Abstract
Osteopontin (OPN) is known to be one of the cytokines that is involved in the vascular inflammation caused by aldosterone (Ald). Previous reports have shown that Ald increases OPN expression, and the mechanisms for this remain to be clarified. In this study, we investigated how Ald increases OPN expression in the vascular smooth muscle cells (VSMCs) of rats. Ald increased OPN expression time dependently as well as dose dependently. This increase was diminished by spironolactone, a mineralocorticoid receptor (MR) antagonist. PD98059, an inhibitor of p42/44 MAPK pathway, and SB203580, an inhibitor of p38 MAPK pathway, suppressed Ald-induced OPN expression and secretion in VSMCs. VSMCs migration stimulated by aldosterone required OPN expression. In conclusion, these data suggest that Ald-induced OPN expression in VSMC is mediated by MR and signaling cascades involving ERK and p38 MAPK. These molecules may represent therapeutic targets for the prevention of pathological vascular remodeling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The proliferative phase of atherosclerotic lesion development involves vascular smooth muscle cell (VSMC) proliferation and migration, accumulation of extracellular matrix, and fibrous cap formation.
Vascular injury induces modulation of VSMCs from a quiescent to a proliferative state characterized by cell division, cell migration into the intima, and secretion of extracellular matrix. This sequence of events is characteristic of the intimal hyperplasia seen in atherosclerosis, and has been implicated in the pathogenesis of vessel restenosis after balloon angioplasty of human coronary arteries.
Osteopontin (OPN) is a multifunctional glycophosphoprotein secreted by many cell types, including VSMCs and endothelial cells [1]. It has been reported that OPN promotes migration, extracellular matrix invasion, and proliferation of VSMC [2, 3].
Cascella et al. [4] demonstrated that in response to injury, OPN concentrations increase in several tissues, and hypoxia, hyperglycemia, and mechanical injury to blood vessels all result in enhanced OPN expression. These increases may be significant for blood vessel growth because overexpression of OPN has been shown to accelerate intimal thickening [5–7].
These findings suggest that OPN serves as a critical mediator during the pro-inflammatory and pro-fibrotic process, thereby contributing to the development and/or progression of cardiovascular diseases.
Aldosterone (Ald) has a well-established pathophysiological role in hypertension and cardiovascular disease. In addition to its primary function in regulating blood pressure and electrolytic balance, aldosterone directly promotes vascular remodeling and profibrotic changes in blood vessels [8, 9]. Numerous experimental studies have postulated the possible direct cardiovascular effects by aldosterone as responsible for the initiation of cardiovascular inflammation and fibrosis (“aldosterone-induced vasculitis”) [10, 11], although the cellular and molecular mechanism(s) by which aldosterone induces such cardiovascular injury remains unknown.
In coronary heart disease patients, aldosterone levels have been reported to be independently associated with plasma OPN levels [12]. Aldosterone has also been shown to stimulate OPN gene transcription in VSMCs [13]. However, there are no studies that have evaluated the signaling pathways involved in OPN induction in cultured rat VSMCs. This study was undertaken to explore the cellular mechanism by which aldosterone induces OPN expression.
Materials and methods
Materials
All experimental protocols were approved by the Animal Care and Use Committee of the University of Jiao Tong. Aldosterone and spironolactone were obtained from Sigma-Aldrich (St. Louis, MO, USA). RU486 was kindly provided by Merck (Rahway, NJ, USA). Anti-phospho-ERK, anti-phospho-JNK, and anti-phospho-p38 antibodies were purchased from Cell Signaling Technology (Cell Signaling, CA, USA), β-actin was purchased from Sigma-Aldrich (St. Louis, MO, USA). The neutralizing OPN antibody was purchased from R&D Systems (Minneapolis, MN, USA).
Cell culture
VSMC were prepared from thoracic aorta of Sprague–Dawley rats by enzyme dispersion and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % bovine serum, as previously described [14]. The cultures were maintained at 37 °C in a 5 % CO2/95 % O2 humidified atmosphere. Cells were between passages 4–15 for all experiments.
Synthesis of antisense oligonucleotides
Phosphorothioate-derived oligodeoxynucleotides was chemically synthesized by MWG Biotech AG (Germany) and was composed of the following sequences: OPN antisense 5′-AACCACTGCCAGTCTCAT-3′, OPN sense: 5′-ATGAGACTGGCAGTGGTT-3′, and OPN scramble sense: 5′-AACTACTATCAGTCTCGT-3′. The OPN sense oligodeoxynucleotide sequence comprised the first five codons of rat OPN mRNA, and antisense OPN oligodeoxynucleotides comprised the complementary sequence.
Western blotting
Whole cell extracts were prepared essentially as before [15].The samples were then resolved on polyacrylamide gels containing SDS and transferred to a nitrocellulose membrane by electroblotting. The membrane was incubated in blocking buffer (TBS Containing 5 % skim milk and 0.1 %Tween20) for 2 h, followed by incubation with the primary antibody diluted in the same buffer. The specific secondary antibody was detected using peroxidase-conjugated anti-IgG at 1:2,000. Relative proteins were detected by the supersignal chemiluminescence system (ECL, Pierce) followed by exposure to autoradiographic film.
Transient transfection and luciferase assay
Transfections were performed with the SuperFect transfection reagent (Qiagen, Hilden, Germany) method. Luciferase activity was detected with a luciferase assay kit (Promega Corporation, Madison, WI).
Migration assays
Cell migration was performed with the Transwell (Costar) system, which allows cells to migrate through 8-μm pore size polycarbonate membrane. The OPN sense, antisense, or its scrambled sequence oligodeoxynucleotides were pretreated to cells at concentrations of 25 μM in DMEM with 0.5 % FBS for 8 h; then trypsinized, washed, and resuspended in serum-free DMEM (5 × 105 cells/mL). This suspension (100 μL) was added to the upper chamber of transwells. The lower chamber was filled with 600 μL serum-free DMEM containing aldosterone (10–7 M) or not containing aldosterone. After 6-h stimulation by aldosterone, filters were removed, and cells remaining on the upper surface of the membrane (that had not migrated through the filter) were removed with a cotton swab. Then, membranes were washed with PBS, and cells present beneath the membrane were fixed with cold methanol for 15 min and stained with Hemalun. Cells were counted in ten high-power microscope fields. Analysis was performed on three wells for each condition, and each experiment was repeated three times [16].
Enzyme-linked immunosorbent assay (ELISA)
Confluent VSMCs in 6-cm collagen-coated plates were incubated with aldosterone alone or along with spironolactone, RU486, PD98059, SB203580, and SP600125, and concentrations of OPN in medium were determined by commercially available ELISA kit (Immuno-Biological Laboratories, Gunma, Japan) according to the manufacturer’s instructions.
Rat carotid balloon injury model
All animals used in this study were treated in accordance with the Guidelines of the Institutional Animal Experimental Committee of JiaoTong University. Male 10-week-old Wistar rats (275–320 g) were anesthetized with sodium pentobarbital (20 mg/kg, intraperitoneally). The left common carotid artery was denuded of endothelium and stretched with a 2 Fr Fogarty balloon catheter (Baxter Healthcare, Deerfield, USA), as described elsewhere [17]. Rats were treated with spironolactone (200 mg/kg/day) in olive oil or only olive oil continuously for 2 weeks using osmotic minipumps (Model 2002; Alzet Corporation, Cupertino, USA) that were subcutaneously implanted at the time of the surgery. Blood pressure was measured every 7 days by the tail-cuff method. Two weeks after balloon injury, rats were euthanized and perfusion-fixed with 4 % paraformaldehyde. The injured arteries were excised and embedded in paraffin.
Immunohistochemistry
A monoclonal antibody against OPN (MPIIIB101; 1:200 dilution) was used as the primary antibody. The sections were visualized using a Vectastain ABC kit (Vector Laboratories, Burlingame, USA), with diaminobenzidine (DAB) as the substrate. Hematoxylin was used as a counter stain. Negative controls were established using nonimmune serum in place of the primary antibody. The degree of OPN immunoreactivity was determined as the percentage of OPN-positive area in the injured vessels.
Statistical analysis
Data were expressed as means ± SEM. Differences between groups were examined for statistical significance using unpaired t test or ANOVA with Dunn’s post hoc test, if they were appropriate. P values less than 0.05 were considered statistically significant.
Results
Role of aldosterone in neointimal formation and OPN expression in the balloon-injured rat carotid artery
Previous studies have indicated that aldosterone plays a critical role in neointimal formation following vascular injury. To investigate the role of aldosterone in OPN expression in injured arteries, rats were treated with spironolactone or vehicle for 2 weeks following balloon injury to the carotid arteries. No significant difference in blood pressure was found between these two groups (117 ± 8 mmHg in the control group, 122 ± 7 mmHg in the spironolactone group). Two weeks after injury, a significant degree of neointimal hyperplasia was observed (Fig. 1a). Treatment with spironolactone reduced the intima/media ratio by 42 %. Spironolactone administration also resulted in decreased OPN expression in the vessel wall following balloon injury by 55 %, indicating that OPN expression in the injured artery is at least partially mediated by aldosterone (Fig. 1b).
Effect of an aldosterone receptor blocker on rat carotid artery neointimal formation following balloon injury in vivo. a Representative cross-sections of an injured carotid artery, an injured carotid artery treated with spironolactone (200 mg/kg/day) for 2 weeks, and an uninjured carotid artery. Arrows indicate the neointima. Original magnification ×100. The graph shows morphometric analysis of the injured carotid arteries. Animals were treated with either vehicle (n = 11) or spironolactone (n = 8). Mean ratios of intima to media (I/M ratio) are represented as the means ± SEM. b Effect of an aldosterone receptor blocker on in vivo OPN expression at 14 days after balloon injury. OPN expression in an injured carotid artery, an injured carotid artery treated with spironolactone, and an uninjured artery are shown. Original magnification, ×200. The graph shows the percentage of OPN-positive area in the injured vessels. Animals were treated with either vehicle (n = 11) or spironolactone (n = 8). Values are the means ± SEM. *P < 0.05 vs. vehicle
OPN is critical for aldosterone-induced vascular smooth muscle cells migration
Our previous study has shown that Aldo increased VSMCs migration. To determine if the migration of VSMCs is OPN dependent, migration experiments with Aldo were performed in the presence of OPN anti-sense. As shown in Fig. 2a, b, we found that cells transfected with OPN anti-sense significantly inhibited Ald-induced OPN expression in VSMCs measured by western blot. Aldosterone significantly increased migration measured by transwell chamber. OPN anti-sense significantly attenuated aldosterone-induced VSMCs migration, whereas OPN sense or scramble sense cannot inhibit aldosterone-induced VSMCs migration, demonstrating that OPN is essential for aldosterone-induced migration in VSMCs. We also found OPN neutralizes antibody-attenuated aldosterone-induced VSMCs migration (data not shown).
Effect of anti-sense for OPN on the Ald-induced migration of vascular smooth muscle. VSMCs were pretreated with OPN sense, antisense, or its scrambled sequence oligodeoxynucleotides for 8 h and subsequently stimulated by Ald for 6 h, the transfect efficiency was measured by western blot. VSMCs pretreated with OPN anti-sense significantly inhibited Ald-induced OPN expression (a). Vascular smooth muscle were treated with Ald (10−7 M) for 8 h and subsequently studied cells migration by using transwell apparatus. The increased migration was blocked by treatment with ant-sense for OPN (b). Each bar represents mean ± SE of cell migrated per high-power fields for three independent experiments. *P < 0.05 vs. vascular smooth muscle in DMEM alone. **P < 0.05 vs. vascular smooth muscle in Ald
Aldosterone induces osteopontin expression and secretion in vascular smooth muscle cells
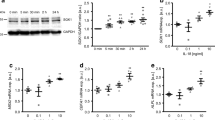
We first examined whether aldosterone directly induces OPN in cultured VSMCs. As shown in Fig. 3, aldosterone induced the OPN expression in a time-dependent manner, with the maximal effect at 24 h (Fig. 3a). Aldosterone dose dependently (10−9–10−7 M) increased steady-state OPN protein levels in VSMCs (Fig. 3b); about 1.8-fold increase (P < 0.05) was induced by as low as 10−9 M and the maximal effect observed at 10−7 M Ald. Likewise, aldosterone dose dependently (10−9–10−7 M) increased the secretion of OPN into media (Fig. 3c); about 1.3-fold increase (P < 0.05) was induced by as low as 10−9 M and about a 4-fold increase by 10−7 M.
Aldosterone induces OPN expression and secretion in vascular smooth muscles. The effect of Ald on OPN expression was time-dependent. The maximal effect of Ald on OPN expression was observed at 24 h (a). Ald-induced expression and secretion of OPN protein in a dose-dependent manner. The concentration for maximal effect of Ald was observed at 10−7 mol/L (b, c). OPN protein expression levels were measured by western blotting. Secretion of OPN in medium was measured by ELISA. Normalized data (bar graphs) are presented as mean ± SEM. *P < 0.05 vs. VSMCs in DMEM alone (control)
Mitogen-activated protein kinases (MAPKs) and mineralocorticoid receptor (MR) receptor were involved in OPN expression
As shown in Fig. 4, aldosterone activates p42/p44 MAPK, JNK1/2, and p38 MAPK with the maximal effect at 5, 30, and 10 min, respectively in VSMCs (Fig. 4a). A selective MR antagonist, spironolactone (10−6 M), blocked the p42/p44 MAPK and p38 MAPK expression induced by aldosterone (Fig. 4b). To further determine the role of aldosterone receptors in OPN expression and whether the MAPK activation was involved in aldosterone-induced OPN expression in VSMCs, VSMCs were pretreated with a selective MR antagonist, spironolactone (10−6 M), a selective GR antagonist, RU486 (10−6 M), p38 MAPK inhibitor SB203580 (20 mM), p42/p44 MAPK inhibitor PD98059 (20 mM), and JNK1/2 inhibitor SP600125. The aldosterone (10−7 M)-induced OPN expression was blocked by MR antagonist, spironolactone, p38 MAPK inhibitor SB203580 (20 mM), and p42/p44 MAPK inhibitor PD98059 (Fig. 4c). In contrast, pretreatment with a JNK1/2 inhibitor SP600125 and GR antagonist RU486 did not block aldosterone-induced OPN expression. These results suggest that aldosterone induced OPN expression through MR, p38 MAPK, and p42/p44 signaling pathway in VSMCs. Likewise, the aldosterone-stimulated OPN secretion was also blocked by MR antagonist, spironolactone, p38 MAPK inhibitor SB203580 (20 mM), and p42/p44 MAPK inhibitor PD98059 (Fig. 4d).
MAPK pathways were involved in Ald-induced OPN expression and secretion in vascular smooth muscle. VSMCs were exposed to 10−7 M aldosterone for the indicated times. The cells were then harvested and 20 μg of the lysates subjected to western blot analysis using antibodies to phosphorylated ERK, JNK, and P38, their activity reached peak at 5, 30, and 10 min, respectively (a). Cells were treated with Ald (10−7 mol/L), alone or along with spironolactone. The cells were then harvested and 20 μg of the lysates subjected to western blot analysis using antibodies to phosphorylated ERK, JNK, and P38 (b). Cells were treated with Ald (10−7 mol/L), alone or along with spironolactone, RU486 for 24 h. Then, cells were preincubated with 20 mM PD98059, SB203580, SP600125 for 1.5 h and then treated with 10−7 mol/L Ald for 24 h (b, c). OPN protein expression was determined by western blotting analysis. Secretion of OPN in medium was measured by ELISA. The gels represent results of three independently performed experiments. Normalized data (bar graphs) are presented as mean ± SEM. *P < 0.05 vs. vascular smooth muscle in DMEM alone (control). **P < 0.05 vs. VSMCs in Ald
Discussion
This study demonstrates that aldosterone directly induced OPN protein expression and secretion in rat VSMCs, whose effects were completely blocked by a selective MR receptor antagonist. The aldosterone-induced upregulation of OPN protein expression and secretion was also completely blocked by p38 MAPK inhibitor and p42/p44 MAPK inhibitor, suggesting that aldosterone-induced OPN expression and secretion is mediated by MR and signaling cascades involving p38 MAPK p42/p44 MAPK.
Two recent clinical trials, the Randomized Aldactone Evaluation Study and the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study, showed that the MR antagonists, spironolactone and eplerenone (Eplr), improved the prognosis of chronic heart failure patients, even at doses less than the threshold that causes significant renal effects [18, 19]. This finding suggests that MR antagonism may have a direct protective effect on the cardiovascular system. MR is distributed in not only distal renal tubules but also nonepithelial tissues, such as the cardiovascular system, including VSMCs [20, 21]. There is a large body of evidence that Ald induces inflammatory changes in the vasculature leading to deterioration in vascular function.
Aldosterone stimulates epidermal growth factor receptor synthesis in VSMCs, and this effect requires MR binding [22, 23]. Irita et al. [24] demonstrated that aldosterone dose and time dependently induced a significant increase in OPN expression in rat renal fibroblasts, and OPN-small interfering RNA completely inhibited the induction of cell proliferation and collagen synthesis in response to aldosterone. Significant relationships have also been identified between aldosterone and OPN in vivo. MR antagonists block OPN synthesis in blood vessels of angiotensin II transgenic mice [25], and OPN knockout mice demonstrate reduced cardiac fibrosis after aldosterone infusion [26]. In humans, changes in plasma OPN concentrations correlate with changes in aldosterone, and patients with primary hyper-aldosteronism have increased plasma levels of OPN [27]. These findings suggest that aldosterone could induce changes in OPN that lead to vascular remodeling. In this study, the aldosterone-induced OPN protein expression and secretion in VSMCs was inhibited by a selective MR antagonist (spironolactone). These results suggest that the aldosterone in its physiological and/or pathophysiological concentrations regulates OPN expression through MR in VSMCs.
MAPKs, a large family of serine-threonine kinases, have important functions as mediators of signal transduction and are activated by a variety extracellular stimuli. Three subgroups of MAPKs have clearly been identified: the extracellular signal-regulated kinases(p42/44 MAPK), the p38 kinase, and the c-jun N-terminal kinases (JNKs). Aldosterone stimulated mesangial cell mitosis and activated ERK1/2 and SAPK/JNK signaling [28].
Aldosterone enhances IGF-I-mediated IRS-1, Akt, MAPK, and p70S6K phosphorylation. Aldosterone also enhances IGF-I-induced VSMC proliferation, migration, and protein synthesis [4]. One of the mechanisms mediating these changes was the induction of OPN production. Aldosterone increases VEGF-A production in human neutrophils through PI3K, ERK1/2, and p38 pathways [29]. Platelet-derived growth factor induced vascular proliferation, migration, and gene expression involving MAPK [30]. Similar to the results, we found that p38 MAPK inhibitor and p42/p44 MAPK inhibitor, but not JNK1/2 inhibitor, significantly blocked Ald-induced OPN protein expression and secretion. MR antagonist RU-28318 blocked aldosterone-induced brain phosphorylation of p44/42 MAPK [31]. Another study showed that adipocyte-conditioned medium (ACM) stimulates p38 MAPK and extracellular signal-regulated kinase 1/2 phosphorylation through MR, glucocorticoid receptors (GR), and angiotensin II type-1 receptor (AT1R); activation of stress-activated protein kinase/JNK involves GR and AT1R [32]. In our study, aldosterone induced upregulation of OPN protein expression through MR. These data suggest that JNKs may be in part regulated through GR.
This study also showed that aldosterone induced VSMC migration and antisense OPN inhibited aldosterone-induced VSMC migration, suggesting that in part, OPN is critical for aldosterone-induced VSMCs migration.
We and other investigators have demonstrated OPN expression in the neointima. In this study, spironolactone attenuated OPN expression and neointimal formation in the balloon-injured rat carotid artery. Accumulating evidence suggests that OPN plays an important role in the development of atherosclerosis [33, 34]. OPN expression in blood vessels has been shown to be induced in response to multiple stimuli, such as aldosterone or during vascular repair or regeneration [35], and to activate macrophages and T-cells to migrate and produce other cytokines through avb3 or CD44 receptors [36]. In vitro studies have demonstrated that OPN promotes migration and proliferation of VSMCs and accumulation of extracellular matrices by directly binding to collagen and fibronectin [2, 37]. Our study showed that OPN is critical for aldosterone-induced VSMCs migration. Aldosterone-induced OPN expression may be involved in the development of atherosclerosis through these biological effects.
Previous study demonstrates that spironolactone modulates the expressions of JNK-2, ERK-2, and p38 in the developing kidney, suggesting that the signaling intermediate through the MR in rat kidney [38]. Aldosterone stimulates collagen gene expression and synthesis through MR-mediated ERK1/2 activation in renal fibroblasts [39].
In our study, the functional link between the MR and the p42/44 and p38 MAPK pathway in VSMCs remains to be further elucidated.
In conclusion, this study demonstrates that aldosterone directly induces OPN expression in VSMCs through MR and signaling cascades involving p38 MAPK p42/p44 MAPK, which may be responsible for the initiation of “aldosterone-induced vasculitis.”
References
E.R. O’Brien, M.R. Garvin, D.K. Stewart, T. Hinohara, J.B. Simpson, S.M. Schwartz, C.M. Giachelli, Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler. Thromb. 14, 1648–1656 (1994)
L. Liaw, M. Almeida, C.E. Hart, S.M. Schwartz, C.M. Giachelli, Osteopontin promotes vascular cell adhesion and spreading and is chemotactic for smooth muscle cells in vitro. Circ. Res. 74, 214–224 (1994)
K. Isoda, K. Nishikawa, Y. Kamezawa, M. Yoshida, M. Kusuhara, M. Moroi, N. Tada, F. Ohsuzu, Osteopontin plays and important role in the development of medial thickening and neointimal formation. Circ. Res. 91, 77–82 (2002)
T. Cascella, Y. Radhakrishnan, L.A. Maile, W.H. Busby Jr., K. Gollahon, A. Colao, D.R. Clemmons, Aldosterone enhances IGF-I-mediated signaling and biological function in vascular smooth muscle cells. Endocrinology 151, 5851–5864 (2010)
M. Han, J.K. Wen, B. Zheng, Z. Liu, Y. Chen, Blockade of integrin β3-FAK signaling pathway activated by osteopontin inhibits neointimal formation after balloon injury. Cardiovasc. Pathol. 16, 283–290 (2007)
M. Kurata, T. Okura, S. Watanabe, T. Fukuoka, J. Higaki, Osteopontin and carotid atherosclerosis in patients with essential hypertension. Clin. Sci. (Lond.) 111, 319–324 (2006)
C.M. Giachelli, N. Bae, M. Almeida, D.T. Denhardt, C.E. Alpers, S.M. Schwartz, Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J. Clin. Invest. 92, 1686–1696 (1993)
R. Rocha, A.E. Rudolph, G.E. Frierdich, D.A. Nachowiak, B.K. Kekec, E.A. Blomme, E.G. McMahon, J.A. Delyani, Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am. J. Physiol. Heart Circ. Physiol. 283, H1802–H1810 (2002)
D. Nagata, M. Takahashi, K. Sawai, T. Tagami, T. Usui, A. Shimatsu, Y. Hirata, M. Naruse, Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO syntheses activity. Hypertension 48, 165–171 (2006)
N. Sukor, Primary aldosteronism: from bench to bedside. Endocrine 41, 31–39 (2012)
R. Rocha, J.W. Funder, The pathophysiology of aldosterone in the cardiovascular system. Ann. N.Y. Acad. Sci. 970, 89–100 (2002)
S. Del Ry, D. Giannessi, M. Maltinti, M. Cabiati, C. Prontera, A. Iervasi, C. Caselli, A.M. Mazzone, D. Neglia, Increased plasma levels of osteopontin are associated with activation of the renin–aldosterone system and with myocardial and coronary micro-vascular damage in dilated cardiomyopathy. Cytokine 49, 325–330 (2010)
A. Kiyosue, D. Nagata, M. Myojo, T. Sato, M. Takahashi, H. Satonaka, R. Nagai, Y. Hirata, Aldosterone-induced osteopontin gene transcription in vascular smooth muscle cells involves glucocorticoid response element. Hypertens. Res. 34, 1283–1287 (2011)
T. Ishida, M. Ishida, J. Suero, M. Takahashi, B.C. Berk, Agonist-stimulated cytoskeletal reorganization and signal transduction at focal adhesions in vascular smooth muscle cells require c-Src. J. Clin. Invest. 103, 789–797 (1999)
X. Jin, X. Song, L. Li, Z. Wang, Y. Tao, L. Deng, M. Tang, W. Yi, Y. Cao, Blockade of AP-1 activity by dominant-negative TAM67 can abrogate the oncogenic phenotype in latent membrane protein 1-positive human nasopharyngeal carcinoma. Mol. Carcinog. 46, 901–911 (2007)
X. Jin, X. Ge, D.L. Zhu, C. Yan, Y.F. Chu, W.D. Chen, J. Liu, P.J. Gao, Expression and function of vascular endothelial growth factor receptors (Flt-1 and Flk-1) in vascular adventitial fibroblasts. J. Mol. Cell. Cardiol. 3, 292–300 (2007)
M. Usui, K. Egashira, K. Ohtani, C. Kataoka, M. Ishibashi, K. Hiasa, M. Katoh, Q. Zhao, S. Kitamoto, A. Takeshita, Anti-monocyte chemoattractant protein-1 gene therapy inhibits restenotic changes (neointimal hyperplasia) after balloon injury in rats and monkeys. FASEB J. 16, 1838–1840 (2002)
B. Pitt, F. Zannad, W.J. Remme, R. Cody, A. Castaigne, A. Perez, J. Palensky, J. Wittes, The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N. Engl. J. Med. 341, 709–717 (1999)
B. Pitt, G. Bakris, L.M. Ruilope, L. DiCarlo, R. Mukherjee, Serum potassium and clinical outcomes in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation 118, 1643–1650 (2008)
L. Pascual-Le Tallec, M. Lombes, The mineralocorticoid receptor: a journey exploring its diversity and specificity of action. Mol. Endocrinol. 19, 2211–2221 (2005)
P.J. Fuller, M.J. Young, Mechanisms of mineralocorticoid action. Hypertension 46, 1227–1235 (2005)
C. Grossmann, M. Gekle, Nongenotropic aldosterone effects and the EGFR: interaction and biological relevance. Steroids 73, 973–978 (2008)
C. Grossmann, A.W. Krug, R. Freudinger, S. Mildenberger, K. Voelker, M. Gekle, Aldosterone-induced EGFR expression: interaction between the human mineralocorticoid receptor and the human EGFR promoter. Am. J. Physiol. Endocrinol. Metab. 292, E1790–E1800 (2007)
J. Irita, T. Okura, M. Kurata, K. Miyoshi, T. Fukuoka, J. Higaki, Osteopontin in rat renal fibroblasts: functional properties and transcriptional regulation by aldosterone. Hypertension 51, 507–513 (2008)
S. Sakurabayashi-Kitade, Y. Aoka, H. Nagashima, H. Kasanuki, N. Hagiwara, M. Kawana, Aldosterone blockade by spironolactone improves the hypertensive vascular hypertrophy and remodeling in angiotensin II overproducing transgenic mice. Atherosclerosis 206, 54–60 (2009)
F. Sam, Z. Xie, H. Ooi, D.L. Kerstetter, W.S. Colucci, M. Singh, K. Singh, Mice lacking osteopontin exhibit increased left ventricular dilation and reduced fibrosis after aldosterone infusion. Am. J. Hypertens. 17, 188–193 (2004)
J. Irita, T. Okura, S. Manabe, M. Kurata, K. Miyoshi, S. Watanabe, T. Fukuoka, J. Higaki, Plasma osteopontin levels are higher in patients with primary aldosteronism than in patients with essential hypertension. Am. J. Hypertens. 19, 293–297 (2006)
H. Otani, F. Otsuka, K. Inagaki, M. Takeda, T. Miyoshi, J. Suzuki, T. Mukai, T. Ogura, H. Makino, Antagonistic effects of bone morphogenetic protein-4 and -7 on renal mesangial cell proliferation induced by aldosterone through MAPK activation. Am. J. Physiol. Renal Physiol. 292, F1513–F1525 (2007)
C. Walczak, F. Gaignier, A. Gilet, F. Zou, S.N. Thornton, A. Ropars, Aldosterone increase VEGF-A production in human neutrophils through PI3K, ERK1/2 and p38 pathways. Biochim. Biophys. Acta 1813, 2125–2132 (2011)
Y. Zhan, S. Kim, Y. Izumi, Y. Izumiya, T. Nakao, H. Miyazaki, H. Iwao, Role of JNK, p38, and ERK in platelet-derived growth factor-induced vascular proliferation, migration and gene expression. Arterioscler. Thromb. Vasc. Biol. 23, 795–801 (2003)
Z.H. Zhang, Y. Yu, S.G. Wei, R.B. Felder, Aldosterone-induced brain MAPK signaling and sympathetic excitation are angiotensin II type-1 receptor dependent. Am. J. Physiol. Heart Circ. Physiol. 302, H742–H751 (2012)
A. Nguyen Dinh Cat, A.M. Briones, G.E. Callera, A. Yogi, Y. He, A.C. Montezano, R.M. Touyz, Adipocyte-derived factors regulate vascular smooth muscle cells through mineralocorticoid and glucocorticoid receptors. Hypertension 58, 479–488 (2011)
K. Isoda, Y. Kamezawa, M. Ayaori, M. Kusuhara, N. Tada, F. Ohsuzu, Osteopontin transgenic mice fed a high-cholesterol diet develop early fatty-streak lesions. Circulation 107, 679–681 (2003)
Y. Matsui, S.R. Rittling, H. Okamoto, M. Inobe, N. Jia, T. Shimizu, M. Akino, T. Sugawara, J. Morimoto, C. Kimura, S. Kon, D. Denhardt, A. Kitabatake, T. Uede, Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 23, 1029–1034 (2003)
D. Panda, G.C. Kundu, B.I. Lee, A. Peri, D. Fohl, I. Chackalaparampil, B.B. Mukherjee, X.D. Li, D.C. Mukherjee, S. Seides, J. Rosenberg, K. Stark, A.B. Mukherjee, Potential roles of osteopontin and alphaVbeta3 integrin in the development of coronary artery restenosis after angioplasty. Proc. Natl. Acad. Sci. U.S.A. 94, 9308–9313 (1997)
J. Irita, T. Okura, M. Jotoku, T. Nagao, D. Enomoto, M. Kurata, V.R. Desilv, K. Miyoshi, Y. Matsui, T. Uede, D.T. Denhardt, S.R. Rittiling, J. Higaki, Osteopontin deficiency protects against aldosterone-induced inflammation, oxidative stress, and interstitial fibrosis in the kidney. Am. J. Physiol. Renal Physiol. 301, F833–F844 (2011)
S.M. Martin, J.L. Schwartz, C.M. Giachelli, B.D. Ratner, Enhancing the biological activity of immobilized osteopontin using a type-1 collagen affinity coating. J. Biomed. Mater. Res. A 70, 10–19 (2004)
H.E. Yim, K.H. Yoo, I.S. Bae, G.Y. Jang, Y.S. Hong, J.W. Lee, Aldosterone regulates cellular turnover and mitogen-activated protein kinase family expression in the neonatal rat kidney. J. Cell. Physiol. 219, 724–733 (2009)
Y. Nagai, K. Miyata, G.P. Sun, M. Rahman, S. Kimura, A. Miyatake, H. Kiyomoto, M. Kohno, Y. Abe, M. Yoshizumi, A. Nishiyama, Aldosterone stimulates collagen gene expression and synthesis via activation of ERK1/2 in rat renal fibroblasts. Hypertension 46, 1039–1045 (2005)
Acknowledgments
This research was supported financially by grants from the National Basic Research Program of China (2011CB503905). We thank Miao Y for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, GX., Xu, CC., Zhong, Y. et al. Aldosterone-induced osteopontin expression in vascular smooth muscle cells involves MR, ERK, and p38 MAPK. Endocrine 42, 676–683 (2012). https://doi.org/10.1007/s12020-012-9675-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9675-2