Abstract
The purpose of this study is to examine the association of A1C with beta-cell dysfunction, insulin resistance, and cardiovascular risk factors in Koreans with the relatively high risk for the future development of diabetes. This cross-sectional study recruited subjects from the pre-diabetic cohort of the Korea National Diabetes Program. Among study subjects (n = 616) aged 21–77 years with a history of hyperglycemia (fasting plasma glucose (FPG) ≥5.5 mmol/mL), analyses were conducted on 504 participants (296 women, 208 men) except for subjects with FPG ≥ 7.0 mmol/L or 120-min post-challenge plasma glucose ≥11.1 mmol/L or A1C ≥ 6.5 %. For insulin sensitivity and β-cell function classified by the categories of A1C levels, ∆Ins30-0/∆Glu30-0 was lower in the highest quartile group than other groups. Although there was no significant difference in HOMA-IR according to the A1C categories, even lowest A1C group (≤5.3 %) already included many subjects with abnormal glucose tolerance. A1C showed a significant association with hsCRP, number of metabolic syndrome (MetS) components and ∆Ins30-0/∆Glu30-0 after adjusting for age, gender, BMI, and medications whereas HOMA-IR was insignificantly associated with A1C. Stepwise regression analysis for A1C showed that A1C is independently and negatively associated with ∆Ins30-0/∆Glu30-0, and positively associated with hsCRP. Our study showed that higher A1C was associated with impaired early-phase insulin secretion, MetS, and low grade inflammation in Koreans with the relatively high risk for the future development of diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of type 2 diabetes is emerging as a serious health issue, especially in Asia [1]. For the primary prevention of diabetes, early identification of persons at high risk is important. Among the variables known to predict type 2 diabetes, fasting plasma glucose (FPG) and glucose levels 2-h after a glucose load are traditionally used for diagnosis and management of diabetes [2]. Because measuring FPG is less expensive, more convenient and more reproducible than performing an oral glucose tolerance test [3], the American Diabetes Association (ADA) recommended moving away from the OGTT to using FPG as diagnostic criterion [4]. Although the use of FPG is simpler and more reproducible [5], the omission of the 2-h plasma glucose will miss a proportion of diabetic and pre-diabetic patients who have normal FPG but elevated 2-h plasma glucose (≥7.8 mmol/l). A1C reflects plasma glucose levels over 2–3 months, is weighted towards more recent levels [6] and is useful for monitoring glycemic control in diabetic patients [7]. New clinical practice recommendations by some international organizations advocate the use of A1C in diagnosis of diabetes, largely on the basis of the established association between A1C and microvascular disease [5]. However, to our knowledge, there are no data on the association of A1C with metabolic parameters including insulin resistance (IR), insulin secretory function and cardiovascular disease (CVD) risk factors in Korean pre-diabetic subjects. During the past few years, it has been established that pre-diabetic conditions of isolated impaired fasting glucose (IFG), isolated impaired glucose tolerance (IGT), and combined fasting and post-challenge hyperglycemia (IFG/IGT) represent distinct pathways to diabetes [8, 9]. Although the primary abnormalities inherent in the different pre-diabetic conditions are still unknown, these pre-diabetic states are characterized by different degrees of insulin sensitivity and IR [10]. Thus, our objective in the current study was to evaluate the contribution of impaired beta-cell function and increase in IR across the range of A1C. Furthermore, this study examines the association of A1C with CVD risk factors and MetS in Korean with pre-diabetes.
Materials and methods
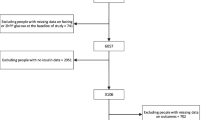
Subjects
This study is part of the Korea National Diabetes Program (KNDP) which is an ongoing epidemiologic study supported by the Ministry for Health, Welfare, and Family Affairs of Republic of Korea. The KNDP is a study of patients with type 2 diabetes and pre-diabetes which has been conducted from March 2005 to understand the characteristics of type 2 diabetes and pre-diabetes and to develop clinical guidelines in Korea. This study was conducted in pre-diabetes cohort. Subjects (n = 616, men: 267, women: 349) aged 21–77 years with a history of hyperglycemia (FPG ≥ 5.5 mmol/mL) were enrolled from July 2006 to December 2009 in the KNDP. After the subjects fasted overnight for >10 h, the standard 75 g glucose for the OGTT was administered according to the recommendations of the National Diabetes Data Group [11]. Blood samples were collected at 0, 30, and 120 min after OGTT. Plasma glucose levels were measured at 0, 30, and 120 min for all subjects and plasma insulin levels at 0 and 30 min were measured. All participants provided written informed consent, and the Korea University Institutional Review Board, in accordance with the Declaration of Helsinki of the World Medical Association approved this study protocol. We excluded patients with (1) previously diagnosed any diabetes or taking any anti-diabetic medication; (2) pregnancy; (3) known liver or renal disease; (4) FPG ≥ 7.0 mmol/L or 120-min post-challenge plasma glucose (PPG120) ≥ 11.1 mmol/L or A1C ≥ 6.5 %. The remaining subjects (n = 504, men: 208, women: 296) were divided into four groups based on their 75 g results: NGT (n = 68; FPG < 5.6 mmol/L and PPG120 < 7.8 mmol/L), isolated IFG (n = 146; 5.6-7.0 mmol/L and < 7.8 mmol/L, respectively), isolated IGT (n = 93; < 5.6 mmol/L and 7.8–11.1 mmol/L, respectively), or combined IFG/IGT (n = 197; 5.6–7.0 mmol/L and 7.8–11.1 mmol/L, respectively) in accordance with the ADA criteria released in 2003 [12].
Clinical and laboratory measurements
The body mass index (BMI) was calculated as the weight/height2 (kg/m2) and the waist circumference was measured at the midpoint between the lower border of the rib cage and the iliac crest. Serum triglycerides and HDL cholesterol levels were determined enzymatically using a chemistry analyzer (Hitachi 747, Tokyo, Japan). The LDL cholesterol concentration was estimated using the Friedewald formula [13]. A glucose oxidase method was employed to measure plasma glucose, and an electrochemiluminescence immunoassay (Roche Diagnostics, Indianapolis, USA) was used to measure insulin levels. The trapezoidal rule was used to calculate the incremental area under the curve for glucose (AUCglucose) for the duration of the OGTT. IR was calculated by the Homeostasis Model Assessment (HOMA). The HOMA-IR was calculated using the updated HOMA2 method (based on the computer model) because it is more accurate than the original HOMA1 method (based on explicit formulas) [14, 15]. ∆Ins30-0/∆Glu30-0, as a measure of insulin secretion (an early secretory response to an oral glucose load), was calculated as reported previously [16]. We also measured ∆Ins30-0/∆Glu30-0/HOMA-IR as an adjusted insulin secretion that accounted for the degree of insulin sensitivity [17]. A1C was measured using high performance liquid chromatography (Bio-Rad Variant II). The CV for glucose was <1.5 % and that for A1C was <3.0 %. High-sensitivity C-reactive protein (hsCRP) levels were measured using a chemiluminescence immunoassay (Beckman, Coulter, USA). A random urine sample was obtained for albumin creatinine ratio (ACR) measurements. Urinary creatinine and albumin were measured using a turbidimetric assay (Cobas Integra, Roche Diagnostics, Mannheim, Germany). MetS was defined according to the criteria established by the National Cholesterol Education Program Adult Treatment Panel III using the adjusted waist circumference for Asians [18]. Accordingly, participants with three or more of the following five criteria were defined as having MetS: (i) abdominal obesity by waist circumference (defined as Asian specific waist circumference cut-off values of ≥90 cm for men and ≥80 cm for women), (ii) systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or on antihypertensive medication, (iii) elevated fasting blood glucose (≥5.6 mmol/L), (iv) hypertriglyceridemia (≥1.7 mmol/L), and (v) low serum HDL–cholesterol <1.03 mmol/L in men and <1.29 mmol/L in women).
Statistical analysis
Data are expressed as means ± SD or median (interquartile range). Discrete variables are presented as the total number (percentage). For non-normally distributed variables, a natural logarithmic transformation was performed before data analysis. Descriptive data were presented according to categories of A1C quartile (<5.3 %, 5.3 to <5.7 %, 5.7 to <6.0, and ≥6.0 %); unequal numbers were found in the quartiles because of decimals, and analyzed with analysis of variance or χ2 test. After adjusting for gender, age, BMI, and medication, Pearson’s partial correlation coefficients were calculated to evaluate the relationship between cardiovascular risk factors, and A1C. Differences in A1C between those with and without MetS were evaluated by the Wilcoxon’s rank-sum test. To determine independent parameters related to A1C levels, multiple stepwise regression analysis was performed. Data were analyzed using the SPSS for Windows (Version 12.0; SPSS Inc.; Chicago, IL, USA). All statistical results were based on two-sided tests. A P value of <0.05 was considered statistically significant.
Results
The study groups included 508 subjects with pre-diabetes (men: 208, women: 296). The age of subjects enrolled ranged from 21 to 77 years with a mean of 51.6 ± 10.3 years of age. Men had larger waist circumferences than women (88.8 ± 7.0 vs. 84.0 ± 9.8 cm, respectively, P < 0.001), higher systolic blood pressure (SBP) (125.6 ± 14.6 vs. 121.3 ± 16.3 mmHg, respectively, P = 0.002), diastolic blood pressure (DBP) (81.3 ± 11.6 vs. 76.9 ± 10.4 mmHg, respectively, P < 0.001), FPG (5.9 ± 0.5 vs. 5.7 ± 0.5 mmol/L, respectively, P < 0.001), and serum triglycerides (4.2 ± 2.7 vs. 3.3 ± 2.4 mmol/L, respectively, P < 0.001) but lower levels of HDL cholesterol (1.2 ± 0.3 vs. 1.4 ± 0.4 mmol/L, respectively, P < 0.001) and A1C (5.6 ± 0.5 vs. 5.7 ± 0.4 %, respectively, P = 0.011).
The clinical and biochemical characteristics of the pre-diabetic group stratified according to quartiles of A1C are shown in Table 1. Gender was not equally distributed in each the quartile group because of a lower number of subjects with the highest quartile of A1C. Age, BMI, waist circumference, hsCRP, number of MetS components, and medication of statin increased significantly with increasing quartiles of A1C values. A1C levels were not correlated with SBP, DBP, total cholesterol, LDL cholesterol, triglycerides, antihypertensive agent, antiplatelet agent, and prevalence of coronary artery disease. Smoking status and alcohol drinking status were higher in individuals with lowest quartile compared with highest quartile, but this was because of the higher proportion of men in the lowest quartile group.
For the variables for glycemia, insulin sensitivity, and β-cell function classified by the preset categories of A1C levels, FPG and 30-min plasma glucose were higher in the highest quartile group than lowest quartile group, whereas ∆Ins30-0/∆Glu30-0 and ∆Ins30-0/∆Glu30-0/HOMA-IR was lower in the highest quartile group (Table 2). There were no significant differences in fasting plasma insulin levels, 30-min plasma insulin levels, and HOMA-IR according to the A1C categories. The majority of study subjects had abnormal glucose tolerance because study subjects had a history of hyperglycemia (FPG ≥ 5.5 mmol/mL) as subjects with a high risk for developing type 2 diabetes. Even 85 % of subjects in the group with lowest quartile of A1C had abnormal glucose tolerance (IFG, IGT, or combined IFG + IGT).
Although AUCglucose was associated with HOMA-IR (r = 0.18, P = 0.005), ∆Ins30-0/∆Glu30-0 (r = −0.27, P < 0.001), and ∆Ins30-0/∆Glu30-0/HOMA-IR (r = −0.37, P < 0.001), A1C was associated with ∆Ins30-0/∆Glu30-0 and ∆Ins30-0/∆Glu30-0/HOMA-IR rather than HOMA-IR. After adjusting for age, gender, BMI, and medication, the partial correlation analysis showed that A1C was significantly associated with hsCRP, number of MetS components, FPG, 30-min plasma glucose levels, AUCglucose, 30-min plasma insulin, ∆Ins30-0/∆Glu30-0, and ∆Ins30-0/∆Glu30-0/HOMA-IR (Table 3). In addition, Women with MetS showed increased A1C (5.7 ± 0.4 vs. 5.6 ± 0.4, P = 0.007) compared to those without MetS, whereas, in men, the difference did not reach statistical significance, probably because of small numbers (5.6 ± 0.4 vs. 5.5 ± 0.4, P = 0.248).
To investigate whether the significant factors shown in Table 3 was related independently to A1C, multiple regression analysis was performed (Table 4). Stepwise regression analysis for A1C showed that A1C is independently and negatively associated with ∆Ins30-0/∆Glu30-0 (P = 0.047), and positively associated with age (P = 0.003), hsCRP (P = 0.001) and FPG (P = 0.007).
Discussion
The main findings of the study are that in Korean with the relatively high risk for the future development of diabetes, A1C showed an association with early-phase insulin secretion assessed by insulinogenic index. However, IR assessed by HOMA-IR was not significantly associated with A1C. We, therefore, suggest that the elevated A1C is more closely related to impaired early-phase insulin secretion than IR in Korean with the relatively high risk for the future development of diabetes.
Type 2 diabetes is a progressive disease of IR and defects in insulin secretion due to loss of β-cell function. At the time of diagnosis, only about 50 % of β-cell function remains, underscoring the progressive nature of the disease and the importance of early diagnosis and prevention [19]. Overt type 2 diabetes is usually preceded by a condition known as pre-diabetes [20]. As with measure of glucose, a continuum of risk for the development of diabetes based on A1C levels has been demonstrated [21, 22]. Thus, the continuum of risk in the subdiabetic glycemic range argues for the elimination of dichotomous subdiabetic classifications, such as IFG and IGT. Although the International Expert Committee did not formally indentify an equivalent intermediate category for A1C [5], incidence of diabetes in people with A1C levels in the range from 6.0 to <6.5 is more than 10 times that of people with lower levels [21, 23–26]. Our findings show that Korean subjects with a A1C value of 6.0 % or higher may be at high risk for the development of diabetes, as a feature of the impaired early-phase insulin secretion.
In normal subjects an improvement in insulin sensitivity is compensated by a decrease in insulin secretion according to the hyperbolic law of glucose tolerance [27]. This hyperbolic relationship between insulin sensitivity and insulin secretion is represented on the curve of the “disposition index (DI).” [28]. DI becomes progressively lower in those along the progression from normal to type 2 diabetes, reflecting a decreased ability of the pancreas to fully compensate for increases in IR, while DI is relatively high in normal individuals [29–31]. Although HOMA-IR, ∆Ins30-0/∆Glu30-0, and ∆Ins30-0/∆Glu30-0/HOMA-IR were evaluated instead of DI in this study, compared with lowest A1C quartile group, highest A1C quartile group had similar degrees of IR but impaired insulin secretion relative to the degree of its IR. Jansen et al. showed that relationship between HOMA-IR and AUCglucose was weak (r 2 = 0.084, P < 0.001). In contrast, there was a strong inverse curvilinear relationship between beta-cell function and AUCglucose (log[∆Ins30-0/∆Glu30-0]: r 2 = 0.29, P < 0.001; log[∆Ins30-0/∆Glu30-0/HOMA-IR]: r 2 = 0.45, P < 0.001) [17]. Subjects with low A1C may already have much IR in group with high risk for the development of diabetes (i.e., a history of hyperglycemia (FPG ≥ 5.5 mmol/ml)) because the group with lowest quartile of A1C had many subjects with abnormal glucose tolerance (IFG, IGT, or combined IFG + IGT). Therefore, worsening degree of glucose intolerance across the A1C spectrum was associated with both IR and impaired beta-cell function, with reduction of early-phage insulin secretion seeming to be more important in determining A1C in subjects with pre-diabetes. In addition, Shin et al. [32] reported that β-cell dysfunction is associated with subsequent development of type 2 diabetes in Koreans, while general obesity and fasting insulin, surrogates for IR, are not. Therefore, the major risk factor for type 2 diabetes in Koreans, who have relatively lower BMI compared with Western counterparts, appears to be impaired insulin secretion rather than worsening IR in pre-diabetic condition.
hsCRP is a sensitive systemic marker of inflammation [33]. Several previous studies have reported that CRP is associated with risk of type 2 diabetes [34–37]. However, few studies have examined the role of serum CRP level in the development of type 2 diabetes among Korean subjects. This study showed that in Korean with the relatively high risk for the future development of diabetes, increasing hsCRP levels were positively associated with the elevated A1C levels, and this association was remained statistically significant, even after adjustment with age, gender, BMI, and medication. Although hsCRP levels are lower in the study subjects, our findings suggested that inflammation may remain to be involved in the pathogenesis in Korean with the relatively high risk for the future development of diabetes.
Numerous studies have examined the ability of the MetS to predict type 2 diabetes. The presence of the MetS increases the risk [38, 39] and is highly predictive of new-onset type 2 diabetes [40, 41]. The risk for incident type 2 diabetes is up to five times higher in individuals with the MetS compared with those without the syndrome [42]. In the current study, number of the components of the MetS increased with increasing quartiles of A1C, and the difference reached statistical significance in the highest quartile (A1C > 6.0 %). We also found that A1C levels in subjects with MetS were significantly higher than that of subjects without MetS. However, in this study, HOMA-IR was not related to A1C. Several reasons are considered. First, The limitation of the validity of the HOMA-IR should be carefully considered in subjects with a lower BMI, a lower beta-cell function, and high fasting glucose levels such as lean type 2 diabetes mellitus with insulin secretory defects [43]. It should also be noted that almost all study participants, including those comprising the NGT group, had a history of hyperglycemia (FPG ≥ 5.5 mmol/ml) before recruitment. Thus, findings with NGT group or lowest quartile group (A1C < 5.3 %) in this study may not reflect a truly normal subject population (i.e., with normal screening (FPG < 5.5 mmol/ml) and normal results on a diagnostic OGTT). These groups may already have higher IR than truly NGT groups.
Our study has several limitations that must be considered. First, the cross-sectional nature of this study did not allow us to identify causal relationships. Second, The classification of glucose tolerance status was based on single OGTTs. Accordingly, it is likely that some individuals may have been misclassified because of normal day-to-day variations in plasma glucose. In addition, the intra-individual variation in serum insulin levels is large [44], affecting the estimates of insulin secretion and action. Small changes in the estimates would therefore be expected if the same measurements were repeated on a separate day. In the future, however, we would confirm the results of current study in our longitudinal studies.
In conclusion, our study showed that subjects with a A1C value of 6.0 % or higher may be at high risk for the development of diabetes, as a feature of the impaired early-phase insulin secretion. Furthermore, A1C was significantly and positively associated with the MetS and low grade inflammation. These results suggest that A1C might be useful for early diagnosis of subjects with high risk for the future development of diabetes, and for estimating the risk stratification of patients with abnormal glucose metabolism.
References
J.C. Chan, V. Malik, W. Jia, T. Kadowaki, C.S. Yajnik, K.H. Yoon, F.B. Hu, Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 301, 2129–2140 (2009)
K.G. Alberti, P.Z. Zimmet, Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 15, 539–553 (1998)
R.G. Barr, D.M. Nathan, J.B. Meigs, D.E. Singer, Tests of glycemia for the diagnosis of type 2 diabetes mellitus. Ann. Intern. Med. 137, 263–272 (2002)
Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 20, 1183–1197 (1997)
International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32, 1327–1334 (2009)
Y. Tahara, K. Shima, Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care 18, 440–447 (1995)
K.H. Tseng, Standards of medical care in diabetes—2006: response to the American Diabetes Association. Diabetes Care 29, 2563–2564 (2006). author reply 2564–2565
C. Meyer, W. Pimenta, H.J. Woerle, T. Van Haeften, E. Szoke, A. Mitrakou, J. Gerich, Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care 29, 1909–1914 (2006)
K. Faerch, A. Vaag, J.J. Holst, T. Hansen, T. Jorgensen, K. Borch-Johnsen, Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance: the Inter99 study. Diabetes Care 32, 439–444 (2009)
M.A. Abdul-Ghani, C.P. Jenkinson, D.K. Richardson, D. Tripathy, R.A. DeFronzo, Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 55, 1430–1435 (2006)
Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes 28, 1039–1057 (1979)
Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 26 Suppl 1, S5–20 (2003)
W.T. Friedewald, R.I. Levy, D.S. Fredrickson, Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502 (1972)
A. Caumo, G. Perseghin, A. Brunani, L. Luzi, New insights on the simultaneous assessment of insulin sensitivity and beta-cell function with the HOMA2 method. Diabetes Care 29, 2733–2734 (2006)
T.M. Wallace, J.C. Levy, D.R. Matthews, Use and abuse of HOMA modeling. Diabetes Care 27, 1487–1495 (2004)
M. Matsuda, R.A. DeFronzo, Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999)
C.C. Jensen, M. Cnop, R.L. Hull, W.Y. Fujimoto, S.E. Kahn, Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the US. Diabetes 51, 2170–2178 (2002)
S.M. Grundy, J.I. Cleeman, S.R. Daniels, K.A. Donato, R.H. Eckel, B.A. Franklin, D.J. Gordon, R.M. Krauss, P.J. Savage, S.C. Smith Jr, J.A. Spertus, F. Costa, Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol. Rev. 13, 322–327 (2005)
U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes 44, 1249–1258 (1995)
V.A. Fonseca, Identification and treatment of prediabetes to prevent progression to type 2 diabetes. Clin. Cornerstone 9, 51–59 (2008). discussion 60-51
D. Edelman, M.K. Olsen, T.K. Dudley, A.C. Harris, E.Z. Oddone, Utility of hemoglobin A1c in predicting diabetes risk. J. Gen. Intern. Med. 19, 1175–1180 (2004)
C. Droumaguet, B. Balkau, D. Simon, E. Caces, J. Tichet, M.A. Charles, E. Eschwege, Use of HbA1c in predicting progression to diabetes in French men and women: data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 29, 1619–1625 (2006)
A.D. Pradhan, N. Rifai, J.E. Buring, P.M. Ridker, Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am. J. Med. 120, 720–727 (2007)
K.K. Sato, T. Hayashi, N. Harita, T. Yoneda, Y. Nakamura, G. Endo, H. Kambe, Combined measurement of fasting plasma glucose and A1C is effective for the prediction of type 2 diabetes: the Kansai Healthcare Study. Diabetes Care 32, 644–646 (2009)
T. Shimazaki, T. Kadowaki, Y. Ohyama, K. Ohe, K. Kubota, Hemoglobin A1c (HbA1c) predicts future drug treatment for diabetes mellitus: a follow-up study using routine clinical data in a Japanese university hospital. Transl. Res. 149, 196–204 (2007)
L.S. Geiss, L. Pan, B. Cadwell, E.W. Gregg, S.M. Benjamin, M.M. Engelgau, Changes in incidence of diabetes in US adults, 1997–2003. Am. J. Prev. Med. 30, 371–377 (2006)
S.E. Kahn, R.L. Prigeon, D.K. McCulloch, E.J. Boyko, R.N. Bergman, M.W. Schwartz, J.L. Neifing, W.K. Ward, J.C. Beard, J.P. Palmer et al., Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42, 1663–1672 (1993)
R.N. Bergman. Orchestration of homeostasis of glucose metabolism. Journ Annu Diabetol Hotel Dieu, 127–138 (2007)
K.M. Utzschneider, R.L. Prigeon, D.B. Carr, R.L. Hull, J. Tong, J.B. Shofer, B.M. Retzlaff, R.H. Knopp, S.E. Kahn, Impact of differences in fasting glucose and glucose tolerance on the hyperbolic relationship between insulin sensitivity and insulin responses. Diabetes Care 29, 356–362 (2006)
J. Hong, W.Q. Gu, Y.F. Zhang, Y.S. Yang, C.F. Shen, M. Xu, X.Y. Li, W.Q. Wang, G. Ning, The interplay of insulin resistance and beta-cell dysfunction involves the development of type 2 diabetes in Chinese obeses. Endocrine 31, 93–99 (2007)
C.A. Slentz, C.J. Tanner, L.A. Bateman, M.T. Durheim, K.M. Huffman, J.A. Houmard, W.E. Kraus, Effects of exercise training intensity on pancreatic beta-cell function. Diabetes Care 32, 1807–1811 (2009)
C.S. Shin, H.K. Lee, C.S. Koh, Y.I. Kim, Y.S. Shin, K.Y. Yoo, H.Y. Paik, Y.S. Park, B.G. Yang, Risk factors for the development of NIDDM in Yonchon County, Korea. Diabetes Care 20, 1842–1846 (1997)
A. Dehghan, I. Kardys, M.P. de Maat, A.G. Uitterlinden, E.J. Sijbrands, A.H. Bootsma, T. Stijnen, A. Hofman, M.T. Schram, J.C. Witteman, Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes 56, 872–878 (2007)
T.S. Han, N. Sattar, K. Williams, C. Gonzalez-Villalpando, M.E. Lean, S.M. Haffner, Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care 25, 2016–2021 (2002)
F.B. Hu, J.B. Meigs, T.Y. Li, N. Rifai, J.E. Manson, Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 53, 693–700 (2004)
S. Nakanishi, K. Yamane, N. Kamei, M. Okubo, N. Kohno, Elevated C-reactive protein is a risk factor for the development of type 2 diabetes in Japanese Americans. Diabetes Care 26, 2754–2757 (2003)
A.D. Pradhan, J.E. Manson, N. Rifai, J.E. Buring, P.M. Ridker, C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286, 327–334 (2001)
R.H. Eckel, S.M. Grundy, P.Z. Zimmet, The metabolic syndrome. Lancet 365, 1415–1428 (2005)
S.M. Grundy, H.B. Brewer Jr, J.I. Cleeman, S.C. Smith Jr, C. Lenfant, Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arter. Thromb. Vasc. Biol. 24, e13–e18 (2004)
D.E. Laaksonen, H.M. Lakka, L.K. Niskanen, G.A. Kaplan, J.T. Salonen, T.A. Lakka, Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am. J. Epidemiol. 156, 1070–1077 (2002)
R.L. Hanson, G. Imperatore, P.H. Bennett, W.C. Knowler, Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes 51, 3120–3127 (2002)
E.S. Ford, C. Li, N. Sattar, Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care 31, 1898–1904 (2008)
E.S. Kang, Y.S. Yun, S.W. Park, H.J. Kim, C.W. Ahn, Y.D. Song, B.S. Cha, S.K. Lim, K.R. Kim, H.C. Lee, Limitation of the validity of the homeostasis model assessment as an index of insulin resistance in Korea. Metabolism 54, 206–211 (2005)
J.M. Mooy, P.A. Grootenhuis, H. de Vries, P.J. Kostense, C. Popp-Snijders, L.M. Bouter, R.J. Heine, Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia 39, 298–305 (1996)
Acknowledgments
This study was supported by a Korean Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. A102065).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, T.N., Park, M.S., Lee, S.K. et al. Elevated A1C is associated with impaired early-phase insulin secretion rather than insulin resistance in Koreans at high risk for developing diabetes. Endocrine 42, 584–591 (2012). https://doi.org/10.1007/s12020-012-9666-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9666-3