Abstract
Diabetes mellitus is a complex chronic disease associated with an absolute insulin deficiency in type 1 diabetes (T1D) and a progressive deterioration of β-cell function in type 2 diabetes (T2D). T2D pathophysiology has numerous defects including incretin deficiency/resistance. Gastrin has demonstrated to be an islet growth factor (like glucagon-like peptide-1, epidermal growth factor, transforming growth factor-α,…) and be able to restore a functional β-cell mass in diabetic animals. This hormone is likely to stimulate insulin secretion during an ordinary protein-rich meal, this is, to have an incretin-like effect. Proton pump inhibitors (PPIs) can raise serum gastrin concentration significantly and therefore, affect to glucose metabolism through promoting β-cell regeneration/expansion and also enhancing insulin secretion. The present paper aims to review studies concerning the effect of PPIs on glucose metabolism. Several research groups have recently explored the potential role of this class of drugs on glycemic control, mainly in T2D. The results show antidiabetic properties for the PPIs with a global glucose-lowering power around 0.6–0.7 % points of HbA1c, but the level of evidence for the available literature is still not high. If these data start to become demonstrated in the ongoing clinical trials, PPIs could become a new antidiabetic agent with a good and safe profile for T2D and even useful for T1D, particularly in the area of islet transplantation to preserve β-cell mass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of diabetes mellitus is 366 million globally (8.3 %), by 2030 this will have risen to 552 million (9.9 %), nearly 95 % of the cases will be type 2 diabetes (T2D) [1]. These predictions indicate a growing burden of diabetes, particularly in developing countries [2]. We face a worldwide epidemic.
T2D is a complex disease, pathophysiology of which includes not only the classical triumvirate—insulin resistance in muscle and liver and progressive β-cell failure—but at least the ominous octet [3]: accelerated lipolysis, incretin deficiency/resistance, hyperglucagonemia, renal increased glucose reabsorption, and brain insulin resistance. At the end, hyperglycemia in both type 1 diabetes (T1D) and T2D results from an absolute or relative deficit in the pancreatic β-cell mass; therefore, β-cell regeneration is an area under active investigation.
The natural history of T2D implies that more than one medication will be necessary for most patients over time and effective treatment requires multiple drugs used in combination to correct multiple pathophysiological defects. Choices of therapies are usually made according to efficacy, safety, tolerability, cost of medications, simplicity, and anticipated degree of patient adherence. Furthermore, it should be noted that some associations have proven safe, but others are not recommended, and other long-term safety is unknown [4], so the therapeutic arsenal is not as broad as it seems and any new therapeutic strategy that takes into account the above features would be welcomed.
Gastrin is the major endocrine regulator of the acid secretory response to a protein meal [5] and has been said to have trophic effects on β-cell mass [6–8]. There is a negative feedback loop between gastric acid and gastrin release.
Proton pump inhibitors (PPIs) are drugs used to treat symptoms of acid-related disorders and for primary prevention of gastroduodenal toxicity [9]. They decrease secretion of acid and therefore produce a modest hypergastrinemia [10], so this effect could be associated with better glycemic control. Based on this hypothesis, a few groups [11–13] have analyzed whether treatment with PPIs is associated with better glycemic control in T2D with positive and similar results in different clinical contexts.
This review examines the in vitro and in vivo evidence of PPIs on glucose metabolism.
Proton pump inhibitors. How they act?
The introduction of PPIs in the late 1980s optimized the medical treatment of acid-related disorders, mainly gastroesophageal reflux disease. Other indicated uses include the treatment and prevention of upper gastrointestinal tract ulcers from nonsteroidal antiinflammatory drugs, as part of therapeutic regimens for eradication of Helicobacter pylori infection, in the management of patients with bleeding peptic ulcer and in the management of functional dyspepsia [14].
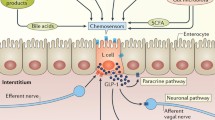
Gastric acid secretion is a complex process regulated by at least three types of receptors on the parietal cell: histamine, gastrin, and acetylcholine [15]. Release of histamine by gastrin from enterochromaffin-like (ECL) cell appears to be the major physiologic mechanism [16], although parietal cells also have gastrin receptors. In addition, the ECL cells integrate stimulatory messages from cholinergic nerves and inhibition by locally released somatostatin (Fig. 1).
Hydrogen ions are actively secreted in exchange for potassium ions by means of an H–K–ATPase, the so-called proton pump located on the apical surface of the parietal cell. H–K–ATPase comprises the final pathway that has been triggered by the stimulation of one or more receptors, by which HCl is secreted into the gastric lumen. There gastric acid serves both to hydrolyze dietary protein and maintain a sterile environment.
PPIs inhibit the final step of acid secretion, the proton pump. All PPIs are prodrugs, and have to be protected against acid degradation. After oral administration of PPIs peak plasma concentrations are achieved within 2–4 h. When PPIs reach the acidic environment of the parietal cells they are entrapped and form an irreversible bond with the hydrogen potassium ATPase, inhibiting its action [17]. Because of the amount of H–K–ATPase present in the parietal cell is greatest after a prolonged fast, PPIs should be administered before the first meal of the day. In most individuals, once-daily dosing is sufficient to produce the desired level of acid inhibition.
There are currently six PPIs available on the market: omeprazole, esomeprazole, lansoprazole, pantoprazole, rabeprazole, and dexlansoprazole. They share a similar core structure, but they differ according to the chemical stability under acidic and neutral pH. Rabeprazole possesses a slightly faster onset of action, while pantoprazole is often touted as being the most “gastro-specific." However, whether these differences in chemical structure translate into differences in clinical efficiency is matter of debate [18].
PPIs are metabolized via hepatic cytochrome P450 enzymes, with CYP2C19 having the dominant role. Pantoprazole has the lowest potential for P450 metabolism and drug interactions. Interactions are uncommon, except for PPIs and clopidrogrel—omeprazole specially—but the recent literature has suggested that there may not be a clinically meaningful interaction [19].
The long-term safety of these drugs has been best established with omeprazole, since it was the first to become available clinically. These data suggest that its use for more than 15 years is safe, although some risks have been described [20]. Hypochlorhydria is of concern because may predispose to infections and malabsorption. Chronic PPI therapy has been associated with Clostridium difficile infection, community-acquired pneumonia, and calcium malabsorption resulting in osteoporosis and increased fracture risk, but with reservations in some aspects because of lack of good methodological studies [21]. There is a risk of hypomagnesemia in patients who have been on PPIs long-term [22] and long-term therapy with omeprazole has been associated with vitamin B12 malabsorption [23].
The secondary hypergastrinemia induced by PPIs created a concern about gastric carcinoid tumors. However, long-term omeprazole therapy (up to 11 years) has shown to be safe [24], but because of this consequence of acid suppression there is a possibility of rebound acid hypersecretion when stopping PPIs after prolonged use [14]. A small risk of atrophic gastritis has been reported [20].
The hormone gastrin was an early incretin candidate
The main production site of gastrin is the antral G-cells, where is synthesized as gatrin-17 (the predominant form) and gastrin-34, also other gastrins are synthesized but in small quantities, in response to a number of luminal stimuli, including the presence of amino acids and dietary amines [25, 26].
Fetal and neonatal G-cells in pancreatic islets produce only sulphated gastrin-17 [27].
The gastrin receptor is the predominant receptor for gastrin and cholecystokinin (CCK) peptides in the central nervous system. It is abundantly expressed on ECL-cells in the stomach [28]. There are data consistent with the view that growth control by gastrin and expression of gastrin/CCK-B receptors is present in fetal and regenerating pancreas [29, 30]. But the expression of this receptor in the pancreas is species-specific [31]; there is an abundant islet cell expression in man and pig, whereas CCK receptor is more abundant in rodents and dogs. Therefore, results on CCK and gastrin obtained from rodents and dogs do not necessarily apply to humans.
Gastrin was an early incretin candidate [32–34], since it was released by oral glucose and potentiates the glucose-induced insulin secretion [35]. However, the amounts of gastrin released by oral glucose were too low to stimulate β-cell secretion significantly, and the interest of gastrin in incretin research decreased for decades until recent studies have shown that interaction between gut hormones, including gastrin, is able to restore normoglycemia in diabetic mice.
But gastrin had shown their effect on insulin secretion several times in old studies [31]. Exogenous gastrin-17 is a potent insulin-releaser together with intravenous glucose and during an ordinary protein-rich meal is likely to stimulate the secretion of insulin significantly. Moreover, studies in endogenous hypergastrinemia support an incretin effect of gastrin in man.
The lack of consideration for the incretin effect of this hormone during decades was motivated by overlooking at least two problems [31]: (1) oral overload of glucose is unphysiological, meals are mixtures of many components that release more than a hundred hormonal bioactive intestinal peptides, (in addition to glucose-dependent insulinotropic peptide—GIP—and glucagon-like peptide-1—GLP-1), while gastrin is mainly delivered by proteins; (2) incretins often have multiple targets, this mean multiple functions, and may have important interactive effects. For instance, GLP-1 in high doses is without effect on pancreatic islet-cell and hyperglycemia in NOD mice. However, when GLP-1 is combined with small doses of gastrin, the β cells grow, insulin is secreted, and the mice become normoglycemic [36].
Gastrin stimulates β-cell neogenesis: evidence from basic research
In the area of pancreatic islet transplantation and regenerative medicine, mechanisms that regulate the pancreatic β-cell mass require deep understanding. Many stimuli influence β-cell neogenesis from pancreatic duct cells in vitro and in vivo. These include growth factors, such as transforming growth factor-α (TGF-α), epidermal growth factor (EGF), and keratinocyte growth factor [37–40]. Gastrointestinal peptides, such as GLP-1 [41] and gastrin [6] can also stimulate β-cell neogenesis.
Rooman et al. [6] reported that a 3-day continuous intravenous gastrin administration further increases expansion of the β-cell mass in the ligated pancreas of the rat and this occurs by the process of neogenesis. In the model of pancreatic duct ligation, no histological changes occur in the unligated part; in the ligated part, exocrine acinar lobules are replaced by ductal complexes and new β-cells are generated from the latter [42]. Duct-like cells start to express TGF-α, gastrin [43], and the high-affinity gastrin/CCK-B receptor, which is not expressed on β-cells [30]. Cells in these ductal complexes proliferate and can differentiate into endocrine cells [42]. Infusion of gastrin from day 7–10 postligation resulted in doubling of the β-cell mass in the ligated part as measured by morphometry. This robust hyperplasia cannot be accounted for by β-cell self-replication, as the fraction of β-cells being engaged in the cell cycle remains very low. This means that gastrin extends the process of neogenesis that was already induced by the procedure of duct ligation. Administration of a selective CCK-B gastrin receptor antagonist completely prevents the duct ligation-induced increase in β-cell mass [44], so gastrin is a crucial factor in the regulation of β-cell neogenesis during regeneration.
Xu et al. [45] reported endocrine differentiation as a result of endogenous hypergastrinemia in a model of subtotal pancreatectomy.
Interesting information concerning regenerative expansion of β-cells has been obtained from various transgenic mouse models. TGF-α overexpressing transgenic mice develop metaplastic acinoductal complexes in the pancreas and show signs of tissue inflammation [46]. When these mice are crossed with transgenic mice overexpressing gastrin hormone under control of the insulin promoter, the double transgenic TGF-α/gastrin mice were found to contain significantly more β-cells (approximately twice over the control group) [47].
Combination therapy with EGF and gastrin increases β-cell mass and reduces hyperglycemia in streptozotocin–diabetic rats [48] and induces islet regeneration and restores normoglycemia in mice treated with alloxan [49]. In this last model, a β-cell growth rate of 30 % per day was observed. Although there was no complete regeneration of the original β-cell number after subtotal destruction, regenerative growth induced by the gastrin and EGF treatment led to the restoration of 30–40 % of the normal β-cell mass within 7 days. The treatment had no effect on β-cell replication, cell size, or apoptosis, and therefore, the regenerative effect could be attributed to neogenesis from precursor cells.
The combination of these two growth factors, EGF and gastrin, also induces neogenesis of adult human islet β-cells from pancreatic duct cells and an increase in functional β-cell mass [8]. EGF stimulated the proliferation of CK19-positive duct cells, whereas gastrin induced the expression of the transcription factor PDX-1 in these duct cells and their differentiation into insulin-positive β-cells. The findings are consonant with the different roles that EGF and gastrin have in islet development.
The gastrointestinal incretin hormone GLP-1 has been repeatedly reported to affect β-cell function, replication, apoptosis, and neogenesis [50, 51]. Suarez-Pinzon et al. [52] demonstrated that combination therapy with GLP-1 and gastrin expands the β-cell mass in human islets implanted in immunodeficient diabetic mice, largely from pancreatic duct cells associated with the islets, and this was sufficient to ameliorate hyperglycemia in the mice. Correction of hyperglycemia was accompanied by increased insulin content in the human pancreatic cell grafts as well as by increased plasma levels of human C-peptide. Immunocytochemical examination revealed a fourfold increase in insulin-positive cells in the human pancreatic cell grafts in mice treated with GLP-1 and gastrin, and most of this increase was accounted for the appearance of CK19-positive pancreatic duct cells expressing insulin.
Finally, specific treatment with a PPI to raise endogenous levels of gastrin in several animal models has shown an improvement in glycemic control.
Suarez-Pinzon et al. [53] demonstrated that combination therapy with a DPP-4 inhibitor to raise endogenous levels of GLP-1, together with a PPI could reverse diabetes in the NOD mouse model of T1D. Treatment with DPP-4 inhibitor restored normoglycemia in 38 % of mice, PPI in 33 %, and combination of DPP-4 inhibitor and PPI in 75 % of animals. Treatments with a single agent did not significantly increase plasma C-peptide or pancreatic insulin content, whereas combined treatment significantly did.
This combination drug therapy, specifically sitagliptin and pantoprazole, induces β-cell neogenesis in adult human pancreatic cells implanted in NOD-severe combined immunodeficient mice. The increased β-cell mass appears to be derived from pancreatic exocrine duct cells [54].
PPIs have also demonstrated a positive effect on glycemic control in a model of type 2 diabetes—Psammomys obesus [55].
In summary, gastrin can be considered as an important regulator of β-cell neogenesis. Neogenesis by the process of transdifferentiation and/or stem cells appears to be activated only after severe injury to endocrine or exocrine pancreatic tissue, or when strong additional stimuli like GLP-1 and/or gastrin are provided. β-Cell self-replication can also be increased by certain stimuli like glucose and other nutrients, insulin and several growth factors, but it apparently cannot lead to an efficient regeneration [56]. Clinical interpretation of β-cell regenerative therapies is limited because of these studies have been almost carried out in rodent models of diabetes or under in vitro conditions.
Somatostatin, proton pump inhibitors and diabetes relationship
Somatostatin (SST) is an inhibitory hormone that regulates numerous biological processes, including insulin and glucagon secretion. SST secretion from islet δ-cells is stimulated by increased extracellular glucose, although the threshold concentration for δ-cells to respond to glucose is lower than for β-cells [57].
Several studies seem to show that administration of omeprazole decreased the antral SST content [58, 59]. In healthy men, administration of omeprazole 40 mg/day increased the antral gastrin content and decrease the antral somatostatin content significantly [58].
In a small interventional study with five healthy volunteers, who were given a single oral administration of omeprazole 20 mg, plasma somatostatin significantly increased after drug ingestion [60]. However, Stoschus et al. [61] found that treatment with omeprazole led to an increase in fasting and postprandial plasma concentrations of xenin, gastrin, and pepsinogens A and C, whereas somatostatin plasma levels remained unchanged.
Studies with animals, specifically in sheep, showed that gastrin can stimulate somatostatin independently of changes in gastric acidity [62] and omeprazole administration had no effect on plasma somatostatin, but antral somatostatin increased significantly [63]. Again species-specific receptor may be the key.
On the one hand, the rare somatostatinoma [64] and somatostatin analogs (SSA) used in acromegaly are followed sometimes by a glucose metabolism imbalance [65]. A recent meta analysis of acromegaly studies [66] shows that SSA decrease fasting serum insulin levels without consistent effects on glucose homeostasis. In most included studies, SSA did not induce any significant change in glucose metabolism, and only a few studies reported an increase in serum HbA1c and glucose during OGTT, whereas fasting plasma glucose was not significantly affected, so the clinical importance may be minor. In fact, current guidelines suggest that the reduction in GH levels usually achieved with SSA tends to outweigh any effect on β-cell function and leads to an overall improvement in insulin resistance [67].
On the other hand, further areas for development of SSA therapy include obesity and diabetes mellitus [68].The most developed thus far is the use of octreotide in hyperinsulinemic forms of obesity [69], SSA can retard weight gain in children with hypothalamic obesity and induce a small amount of weight loss in some adults with hyperinsulinemic obesity. The therapeutic potential of somatostatin receptor ligands to control the excess of pro-angiogenic factors in diabetes-associated retinal complications has also been tested [70]. Nevertheless, for the treatment of T2D, use of somatostatin per se would be counterproductive due to its broad spectrum of inhibitory actions, including, of course, inhibition of insulin secretion [71]. Considerable effort has been put forth over the years in identifying the receptor subtypes responsible for the various inhibitory actions of somatostatin, and some subtype-selective analogs have been identified [72]. However, there appears to be species-specific receptor and receptor subtype overlap. For instance, somatostatin receptor subtype 2 (SSTR2) appears to mediate the effects of somatostatin on GH [72] and inhibits the release of glucagon, but not that of insulin, via SSTR2 [73]. In isolated perfused human pancreas, infusion of SSTR2 agonist into the isolated perfused human pancreas resulted in a significant inhibition of insulin and C-peptide secretion [74].
It can be said based on these papers that the relationship between SST and PPIs is not clear at all, but some kind of relationship with the pancreas activity exists that needs necessarily further investigation.
Proton pump inhibitors delay gastric emptying: underlying mechanism?
The delayed emptying of solids due to PPI therapy may have clinical implications in the management of gastroesophageal reflux disease, functional dyspepsia, as well as diabetes. Sanaka et al. [75] reviewed several studies (from 1979 to 2009) that investigated the rate of gastric emptying in PPIs users. The underlying mechanisms for this effect remain mostly hypothetic. Gastric emptying of solids involves a process of peptic hydrolysis. PPIs impair the hydrolytic digestion by inhibiting acid-dependent peptic activity, thereby delaying the solid emptying. Gastric emptying of liquids largely depends on volume and energy density of intragastric contents. These medications variably modify the volume and the energy density by reducing gastric fluid secretion.
Hypergastrinemia has also been considered to delay gastric emptying, but it seems of minor importance.
Treatment with proton pump inhibitors improves glycemic control in humans: a retrospective look
There are few data about the effect of PPIs on glucose metabolism in humans. Below we are going to review them (Table 1).
First, Mefford et al. [11] compared HbA1c levels from type 2 diabetic patients taking a PPI (7.0 %) and type 2 diabetic patients not taking (7.6 %), obtaining significant differences. Then, they performed a sub-analysis based on diabetes treatment being aware of the risk the treatment groups become so small as to have lack of statistical power. The result was a trend to have lower means of HbA1c for the different antidiabetic therapies combined with a PPI, although as expected, without getting statistical significance except for the sulfonylureas group (in combination with or without metformin and/or thiazolidenedione) that got a reduction in HbA1c of 1.4 %—respect those patients who did not consume a PPI. Limitations of this study include, besides of the study design ones, the heterogeneity of the comparative groups.
After this, Boj-Carceller et al., personal communication—in a smaller study with diabetic in-patients with poor glycemic control (average HbA1c of 9.2 %), where 33.8 % had T1D, found that those who were using a PPI showed lower HbA1c levels, specially if they were T2D not receiving insulin treatment yet.
Hove et al. [13] published the results of a small case–control study conducted to investigate whether treatment with esomeprazole (prescribed for unspecified gastric acid related disorders with a mean duration of treatment of 11 months) improved HbA1c levels in a group of T2D patients receiving insulin as treatment. They found a borderline significant reduction of HbA1c by 0.7 % in patients with T2D treated with the PPI compared with a reduction of 0.2 % in the control group (matched for gender, age, duration of diabetes, treatment, and diabetic complications). Interestingly, in patients with poor glycemic control (HbA1c >9 %) the beneficial effects of esomeprazole were more pronounced. For these patients HbA1c dropped significantly by 1.2 % in the esomprazole-treated group (p = 0.004) compared with only 0.2 % in the control group (p = 0.287). Authors conclude since the control group with elevated HbA1c levels did not experience a significative decline in HbA1c, it is likely that the observation is related to the esomeprazole treatment, but due to the retrospective nature of this study, the results should be interpreted with caution.
Then, Boj-Carceller et al. [12] selected consecutive patients with T2D who had been admitted to hospital in any department, but excluding those with a diagnosis of hyperglycemic decompensation, diabetic onset or pregnancy. They compared HbA1c levels of patients taking a PPI and those not taking. HbA1c was significantly lower in individuals who took PPI: −0.6 % (p = 0.018), 95 % CI: −0.12 to −0.83. When they subdivided these two groups based on diabetes treatment, those taking insulin and concurrent PPI had better glycemic control; HbAc1 of −0.8 % points (p = 0.022), 95 % CI: −0.12 to −1.48, compared with those taking insulin but not the study drug. For the rest of comparisons, there was a lack of statistical significance, although the trend for lower HbA1c was constant in all groups taking a PPI.
It is interesting to note that patients retrieved for this study had good glycemic control. Previously, they had found similar results in diabetic in-patients with poor glycemic control. This may be an independent effect of the severity of disease.
The last paper has been published by Crouch et al. [76]. They reviewed electronic medical records of patients with T2D for whom PPIs were prescribed. Patients who were on insulin or prolonged corticosteroid therapy were excluded. Authors compared HbA1c during time periods on PPI therapy (7.1 %) versus time periods off PPI therapy (7.7 %). Unfortunately, they do not mention the mean duration of the PPI treatment (and they do not use appropriate tests for paired data in the statistical analysis.)
When considering confounding factors, the group of Hove et al. mentioned the possibility that general improved well-being caused by relief of gastro-intestinal symptoms could be beyond the results because their patients had been treated with esomeprazole due to unspecified gastric acid related disorders. From our experience many patients are receiving regular PPI treatment for poorly defined reasons or for conditions where PPIs have not been shown to be useful. Current evidence also suggests PPIs are often over used, only half of patients admitted to medical wards in a university hospital had an appropriate indication [77], so probably this third variable is not affecting significantly the results of transversal studies. Moreover, the effect could be underestimated because this drug has become so routine that people sometimes forget to declare its use during anamnesis.
All these studies are retrospective, as expected in the early stage of a research; it is the classical architecture: from general, exploratory studies, toward highly focused studies. Cross-sectional studies are cost-effective ones and case–control studies represent a step in the ability to prove causation but with more probability of bias. The uncontrolled before and after study design is relatively simple to conduct and superior to observational studies [78], but the study of Crouch and coworkers had a retrospective nature and that is not an authentic uncontrolled before and after study.
In conclusion, the level of evidence for the state of the art in this topic is not really high, nevertheless the consistence of the results—positive and with a similar magnitude in spite of the different context—points in a good direction.
Ongoing trials focus on the potential for proton pump inhibitors in the diabetes treatment
The available studies seem to support the hypothesis that PPIs are associated with better glycemic control in T2D with a global glucose-lowering effectiveness around 0.6–0.7 % points of HbA1c, though this idea has to be tested in randomized clinical trials to be the PPI accepted as a new class of anti-diabetic agent.
Currently, different groups are carrying out interventional studies [79–84] focus mainly on T1D and the potential role for PPIs to promote β-cell regeneration/expansion, in contrast with the retrospective studies that mostly heed T2D, although it is also being explored the effect on insulin secretion in patients with T2D treated with a particular PPI (Table 2).
Summary
It has been reviewed the sparse available literature to date developed to assess the new hypothesis that PPIs could be associated with better glycemic control in diabetes, mainly T2D.
All the studies found are retrospective, with their well-known intrinsic limitations, so causality cannot have been proved, but it was the logical beginning to explore a new field of research. Nevertheless, Bradford Hill “viewpoints” [85] for inferring causation are quite respected, specially: strength of association, consistency (similar results in terms of decrease of HbA1c (%) between different studies), plausibility (the mechanism would be through the secondary hypergastrinemia induced by PPIs), coherence (between epidemiological and laboratory findings), and analogy (H. pylori infection elevates serum gastrin and it has been told to lower fasting plasma glucose concentrations [86]).
The gastrin incretin effect does exist since during an ordinary protein-rich meal this hormone stimulates the secretion of insulin. Besides, PPIs may share most of the glucoregulatory effects of incretin-based therapies: potential for improvement of β-cell mass/function, slowing gastric emptying, no weight gain, and even adverse events such as no hypoglycemia or susceptibility to infections. Moreover, the glucose-lowering power seems to be inside the range of dipeptidyl peptidase-4 inhibitors (HbA1c reduction ~0.5–1 %) [87].
Nowadays, interventional trials are being performed to prove PPIs ability to promote β-cell regeneration/expansion in subjects with T1D and to study insulin secretion in T2D, so it is expected to get data of their impact on glucose metabolism from the strongest evidence in a near future.
References
International Diabetes Federation. Diabetes and impaired glucose tolerance: global burden: prevalence and projections, 2010 and 2030. International Diabetes Federation Website. http://www.idf.org/diabetesatlas/5e/the-global-burden (2011). Accessed 9 Apr 2012
J.E. Shaw, R.A. Sicree, P.Z. Zimmet, Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 87, 4–14 (2010)
R.A. Defronzo, Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58, 773–795 (2009)
Menéndez Torre E., Lafita Tejedor F.J., Artola Menéndez S., Millán Núñez-Cortés J., Alonso García Á., Puig Domingo M. et al. Working Group for Consensus and Clinical Guidelines of the Spanish Diabetes Society. Recommendations for the pharmacological treatment of hyperglycemia in type 2 diabetes. Endocrinol. Nutr. 58, 112–120 (2011)
G. Dockray, R. Dimaline, A. Varro, Gastrin: old hormone, new functions. Pflugers Arch. 449, 344–355 (2005)
I. Rooman, J. Lardon, L. Bouwens, Gastrin stimulates β-cell neogenesis and increases islet mass from transdifferentiated but not from normal exocrine pancreas tissue. Diabetes 51, 686–690 (2002)
W.L. Suarez-Pinzon, Y. Yan, R. Power, S.J. Brand, A. Rabinovitch, Combination therapy with epidermal growth factor and gastrin increases β-cell mass and reverses hyperglycemia in diabetic NOD mice. Diabetes 54, 2596–2601 (2005)
W.L. Suarez-Pinzon, J.R. Lakey, S.J. Brand, A. Rabinovitch, Combination therapy with epidermal growth factor and gastrin induces neogenesis of human islet β-cells from pancreatic duct cells and an increase in functional β-cell mass. J. Clin. Endocrinol. Metab. 90, 3401–3409 (2005)
E. Sheen, G. Triadafilopoulos, Adverse effects of long-term proton pump inhibitor therapy. Dig. Dis. Sci. 56, 931–950 (2011)
M.S. McDonagh, S. Carson, S. Thakurta, Drug Class Review: Proton Pump Inhibitors. Final Report Update 5 (Oregon Health and Science University, Portland, 2009)
I.N. Mefford, E.U. Wade, Proton pump inhibitors as a treatment method for type II diabetes. Med. Hypotheses 73, 29–32 (2009)
D. Boj-Carceller, P. Bocos-Terraz, M. Moreno-Vernis, A. Sanz-Paris, P. Trincado-Aznar, R. Albero-Gamboa, Are proton pump inhibitors a new antidiabetic drug? A cross sectional study. World J. Diabetes 2, 217–220 (2011)
K.D. Hove, K. Færch, T.B. Bödvarsdóttir, A.E. Karlsen, J.S. Petersen, A. Vaag, Treatment with a proton pump inhibitor improves glycaemic control in type 2 diabetic patients—a retrospective analysis. Diabetes Res. Clin. Pract. 90, e72–e74 (2010)
N. Parikh, C.W. Howden, The safety of drugs used in acid-related disorders and functional gastrointestinal disorders. Gastroenterol. Clin. N. Am. 39, 529–542 (2010)
M. Robinson, Review article: the pharmacodynamics and pharmacokinetics of proton pump inhibitors—overview and clinical implications. Aliment. Pharmacol. Ther. 20, 1–10 (2004)
M.M. Wolfe, A.H. Soll, The physiology of gastric acid secretion. N. Engl. J. Med. 319, 1707–1715 (1988)
M.M. Wolfe, G. Sachs, Acid suppression: optimizing therapy for gastroduodenal ulcer healing, gastroesophageal reflux disease, and stress-related erosive syndrome. Gastroenterology 118, S9–S31 (2000)
J. Horn, Review article: relationship between the metabolism and efficacy of proton pump inhibitors-focus on rabeprazole. Aliment. Pharmacol. Ther. 20, 11–19 (2004)
R.W. Harrison, K.W. Mahaffey, Clopidogrel and PPI interaction: clinically relevant or not? Curr. Cardiol. Rep. 14, 49–58 (2012)
A.S. Raghunath, C. O’Morain, R.C. McLoughlin, Review article: the long-term use of proton-pump inhibitors. Aliment. Pharmacol. Ther. 22, 55–63 (2005)
A.B. Thomson, M.D. Sauve, N. Kassam, H. Kamitakahara, Safety of the long-term use of proton pump inhibitors. World J. Gastroenterol. 16, 2323–2330 (2010)
J. Chen, Y.C. Yuan, G.I. Leontiadis, C.W. Howden, Recent safety concerns with proton pump inhibitors. J. Clin. Gastroenterol. 46, 93–114 (2012)
S.P. Marcuard, L. Albernaz, P.G. Khazanie, Omeprazole therapy causes malabsorption of cyanocobalamin (vitamin B12). Ann. Intern. Med. 120, 211–215 (1994)
E.C. Klinkenberg-Knol, F. Nelis, J. Dent, P. Snel, B. Mitchell, P. Prichard et al., Long-term omeprazole treatment in resistant gastroesophageal reflux disease: efficacy, safety, and influence on gastric mucosa. Gastroenterology 118, 661–669 (2000)
R.A. Gregory, H.J. Tracy, J.I. Harris, M.J. Runswick, S. Moore, G.W. Kenner et al., Minigastrin; corrected structure and synthesis. Hoppe Seylers Z. Physiol. Chem. 360, 73–80 (1979)
M.D. Burkitt, A. Varro, D.M. Pritchard, Importance of gastrin in the pathogenesis and treatment of gastric tumors. World J. Gastroenterol. 15, 1–16 (2009)
S.J. Brand, B.N. Andersen, J.F. Rehfeld, Complete tyrosine-o-sulphation of gastrin in neonatal rat pancreas. Nature 6, 456–458 (1984)
D. Chen, C.M. Zhao, R. Håkanson, L.C. Samuelson, J.F. Rehfeld, L. Friis-Hansen, Altered control of gastric acid secretion in gastrin-cholecystokinin double mutant mice. Gastroenterology 126, 476–487 (2004)
C. Saillan-Barreau, M. Dufresne, P. Clerc, D. Sanchez, H. Corominola, C. Moriscot et al., Evidence for a functional role of the cholecystokinin-B/gastrin receptor in the human fetal and adult pancreas. Diabetes 48, 2015–2021 (1999)
I. Rooman, J. Lardon, D. Flamez, F. Schuit, L. Bouwens, Mitogenic effect of gastrin and expression of gastrin receptors in duct-like cells of rat pancreas. Gastroenterology 121, 940–949 (2001)
J.F. Rehfeld, Incretin physiology beyond glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide: cholecystokinin and gastrin peptides. Acta Physiol. (Oxf). 201, 405–411 (2011)
J. Dupre, J.D. Curtis, R.H. Unger, R.W. Waddell, J.C. Beck, Effects of secretin, pancreozymin, or gastrin on the response of the endocrine pancreas to administration of glucose or arginine in man. J. Clin. Invest. 48, 745–757 (1969)
A. Kaneto, Y. Tasaka, K. Kosaka, K. Nakao, Stimulation of insulin secretion by the C-terminal tetrapeptide amide of gastrin. Endocrinology 84, 1098–1106 (1969)
H. Ohgawara, Y. Mizuno, Y. Tasaka, K. Kosaka, Effect of the C-terminal tetrapeptide amide of gastrin on insulin secretion in man. J. Clin. Endocrinol. Metab. 29, 1261–1262 (1969)
J.F. Rehfeld, F. Stadil, The effect of gastrin on basal- and glucose-stimulated insulin secretion in man. J. Clin. Invest. 52, 1415–1426 (1973)
W.L. Suarez-Pinzon, R.F. Power, Y. Yan, C. Wasserfall, M. Atkinson, A. Rabinovitch, Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes 57, 3281–3288 (2008)
S.Y. Song, M. Gannon, M.K. Washington, C.R. Scoggins, I.M. Meszoely, J.R. Goldenring et al., Expansion of PDX-1-expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor α. Gastroenterology 117, 1416–1426 (1999)
K. Yamamoto, J. Miyagawa, M. Waguri, R. Sasada, K. Igarashi, M. Li et al., Recombinant human betacellulin promotes the neogenesis of β-cells and ameliorates glucose intolerance in mice with diabetes induced by selective alloxan perfusion. Diabetes 49, 2021–2027 (2000)
C. Cras-Meneur, L. Elghazi, P. Czernichow, R. Scharfmann, Epidermal growth factor increases undifferentiated pancreatic embryonic cells in vitro: a balance between proliferation and differentiation. Diabetes 50, 1571–1579 (2001)
M.L. Krakowski, M.R. Kritzik, E.M. Jones, T. Krachl, J. Lee, M. Arnush et al., Transgenic expression of epidermal growth factor and keratinocyte growth factor in β-cells results in substantial morphological changes. J. Endocrinol. 162, 167–175 (1999)
G. Xu, D.A. Stoffers, J.F. Habener, S. Bonner-Weir, Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48, 2270–2276 (1999)
R.N. Wang, G. Klöppel, L. Bouwens, Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia 38, 1405–1411 (1995)
R.N. Wang, J.F. Rehfeld, F.C. Nielsen, G. Klöppel, Expression of gastrin and transforming growth factor-alpha during duct to islet cell differentiation in the pancreas of duct-ligated adult rats. Diabetologia 40, 887–893 (1997)
I. Rooman, L. Bouwens, Islet neogenesis in the regeneration model of rat pancreatic duct ligation requires endogenous gastrin action via CCK2 receptors. Diabetologia 45(Suppl. 2), A26 (2002)
G. Xu, S. Sumi, M. Koike, K. Tanigawa, Y. Nio, K. Tamura, Role of endogenous hypergastrinemia in regenerating endocrine pancreas after partial pancreatectomy. Dig. Dis. Sci. 41, 2433–2439 (1996)
E.P. Sandgren, N.C. Luetteke, R.D. Palmiter, R.L. Brinster, D.C. Lee, Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell 61, 1121–1135 (1990)
T.C. Wang, S. Bonner-Weir, P.S. Oates, M. Chulak, B. Simon, G.T. Merlino et al., Pancreatic gastrin stimulates islet differentiation of transforming growth factor alpha-induced ductular precursor cells. J. Clin. Invest. 92, 1349–1356 (1993)
S.J. Brand, S. Tagerud, P. Lambert, S.G. Magil, K. Tartarkiewicz, K. Doiron et al., Pharmacological treatment of chronic diabetes by stimulating pancreatic β-cell regeneration with systemic co-administration of EGF and gastrin. Pharmacol. Toxicol. 91, 414–420 (2002)
I. Rooman, L. Bouwens, Combined gastrin and epidermal growth factor treatment induces islet regeneration and restores normoglycemia in C57BL6/J mice treated with alloxan. Diabetologia 47, 259–265 (2004)
P.L. Brubaker, D.J. Drucker, Minireview: glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology 145, 2653–2659 (2004)
A. Bulotta, L. Farilla, H. Hui, R. Perfetti, The role of GLP-1 in the regulation of islet cell mass. Cell Biochem. Biophys. 40, 65–78 (2004)
W.L. Suarez-Pinzon, J.R. Lakey, A. Rabinovitch, Combination therapy with glucagon-like peptide-1 and gastrin induces beta-cell neogenesis from pancreatic duct cells in human islets transplanted in immunodeficient diabetic mice. Cell Transplant. 17, 631–640 (2008)
W.L. Suarez-Pinzon, G.S. Cembrowski, A. Rabinovitch, Combination therapy with a dipeptidyl peptidase-4 inhibitor and a proton pump inhibitor restores normoglycaemia in non-obese diabetic mice. Diabetologia 52, 1680–1682 (2009)
W.L. Suarez-Pinzon, A. Rabinovitch, Combination therapy with a dipeptidyl peptidase-4 inhibitor and a proton pump inhibitor induces β-cell neogenesis from adult human pancreatic duct cells implanted in immunodeficient mice. Cell Transplant. 20, 1343–1349 (2011)
T.B. Bödvarsdóttir, K.D. Hove, C.F. Gotfredsen, L. Pridal, A. Vaag, A.E. Karlsen, J.S. Petersen, Treatment with a proton pump inhibitor improves glycaemic control in Psammomys obesus, a model of type 2 diabetes. Diabetologia 53, 2220–2223 (2010)
L. Bouwens, I. Rooman, Regulation of pancreatic beta-cell mass. Physiol. Rev. 85, 1255–1270 (2005)
A.C. Hauge-Evans, A.J. King, D. Carmignac, C.C. Richardson, I.C. Robinson, M.J. Low et al., Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 58, 403–411 (2009)
M. Sumii, K. Sumii, A. Tari, M. Yoshihara, K. Haruma, G. Kaji-yama, Regulation of antral peptides by administration of omeprazole to healthy men. Am. J. Gastroenterol. 89, 2033–2037 (1994)
S.J. Brand, D. Stone, Reciprocal regulation of antral gastrin and somatostatin gene expression by omeprazole-induced achlorhydria. J. Clin. Invest. 82, 1059–1066 (1988)
F. Katagiri, S. Inoue, H. Itoh, M. Takeyama, Omeprazole raises somatostatin and motilin in human plasma. Biol. Pharm. Bull. 28, 370–373 (2005)
B. Stoschus, G. Hamscher, S. Ikonomou, G. Partoulas, C. Eberle, T. Sauerbruch et al., Effect of omeprazole treatment on plasma concentrations of the gastric peptides, xenin, gastrin and somatostatin, and of pepsinogen. J. Pept. Res. 52, 27–33 (1998)
A. Shulkes, M. Read, Regulation of somatostatin secretion by gastrin- and acid-dependent mechanisms. Endocrinology 129, 2329–2334 (1991)
M.A. Read, D.M. Read, M. Kapuscinski, A. Shulkes, Achlorhydria induced changes in gastrin, somatostatin, H+/K(+)-ATPase and carbonic anhydrase in the sheep. Regul. Pept. 40, 13–27 (1992)
A. Theodoraki, B. Khoo, A. Hamda, F. Grillo, T. Meyer, P.M. Bou-loux, Malignant somatostatinoma presenting with diabetic ketoacidosis and inhibitory syndrome: pathophysiologic considerations. Endocr. Pract. 16, 835–837 (2010)
E. Resmini, F. Minuto, A. Colao, D. Ferone, Secondary diabetes associated with principal endocrinopathies: the impact of new treatment modalities. Acta Diabetol. 46, 85–95 (2009)
G. Mazziotti, I. Floriani, S. Bonadonna, V. Torri, P. Chanson, A. Giustina, Effects of somatostatin analogs on glucose homeostasis: a metaanalysis of acromegaly studies. J. Clin. Endocrinol. Metab. 94, 1500–1508 (2009)
S. Melmed, A. Colao, A. Barkan, M. Molitch, A.B. Grossman, D. Kleinberg et al., Guidelines for acromegaly management: an update. J. Clin. Endocrinol. Metab. 94, 1509–1517 (2009)
B.O. Boehm, The therapeutic potential of somatostatin receptor ligands in the treatment of obesity and diabetes. Expert Opin. Investig. Drugs 12, 1501–1509 (2003)
T. Tzotzas, K. Papazisis, P. Perros, G.E. Krassas, Use of somatostatin analogues in obesity. Drugs 68, 1963–1973 (2008)
B.O. Boehm, R.H. Lustig, Use of somatostatin receptor ligands in obesity and diabetic complications. Best Pract. Res. Clin. Gastroenterol. 16, 493–509 (2002)
B.E. Dunning, J.E. Gerich, The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr. Rev. 28, 253–283 (2007)
L. Yang, S.C. Berk, S.P. Rohrer, R.T. Mosley, L. Guo, D.J. Underwood et al., Synthesis and biological activities of potent peptidomimetics selective for somatostatin receptor subtype 2. Proc. Natl. Acad. Sci. USA 95, 10836–10841 (1998)
K. Cejvan, D.H. Coy, S. Efendic, Intra-islet somatostatin regulates glucagon release via type 2 somatostatin receptors in rats. Diabetes 52, 1176–1181 (2003)
S. Moldovan, A. Atiya, T.E. Adrian, R.M. Kleinman, K. Lloyd, K. Olthoff et al., Somatostatin inhibits B-cell secretion via a subtype-2 somatostatin receptor in the isolated perfused human pancreas. J. Surg. Res. 59, 85–90 (1995)
M. Sanaka, T. Yamamoto, Y. Kuyama, Effects of proton pump inhibitors on gastric emptying: a systematic review. Dig. Dis. Sci. 55, 2431–2440 (2010)
M.A. Crouch, I.N. Mefford, E.U. Wade, Proton pump inhibitor therapy associated with lower glycosylated hemoglobin levels in type 2 diabetes. J. Am. Board Fam. Med. 25, 50–54 (2012)
B.T. Batuwitage, J.G. Kingham, N.E. Morgan, R.L. Bartlett, Inappropriate prescribing of proton pump inhibitors in primary care. Postgrad. Med. J. 83, 66–68 (2007)
M. Eccles, J. Grimshaw, M. Campbell, C. Ramsay, Research designs for studies evaluating the effectiveness of change and improvement strategies. Qual. Saf. Health Care 12, 47–52 (2003)
Steno Diabetes Center. The effect of nexium and probiotics on insulin secretion and cardiovascular risk factors in patients with type 2 diabetes. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). http://clinicaltrials.gov/show/NCT00699426NLMIdentifier:NCT00699426 (2000). Accessed 6 June 2012
City of Hope Medical Center. Plasm gastrin concentrations in response to nexium administration in healthy volunteers. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). http://clinicaltrials.gov/show/NCT01135472NLMIdentifier:NCT01135472 (2000). Accessed 6 June 2012
Sanford Health. Combination therapy with sitagliptin and lansoprazole to restore pancreatic β cell function in recent-onset type 1 diabetes. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). http://clinicaltrials.gov/show/NCT01155284NLMIdentifier:NCT01155284 (2000). Accessed 6 June 2012
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Novel therapy combining regenerative stimuli immunomodulation to preserve β cell function in new onset type 1 diabetes. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). http://clinicaltrials.gov/show/NCT00837759NLMIdentifierNCT00837759 (2000). Accessed 6 June 2012
Coordinación de Investigación en Salud, Mexico. Effect of pantoprazole on insulin secretion in patients with type 2 diabetes. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). http://clinicaltrials.gov/show/NCT01541735NLMIdentifier:NCT01541735 (2000). Accessed 8 June 2012
University of Alberta. Pilot study of safety and efficacy of combined use of dipeptidyl-peptidase inhibitor (sitagliptin) and proton pump inhibitor (pantoprazole) to prevent β -cell apoptosis and promote islet regeneration in islet transplant recipients with early graft dysfunction. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). http://clinicaltrials.gov/show/NCT00768651NLMIdentifier:NCT00768651 (2000). Accessed 8 June 2012
A.B. Hill, The environment and disease: association or causation? Proc. R. Soc. Med. 58, 295–300 (1965)
H.G. Peach, N.E. Barnett, Helicobacter pylori infection and fasting plasma glucose concentration. J. Clin. Pathol. 54, 466–469 (2001)
S.E. Inzucchi, R.M. Bergenstal, J.B. Buse, M. Diamant, E. Ferrannini, M. Nauck et al., Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35, 1364–1379 (2012)
D. Boj-Carceller, J. Playán-Usón, P. Trincado-Aznar, F.J. Acha-Pérez, R. Albero-Gamboa. Proton pump inhibitors for the treatment of diabetes mellitus? Av Diabetol 26, 45–46 (2010)
Conflict of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boj-Carceller, D. Proton pump inhibitors: impact on glucose metabolism. Endocrine 43, 22–32 (2013). https://doi.org/10.1007/s12020-012-9755-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9755-3