour with acromegaly in two patients. J. Clin. Endocrinol. Metab. 38, 329–333 (1974)
P.H. Sonksen, A.B. Ayres, M. Braimbridge, B. Corrin, D.R. Davies, G.M. Jeremiah, S.W. Oaten, C. Lowy, T.E. West, Acromegaly caused by pulmonary carcinoid tumours. Clin. Endocrinol. (Oxf.) 5, 503–513 (1976)
M. Saeed uz Zafar, R.C. Mellinger, G. Fine, M. Szabo, L.A. Frohman, Acromegaly associated with a bronchial carcinoid tumor: evidence for ectopic production of growth hormone-releasing activity. J. Clin. Endocrinol. Metab. 48, 66–71 (1979)
S.M. Shalet, C.G. Beardwell, I.A. MacFarlane, M.L. Ellison, C.M. Norman, L.H. Rees, M. Hughes, Acromegaly due to production of a growth hormone releasing factor by a bronchial carcinoid tumor. Clin. Endocrinol. (Oxf.) 10, 61–67 (1979)
B.W. Scheithauer, P.C. Carpenter, B. Bloch, P. Brazeau, Ectopic secretion of a growth hormone-releasing factor. Report of a case of acromegaly with bronchial carcinoid tumor. Am. J. Med. 76, 605–616 (1984)
F. Hawkins, V. Sanchez Moro, M. Aguirre, M. Leon, D. Rigopoulou, J.L. Martin de Nicolas, J. Toledo, Acromegaly and bronchial carcinoid. Effect of removal of the latter. Chest 88, 149–152 (1985)
R. Boizel, S. Halimi, F. Labat, R. Cohen, I. Bachelot, Acromegaly due to a growth hormone-releasing hormone-secreting bronchial carcinoid tumor: further information on the abnormal responsiveness of the somatotroph cells and their recovery after successful treatment. J. Clin. Endocrinol. Metab. 64, 304–308 (1987)
P.P. Garcia-Luna, A. Leal-Cerro, C. Montero, B.W. Scheithauer, A. Campanario, C. Dieguez, R. Astorga, K. Kovacs, A rare cause of acromegaly: ectopic production of growth hormone-releasing factor by a bronchial carcinoid tumor. Surg. Neurol. 27, 563–568 (1987)
D.G. Carroll, J.W. Delahunt, C.A. Teague, R.R. Cooke, E.F. Adams, N.D. Christofides, S.R. Bloom, G. Terenghi, J.M. Polak, Resolution of acromegaly after removal of a bronchial carcinoid shown to secrete growth hormone releasing factor. Aust. N. Z. J. Med. 17, 63–67 (1987)
A.L. Barkan, Y. Shenker, R.J. Grekin, W.W. Vale, R.V. Lloyd, T.F. Beals, Acromegaly due to ectopic growth hormone (GH)-releasing hormone (GHRH) production: dynamic studies of GH and ectopic GHRH secretion. J. Clin. Endocrinol. Metab. 63, 1057–1064 (1986)
S. Melmed, F.H. Ziel, G.D. Braunstein, T. Downs, L.A. Frohman, Medical management of acromegaly due to ectopic production of growth hormone-releasing hormone by a carcinoid tumor. J. Clin. Endocrinol. Metab. 67, 395–399 (1988)
D.E. Moller, A.C. Moses, K. Jones, M.O. Thorner, M.L. Vance, Octreotide suppresses both growth hormone (GH) and GH-releasing hormone (GHRH) in acromegaly due to ectopic GHRH secretion. J. Clin. Endocrinol. Metab. 68, 499–504 (1989)
P.E. Harris, P.M. Bouloux, J.A. Wass, G.M. Besser, Successful treatment by chemotherapy for acromegaly associated with ectopic growth hormone releasing hormone secretion from a carcinoid tumour. Clin. Endocrinol. (Oxf.) 32, 315–321 (1990)
V. Popovic, D. Micic, S. Damjanovic, M. Petakov, D. Manojlovic, J. Micic, Discordance between growth hormone responses after growth hormone-releasing hormone (GHRH) and insulin hypoglycemia in ectopic GHRH syndrome. Endocrinol. Exp. 24, 167–173 (1990)
R.J. Barth, R.B. Constant, M.W. Parker, G.L. Mueller, K. Kovacs, M.O. Thorner, Preoperative diagnosis of acromegaly by growth hormone-releasing factor radioimmunoassay. Mil. Med. 156, 375–378 (1991)
M. Glikson, I. Gil-Ad, E. Galun, R. Dresner, S. Zilberman, Y. Halperin, E. Okon, Z. Laron, A. Rubinow, Acromegaly due to ectopic growth hormone-releasing hormone secretion by a bronchial carcinoid tumour. Dynamic hormonal responses to various stimuli. Acta Endocrinol. (Copenh) 125, 366–371 (1991)
J. Bertherat, G. Turpin, C. Rauch, J.Y. Li, J. Epelbaum, G. Sassolas, G. Schaison, Presence of somatostatin receptors negatively coupled to adenylate cyclase in ectopic growth hormone-releasing hormone- and alpha-subunit-secreting tumors from acromegalic patients responsive to octreotide. J. Clin. Endocrinol. Metab. 79, 1457–1464 (1994)
S. Ezzat, S.L. Asa, L. Stefaneanu, R. Whittom, H.S. Smyth, E. Horvath, K. Kovacs, L.A. Frohman, Somatotroph hyperplasia without pituitary adenoma associated with a long standing growth hormone-releasing hormone-producing bronchial carcinoid. J. Clin. Endocrinol. Metab. 78, 555–560 (1994)
S. Lefebvre, L. De Paepe, R. Abs, J. Rahier, P. Selvais, D. Maiter, Subcutaneous octreotide treatment of a growth hormone-releasing hormone-secreting bronchial carcinoid: superiority of continuous versus intermittent administration to control hormonal secretion. Eur. J. Endocrinol. 133, 320–324 (1995)
J.K. Platts, D.F. Child, P. Meadows, J.N. Harvey, Ectopic acromegaly. Postgrad. Med. J. 73, 349–351 (1997)
M.R. Drange, S. Melmed, Long-acting lanreotide induces clinical and biochemical remission of acromegaly caused by disseminated growth hormone-releasing hormone-secreting carcinoid. J. Clin. Endocrinol. Metab. 83, 3104–3109 (1998)
J. Krassowski, W. Zgliczynski, W. Jeske, S. Zgliczynski, Comment of long-acting lanreotide inducing clinical and biochemical remission of acromegaly caused by disseminated GHRH secreting carcinoid. J. Clin. Endocrinol. Metab. 84, 1761–1762 (1999)
N.H. Othman, S. Ezzat, K. Kovacs, E. Horvath, E. Poulin, H.S. Smyth, S.L. Asa, Growth hormone-releasing hormone (GHRH) and GHRH receptor (GHRH-R) isoform expression in ectopic acromegaly. Clin. Endocrinol. (Oxf.) 55, 135–140 (2001)
M. Bolanowski, J. Schopohl, M. Marciniak, M. Rzeszutko, K. Zatonska, J. Daroszewski, A. Milewicz, J. Malczewska, R. Badowski, Acromegaly due to GHRH-secreting large bronchial carcinoid. Complete recovery following tumor surgery. Exp. Clin. Endocrinol. Diabetes 110, 188–192 (2002)
A. Bhansali, S.S. Rana, S. Bhattacharya, R. Muralidharan, R.J. Dash, A.K. Banerjee, Acromegaly: a rare manifestation of bronchial carcinoid. Asian Cardiovasc. Thorac. Ann. 10, 273–274 (2002)
G. Osella, F. Orlandi, P. Caraci, M. Ventura, D. Deandreis, M. Papotti, M. Bongiovanni, A. Angeli, M. Terzolo, Acromegaly due to ectopic secretion of GHRH by bronchial carcinoid in a patient with empty sella. J. Endocrinol. Invest. 26, 163–169 (2003)
V. Reuters, E. Dias, M.S.R.S. Pupo, M.R. Gadelha, Ectopic growth hormone-releasing hormone-secreting bronchial carcinoid cured after pneumectomy. Endocrinologist 13, 376–379 (2003)
K. Athanassiadi, D. Exarchos, S. Tsagarakis, I. Bellenis, Acromegaly caused by ectopic growth hormone-releasing hormone secretion by a carcinoid bronchial tumor: a rare entity. J. Thorac. Cardiovasc. Surg. 128, 631–632 (2004)
M.B. Fessler, C.D. Cool, Y.E. Miller, M.I. Schwarz, K.K. Brown, Idiopathic diffuse hyperplasia of pulmonary neuroendocrine cells in a patient with acromegaly. Respirology 9, 274–277 (2004)
O. Morel, P. Giraud, M.O. Bernier, J.J. Le Jeune, V. Rohmer, P. Jallet, Ectopic acromegaly: localization of the pituitary growth hormone-releasing hormone producing tumor by In-111 pentetreotide scintigraphy and report of two cases. Clin. Nucl. Med. 29, 841–843 (2004)
M.C. Zatelli, P. Maffei, D. Piccin, C. Martini, F. Rea, D. Rubello, A. Margutti, M.D. Culler, N. Sicolo, E.C. degli Uberti, Somatostatin analogs in vitro effects in a growth hormone-releasing hormone-secreting bronchial carcinoid. J. Clin. Endocrinol. Metab. 90, 2104–2109 (2005)
C. Nasr, A. Mason, M. Mayberg, S.M. Staugaitis, S.L. Asa, Acromegaly and somatotroph hyperplasia with adenomatous transformation due to pituitary metastasis of a growth hormone-releasing hormone-secreting pulmonary endocrine carcinoma. J. Clin. Endocrinol. Metab. 91, 4776–4780 (2006)
N.R. Biermasz, J.W. Smit, A.M. Pereira, M. Frolich, J.A. Romijn, F. Roelfsema, Acromegaly caused by growth hormone-releasing hormone-producing tumors: long-term observational studies in three patients. Pituitary 10, 237–249 (2007)
P. Fainstein Day, L. Frohman, H. Garcia Rivello, J.C. Reubi, G. Sevlever, M. Glerean, T. Fernandez Gianotti, M. Pietrani, A. Rabadan, S. Racioppi, M. Bidlingmaier, Ectopic growth hormone-releasing hormone secretion by a metastatic bronchial carcinoid tumor: a case with a non hypophysial intracranial tumor that shrank during long acting octreotide treatment. Pituitary 10, 311–319 (2007)
C.M. de Jager, L.J. de Heide, G. van den Berg, A. Wolthuis, W.D. van Schelven, Acromegaly caused by a growth hormone-releasing hormone secreting carcinoid tumour of the lung: the effect of octreotide treatment. Neth. J. Med. 65, 263–266 (2007)
M. van Hoek, L.J. Hofland, Y.B. de Rijke, F.H. van Nederveen, R.R. de Krijger, P.M. van Koetsveld, S.W. Lamberts, A.J. van der Lely, W.W. de Herder, R.A. Feelders, Effects of somatostatin analogs on a growth hormone-releasing hormone secreting bronchial carcinoid, in vivo and in vitro studies. J. Clin. Endocrinol. Metab. 94, 428–433 (2009)
P.W. Butler, C.S. Cochran, M.J. Merino, D.M. Nguyen, D.S. Schrump, P. Gorden, Ectopic growth hormone-releasing hormone secretion by a bronchial carcinoid tumor: clinical experience following tumor resection and long-acting octreotide therapy. Pituitary 15, 260–265 (2012)
E. Verrua, C.L. Ronchi, E. Ferrante, D.I. Ferrari, S. Bergamaschi, S. Ferrero, M.C. Zatelli, V. Branca, A. Spada, P. Beck-Peccoz, A.G. Lania, Acromegaly secondary to an incidentally discovered growth-hormone-releasing hormone secreting bronchial carcinoid tumour associated to a pituitary incidentaloma. Pituitary 13, 289–292 (2010)
T. Gudbjartsson, B.A. Agnarsson, P.S. Palsson, A. Johannesson, Acromegaly caused by ectopic growth hormone-releasing hormone production from a bronchial carcinoid tumor. Thorac. Cardiovasc. Surg. 59, 184–185 (2011)
L. Garby, P. Caron, F. Claustrat, P. Chanson, A. Tabarin, V. Rohmer, G. Arnault, F. Bonnet, O. Chabre, S. Christin-Maitre, H. du-Boullay, A. Murat, I. Nakib, J.L. Sadoul, G. Sassolas, B. Claustrat, G. Raverot, F. Borson-Chazot, Clinical Characteristics and Outcome of Acromegaly Induced by Ectopic Secretion of Growth Hormone-Releasing Hormone (GHRH): a French Nationwide Series of 21 Cases. J. Clin. Endocrinol. Metab. 97, 2093–2104 (2012)
R.H. Caplan, L. Koob, R.M. Abellera, A.S. Pagliara, K. Kovacs, R.V. Randall, Cure of acromegaly by operative removal of an islet cell tumor of the pancreas. Am. J. Med. 64, 874–882 (1978)
M.O. Thorner, R.L. Perryman, M.J. Cronin, A.D. Rogol, M. Draznin, A. Johanson, W. Vale, E. Horvath, K. Kovacs, Somatotroph hyperplasia. Successful treatment of acromegaly by removal of a pancreatic islet tumor secreting a growth hormone-releasing factor. J. Clin. Invest. 70, 965–977 (1982)
G. Berger, J. Trouillas, B. Bloch, G. Sassolas, F. Berger, C. Partensky, J.A. Chayvialle, P. Brazeau, B. Claustrat, F. Lesbros et al., Multihormonal carcinoid tumor of the pancreas. Secreting growth hormone-releasing factor as a cause of acromegaly. Cancer 54, 2097–2108 (1984)
J.L. Ch’ng, N.D. Christofides, M.E. Kraenzlin, A. Keshavarzian, J.M. Burrin, I.L. Woolf, H.J. Hodgson, S.R. Bloom, Growth hormone secretion dynamics in a patient with ectopic growth hormone-releasing factor production. Am. J. Med. 79, 135–138 (1985)
H.M. Schulte, G. Benker, R. Windeck, T. Olbricht, D. Reinwein, Failure to respond to growth hormone releasing hormone (GHRH) in acromegaly due to a GHRH secreting pancreatic tumor: dynamics of multiple endocrine testing. J. Clin. Endocrinol. Metab. 61, 585–587 (1985)
D.M. Wilson, G.P. Ceda, D.G. Bostwick, R.J. Webber, J.R. Minkoff, A. Pont, R.L. Hintz, K.G. Bensch, F.B. Kraemer, R.G. Rosenfeld et al., Acromegaly and Zollinger–Ellison syndrome secondary to an islet cell tumor: characterization and quantification of plasma and tumor human growth hormone-releasing factor. J. Clin. Endocrinol. Metab. 59, 1002–1005 (1984)
J.A. Ramsay, K. Kovacs, S.L. Asa, M.J. Pike, M.O. Thorner, Reversible sellar enlargement due to growth hormone-releasing hormone production by pancreatic endocrine tumors in a acromegalic patient with multiple endocrine neoplasia type I syndrome. Cancer 62, 445–450 (1988)
R. Yamasaki, H. Saito, T. Sano, K. Kameyama, K. Yoshimoto, E. Hosoi, M. Matsumura, K. Harada, S. Saito, Ectopic growth hormone-releasing hormone (GHRH) syndrome in a case with multiple endocrine neoplasia type I. Endocrinol. Jpn. 35, 97–109 (1988)
A. Sasaki, S. Yumita, S. Kimura, Y. Miura, K. Yoshinaga, Immunoreactive corticotropin-releasing hormone, growth hormone-releasing hormone, somatostatin, and peptide histidine methionine are present in adrenal pheochromocytomas, but not in extra-adrenal pheochromocytoma. J. Clin. Endocrinol. Metab. 70, 996–999 (1990)
D.E. Price, S.R. Absalom, K. Davidson, A. Bolia, P.R. Bell, T.A. Howlett, A case of multiple endocrine neoplasia: hyperparathyroidism, insulinoma, GRF-oma, hypercalcitoninaemia and intractable peptic ulceration. Clin. Endocrinol. (Oxf.) 37, 187–188 (1992)
Y. Shintani, K. Yoshimoto, H. Horie, T. Sano, Y. Kanesaki, E. Hosoi, Y. Yokogoshi, H. Bando, H. Iwahana, S. Kannuki et al., Two different pituitary adenomas in a patient with multiple endocrine neoplasia type 1 associated with growth hormone-releasing hormone-producing pancreatic tumor: clinical and genetic features. Endocr. J. 42, 331–340 (1995)
S.W. Liu, C.J. van de Velde, J.M. Heslinga, J. Kievit, F. Roelfsema, Acromegaly caused by growth hormone-relating hormone in a patient with multiple endocrine neoplasia type I. Jpn. J. Clin. Oncol. 26, 49–52 (1996)
S. Kawa, T. Ueno, A. Iijima, T. Midorikawa, Y. Fujimori, M. Tokoo, H. Oguchi, K. Kiyosawa, Y. Imai, G. Kaneko, T. Kuroda, K. Hashizume, R.Y. Osamura, H. Katakami, Growth hormone-releasing hormone (GRH)-producing pancreatic tumor with no evidence of multiple endocrine neoplasia type 1. Dig. Dis. Sci. 42, 1480–1485 (1997)
T. Sano, R. Yamasaki, H. Saito, T. Hirose, E. Kudo, K. Kameyama, K. Hiraishi, S. Saito, K. Hizawa, Growth hormone-releasing hormone (GHRH)-secreting pancreatic tumor in a patient with multiple endocrine neoplasia type I. Am. J. Surg. Pathol. 11, 810–819 (1987)
K. Suga, N. Yamashita, K. Chiba, T. Ito, Y. Kaziwara, N. Yokoyama, Multiple endocrine neoplasia type 1 producing growth hormone-releasing factor in an endocrine pancreatic tumor. Acta. Med. Nagasaki. 47, 55–61 (2002)
L. Agha, P. Farrell, P. Downey, P. Keeling, E. Leen, S. Sreenan, Acromegaly secondary to growth hormone releasing hormone secretion. Ir. J. Med. Sci. 173, 215–216 (2004)
H. Sugihara, T. Shibasaki, A. Tatsuguchi, F. Okajima, S. Wakita, Y. Nakajima, K. Tanimura, H. Tamura, S. Ishii, J. Kamegai, H. Akasu, W. Kitagawa, K. Shimizu, Y. Nakamura, E. Uchida, T. Tajiri, Z. Naito, H. Katakami, S. Oikawa, A non-acromegalic case of multiple endocrine neoplasia type 1 accompanied by a growth hormone-releasing hormone-producing pancreatic tumor. J. Endocrinol. Invest. 30, 421–427 (2007)
D.E. Weiss, H. Vogel, M.B. Lopes, S.D. Chang, L. Katznelson, Ectopic acromegaly due to a pancreatic neuroendocrine tumor producing growth hormone-releasing hormone. Endocr. Pract. 17, 79–84 (2011)
S.A. Leveston, D.W. McKeel Jr, P.J. Buckley, K. Deschryver, M.H. Greider, B.M. Jaffe, W.H. Daughaday, Acromegaly and Cushing’s syndrome associated with a foregut carcinoid tumor. J. Clin. Endocrinol. Metab. 53, 682–689 (1981)
K. von Werder, O.A. Muller, R. Hartl, M. Losa, G.K. Stalla, Growth hormone releasing factor (hpGRF)-stimulation test in normal controls and acromegalic patients. J. Endocrinol. Invest. 7, 185–191 (1984)
A. Van den Bruel, J. Fevery, J. Van Dorpe, L. Hofland, R. Bouillon, Hormonal and volumetric long term control of a growth hormone-releasing hormone-producing carcinoid tumor. J. Clin. Endocrinol. Metab. 84, 3162–3169 (1999)
J. Furrer, A. Hattenschwiler, P. Komminoth, T. Pfammatter, P. Wiesli, Carcinoid syndrome, acromegaly, and hypoglycemia due to an insulin-secreting neuroendocrine tumor of the liver. J. Clin. Endocrinol. Metab. 86, 2227–2230 (2001)
T.J. Altstadt, B. Azzarelli, C. Bevering, J. Edmondson, P.B. Nelson, Acromegaly caused by a growth hormone-releasing hormone-secreting carcinoid tumor: case report. Neurosurgery 50, 1356–1359 (2002). discussion 1360
N. Colak Ozbey, Y. Kapran, A. Bozbora, Y. Erbil, C. Tascioglu, S.L. Asa, Ectopic growth hormone-releasing hormone secretion by a neuroendocrine tumor causing acromegaly: long-term follow-up results. Endocr. Pathol. 20, 127–132 (2009)
J.O. Jansson, J. Svensson, B.A. Bengtsson, L.A. Frohman, H. Ahlman, B. Wangberg, O. Nilsson, M. Nilsson, Acromegaly and Cushing’s syndrome due to ectopic production of GHRH and ACTH by a thymic carcinoid tumour: in vitro responses to GHRH and GHRP-6. Clin. Endocrinol. (Oxf.) 48, 243–250 (1998)
E. Boix, A. Pico, R. Pinedo, I. Aranda, K. Kovacs, Ectopic growth hormone-releasing hormone secretion by thymic carcinoid tumour. Clin. Endocrinol. (Oxf.) 57, 131–134 (2002)
K.A. Roth, D.M. Wilson, J. Eberwine, R.I. Dorin, K. Kovacs, K.G. Bensch, A.R. Hoffman, Acromegaly and pheochromocytoma: a multiple endocrine syndrome caused by a plurihormonal adrenal medullary tumor. J. Clin. Endocrinol. Metab. 63, 1421–1426 (1986)
L. Vieira Neto, G.F. Taboada, L.L. Correa, J. Polo, A.F. Nascimento, L. Chimelli, K. Rumilla, M.R. Gadelha, Acromegaly secondary to growth hormone-releasing hormone secreted by an incidentally discovered pheochromocytoma. Endocr. Pathol. 18, 46–52 (2007)
H.J. Southgate, G.P. Archbold, M.E. el-Sayed, J. Wright, V. Marks, Ectopic release of GHRH and ACTH from an adenoid cystic carcinoma resulting in acromegaly and complicated by pituitary infarction. Postgrad. Med. J. 64, 145–148 (1988)
A. Matsuno, H. Katakami, N. Sanno, Y. Ogino, R.Y. Osamura, S. Matsukura, N. Shimizu, T. Nagashima, Pituitary somatotroph adenoma producing growth hormone (GH)-releasing hormone (GHRH) with an elevated plasma GHRH concentration: a model case for autocrine and paracrine regulation of GH secretion by GHRH. J. Clin. Endocrinol. Metab. 84, 3241–3247 (1999)
Pacak, K., Timmers, H.J.L.M., Eisenhofer, G.: Pheochromocytoma. In: Jameson, J.L., DeGroot, L.J., Series (Eds) Pheochromocytoma, 1990-2018. 2010
H. Saito, T. Sano, R. Yamasaki, S. Mitsuhashi, E. Hosoi, S. Saito, Demonstration of biological activity of a growth hormone-releasing hormone-like substance produced by a pheochromocytoma. Acta Endocrinol (Copenh) 129, 246–250 (1993)
H.P. Neumann, C. Eng, The approach to the patient with paraganglioma. J. Clin. Endocrinol. Metab. 94, 2677–2683 (2009)
W.F. Young Jr, Paragangliomas: clinical overview. Ann. N. Y. Acad. Sci. 1073, 21–29 (2006)
M.L. Brown, G.E. Zayas, M.D. Abel, W.F. Young Jr, H.V. Schaff, Mediastinal paragangliomas: the mayo clinic experience. Ann. Thorac. Surg. 86, 946–951 (2008)
D. Erickson, Y.C. Kudva, M.J. Ebersold, G.B. Thompson, C.S. Grant, J.A. van Heerden, W.F. Young Jr, Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. J. Clin. Endocrinol. Metab. 86, 5210–5216 (2001)
K.P. Koopmans, P.L. Jager, I.P. Kema, M.N. Kerstens, F. Albers, R.P. Dullaart, 111In-octreotide is superior to 123I-metaiodobenzylguanidine for scintigraphic detection of head and neck paragangliomas. J. Nucl. Med. 49, 1232–1237 (2008)
A.N. Van Der Horst-Schrivers, P.L. Jager, H.M. Boezen, J.P. Schouten, I.P. Kema, T.P. Links, Iodine-123 metaiodobenzylguanidine scintigraphy in localising phaeochromocytomas–experience and meta-analysis. Anticancer Res. 26, 1599–1604 (2006)
H.J. Timmers, A. Kozupa, C.C. Chen, J.A. Carrasquillo, A. Ling, G. Eisenhofer, K.T. Adams, D. Solis, J.W. Lenders, K. Pacak, Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J. Clin. Oncol. 25, 2262–2269 (2007)
K.S. King, C.C. Chen, D.K. Alexopoulos, M.A. Whatley, J.C. Reynolds, N. Patronas, A. Ling, K.T. Adams, P. Xekouki, H. Lando, C.A. Stratakis, K. Pacak, Functional imaging of SDHx-related head and neck paragangliomas: comparison of 18F-fluorodihydroxyphenylalanine, 18F-fluorodopamine, 18F-fluoro-2-deoxy-d-glucose PET, 123I-metaiodobenzylguanidine scintigraphy, and 111In-pentetreotide scintigraphy. J. Clin. Endocrinol. Metab. 96, 2779–2785 (2011)
G. Treglia, P. Castaldi, G. Rindi, A. Giordano, V. Rufini, Diagnostic performance of Gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: a meta-analysis. Endocrine 42, 80–87 (2012)
K. Oberg, Gallium-68 somatostatin receptor PET/CT: is it time to replace (111)Indium DTPA octreotide for patients with neuroendocrine tumors? Endocrine 42, 3–4 (2012)
K. von Werder, M. Losa, O.A. Muller, L. Schweiberer, R. Fahlbusch, E. Del Pozo, Treatment of metastasising GRF-producing tumour with a long-acting somatostatin analogue. Lancet 2, 282–283 (1984)
S. Genka, H. Soeda, M. Takahashi, H. Katakami, N. Sanno, Y. Osamura, T. Fuchinoue, A. Teramoto, Acromegaly, diabetes insipidus, and visual loss caused by metastatic growth hormone-releasing hormone-producing malignant pancreatic endocrine tumor in the pituitary gland. Case report. J. Neurosurg. 83, 719–723 (1995)
I. Shimon, M. Hadani, D. Nass, S.T. Zwas, Malignant bronchial carcinoid tumor metastatic to the pituitary in a thyroid carcinoma patient: successful treatment with surgery, radiotherapy and somatostatin analog. Pituitary 7, 51–57 (2004)
A. Giustina, A. Barkan, F.F. Casanueva, F. Cavagnini, L. Frohman, K. Ho, J. Veldhuis, J. Wass, K. Von Werder, S. Melmed, Criteria for cure of acromegaly: a consensus statement. J. Clin. Endocrinol. Metab. 85, 526–529 (2000)
A. Giustina, P. Chanson, M.D. Bronstein, A. Klibanski, S. Lamberts, F.F. Casanueva, P. Trainer, E. Ghigo, K. Ho, S. Melmed, A consensus on criteria for cure of acromegaly. J. Clin. Endocrinol. Metab. 95, 3141–3148 (2010)
R.M. Huber, J. Schopohl, M. Losa, G. Wolfram, O. Thetter, W. Permanetter, K. v Werder, Growth-hormone releasing hormone in a bronchial carcinoid. Cancer 67, 2538–2542 (1991)
J. Rothenstein, S.P. Cleary, G.R. Pond, D. Dale, S. Gallinger, M.J. Moore, J. Brierley, L.L. Siu, Neuroendocrine tumors of the gastrointestinal tract: a decade of experience at the Princess Margaret Hospital. Am. J. Clin. Oncol. 31, 64–70 (2008)
C. Mao, P. Carter, P. Schaefer, L. Zhu, J.M. Dominguez, D.J. Hanson, H.E. Appert, K. Kim, J.M. Howard, Malignant islet cell tumor associated with hypercalcemia. Surgery 117, 37–40 (1995)
K.K. Kazanjian, H.A. Reber, O.J. Hines, Resection of pancreatic neuroendocrine tumors: results of 70 cases. Arch. Surg. 141, 765–769 (2006). discussion 769–70
K.L. Yim, Role of biological targeted therapies in gastroenteropancreatic neuroendocrine tumours. Endocrine 40, 181–186 (2011)
K.I. Alexandraki, G. Kaltsas, Gastroenteropancreatic neuroendocrine tumors: new insights in the diagnosis and therapy. Endocrine 41, 40–52 (2012)
Acknowledgments
This work was supported by National Institutes of Health Grant K23DK085148 (O.C.) and Grant CA75979 (S.M.).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghazi, A.A., Amirbaigloo, A., Dezfooli, A.A. et al. Ectopic acromegaly due to growth hormone releasing hormone. Endocrine 43, 293–302 (2013). https://doi.org/10.1007/s12020-012-9790-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9790-0