Abstract
The issue of how rapid frequency GnRH pulses selectively stimulate LH transcription is not fully understood. The rat LHβ promoter contains two GnRH-responsive regions: the proximal region has binding elements for SF1, and the distal site contains a CArG box, which binds SRF. This study determined whether GnRH stimulates pituitary SF1, DAX1 (an endogenous SF1 inhibitor), and SRF transcription in vivo, and whether regulation is frequency dependent. Male rats were pulsed with 25 ng GnRH i.v. every 30 min or every 240 min for 1–24 h, and primary transcripts (PTs) and mRNAs were measured by real time PCR. Fast frequency GnRH pulses (every 30 min) increased SF1 PT (threefold) within 1 h, and then declined after 6 h. SF1 mRNA also increased within 1 h and remained elevated through 24 h. Fast frequency GnRH also stimulated a transient increase in DAX1 PT (twofold after 1 h) and mRNA (1.7-fold after 6 h), while SRF mRNA rose briefly at 1 h. Slow frequency pulses did not affect gene expression of SF1, DAX1, or SRF. These findings support a mechanistic link between SF1 in the frequency regulation of LHβ transcription by pulsatile GnRH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gonadotropins, LH and FSH, are dimeric protein hormones composed of a common α subunit (α) and a unique β subunit [1]. The subunit genes are regulated by hypothalamic GnRH in a coordinate as well as a differential manner. GnRH regulates subunit mRNA synthesis via changes in pulse frequency, with faster-intermediate GnRH pulse frequencies (8–60 min pulse intervals) favoring common glycloprotein α subunit, and LHβ and slower frequency pulses (≥120 min pulse intervals) favoring FSHβ [1].
The signal transduction mechanisms responsible for interpreting GnRH pulse frequency and differentially regulating β-subunit gene expression are not well understood. The GnRH receptor (GnRHR) is a member of the G protein-coupled receptor family [2], and GnRHR binding activates several G proteins, including Gq and G11 [2]. GnRH also stimulates the activation of various signal transduction pathways that regulate gonadotropin subunit transcription, including protein kinase C (PKC), calcium/calmodulin kinase II (CAMK2) and members of the mitogen-activated protein kinase (MAPK) signaling cascades [extracellular signal-regulated kinase (ERK, aka MAPK1/3), c-Jun N-terminal Kinase (JNK, aka MAPK8/9), and p38 (MAPK 11/12/13/14)] [2]. The role that these pathways play in differential responses to GnRH pulse frequency remains to be fully characterized. The author and other groups have reported that slow frequency GnRH pulses preferentially stimulates ERK activation (phosphorylation), suggesting a role for ERK in FSHβ; however, blocking ERK activation (with MEK inhibitors, or gonadotrope-specific gene knockout) results in loss of induction of both LHβ and FSHβ gene expression by GnRH [3–6]. In Addition, we found that CAMK2 and JNK are not activated by pulsatile GnRH in a frequency-dependant manner [5]. Thus, it is unlikely that fast frequency GnRH pulses regulate LHβ transcription via selective activation of one signaling cascade versus another.
The rat LHβ promoter contains two GnRH responsive regions [7]. The proximal region included binding elements for steroidogenic factor 1 (SF1, aka NR5A1), early growth response 1 (Egr1) , and pituitary homeobox 1 (PITX1). Although PITX1 binding is critical for basal LHβ transcription [8, 9], the effects of GnRH are primarily through Egr1 and SF1. Egr1- and pituitary-specific SF1 knockout mice are either completely infertile or subfertile, due to a lack of LHβ synthesis [10–13]. Two Egr1-binding sites have been identified in the proximal GnRH responsive region of the LHβ promoter, which are highly conserved across species [11, 14–18], and mutations within these Egr1-binding sites abrogate the GnRH induction of LHβ promoter reporter constructs in gonadotrope-derived cell lines [18–21]. However, recently we did not find that pituitary Egr1 (transcripts or protein) or the two Egr1 inhibitors (NAB1 and/or 2) were differentially regulated by GnRH pulse frequency in rats in vivo [22]. Adjacent to the two Egr1-binding sites on the LHβ promoter are two SF1-binding sites [14, 16, 17, 23]. Both SF1 sites are required for maximal induction of LHβ by GnRH [12, 13] and over-expression analyses indicate that Egr1 and SF1 act in tandem to stimulate LHβ transcription [16–18, 20].
SF1 is an orphan nuclear receptor with a modular domain structure is composed of an N-terminal zinc finger DNA-binding, a ligand-binding, a C-terminal AF-2 activation domains, and an intervening proline-rich hinge region that has AF-1-like activation activity [24]. It has been reported that GnRH does not stimulate SF1 gene expression in gonadotrope-derived LβT2 cells [19, 20, 25]. However, in rats, we showed that gonadectomy increased pituitary SF1 mRNA two to threefold, which was reversed by suppression of endogenous GnRH, and that SF1 mRNA was increased by GnRH pulses administered every 30 min [26]. Recent findings suggest that SF1 transcriptional activity can be regulated by its corepressor DAX1 (dosage-sensitive sex reversal-AHC critical region on the X chromosome gene 1, aka NR0B1). Specifically, DAX1 over-expression was found to inhibit LHβ promoter activity, and DAX1 mRNA is selectively stimulated by slow frequency GnRH pulses in LβT2 cells [25].
In addition to the critical elements in the proximal LHβ promoter, the distal GnRH-responsive region of the rat LHβ promoter contains two specificity protein 1 (SP1)-binding sites and a CArG box which overlaps with the 5′ SF1 site [7, 16]. Mutation or deletion of either the SP1 or CArG elements reduced basal LHβ transcription, but disruption of the CArG box specifically eliminated the response to pulsatile GnRH [7, 16]. Serum Response Factor (SRF) is a MADS box transcription factor that typically binds to CArG box elements [27], often in conjunction with an ETS-binding protein [CArG box + ETS-binding site = serum response element (SRE)]. GnRH has been reported to stimulate SRE promoter–reporter constructs in gonadotrope-derived LβT2 cells [28], and SRF-mediated SRE activity in LβT2 cells is both ERK and protein kinase A (PKA) regulated [29].
Thus, the aim of this study was to investigate whether GnRH pulse frequency differentially regulates SF1, DAX1, and SRF gene and protein expressions in normal rat pituitary cells, and to correlate those effects with LHβ gene transcription.
Materials and methods
In vivo studies
Adult (225–250 g) male Sprague Dawley rats (Harlan Sprague Dawley, Inc., Indianapolis, IN) were used for all experiments. Animals were housed in a light (lights on 0500–1700 h)- and temperature (25°C)-controlled room and allowed access to food and water ad libitum. All surgeries were performed under isoflurane (2.5% isoflurane, balance O2; ISO-THESIA, Vetus Animal Heath, Burns Veterinary Supply, Inc, Westbury, NY) anesthesia. At the completion of experiments, rats were euthanized by decapitation under anesthesia. All experimental procedures were conducted in accordance with the NRC publication—Guide for Care and Use of Laboratory Animals and were approved by the University of Virginia Animal Care and Use Committee.
To study the effects of GnRH pulse frequency, we used a GnRH-deficient [castrate + testosterone (T) replaced] rat model. Animals were castrated, and two 20 mm T-containing silastic implants were inserted s.c. (serum T levels were 4–5 ng/ml, 24-h post-implant insertion), as previously described [30]. An indwelling right jugular cannula was also inserted at the time of castration for i.v. GnRH administration, 24 h after castration. Rats (n = 5–7/group) received 25 ng GnRH pulses (in 0.25 ml 0.9% saline–0.1% BSA) either every 30 min or every 240 min for 1–24 h. Controls were pulsed with vehicle only. Animals were euthanized 5 min after the last pulse, based on the previous data in rats showing that LHβ and FSHβ primary transcript (PT) responses to GnRH are maximal 5 min after a pulse [31]. Pituitaries were collected, snap frozen in liquid nitrogen, and stored at −70°C until RNA extraction.
In studies to determine the effects of GnRH pulse frequency on SF1, DAX1, and SRF protein levels, 6–7 rats per group received GnRH pulses (25 ng, i.v.) every 30 min or every 240 min for 8 h (controls were pulsed with vehicle only). Animals were euthanized 15 min after the last pulse; based on previous in vivo data showing that activation/phosphorylation events in response to GnRH for various signaling proteins were maximal at this time point [32] The 8-h treatment duration was chosen as it was after all the changes in mRNA had occurred, and the previous studies revealed that changes in other transcription factor proteins, such as Egr1, occur within 8 h [22].
RNA preparation and measurement of SF1, DAX1, and SRF transcripts
Total pituitary RNA was extracted using the acid guanidinium method [33]. Residual genomic DNA was removed by treatment with 1 U RNase-free DNase I/µg RNA (Roche Molecular Biochemicals, Indianapolis, IN) at 37°C for 1 h. RNA preparations were confirmed to be DNA free by PCR in the absence of a preceding reverse transcriptase (RT) reaction. Primary transcripts and mRNAs were measured by real-time PCR as previously described [34]. PCR assay primers were (5′–3′) SF1 PT FWD AGAGGGTGATGGGCTGCT, SF1 PT/mRNA REV ACCTCCACCAGGCACAATAG, SF1 mRNA FWD CGCCAGGAGTTTGTCTGTCT, DAX1 PT/mRNA FWD TCCAGGCCATCAAGAGTTTC, DAX1 PT REV AAGCTCACCCACTTGACCAC, DAX1 mRNA REV TGTGCTCAGTGAGGATCTGC, SRF PT FWD CCTTCCTTACAGATGGCTGTG, SRF PT/mRNA REV GCGGATCATTCACTCTTGGT, SRF mRNA FWD GCTCAATGCCTTCTCTCAGG.
To confirm amplification of a single product, the PCR reaction was followed by melt curve analysis. To create a standard for each subscript, RNA from a pooled rat pituitary cDNA sample was amplified by PCR using the aforementioned primers and then subcloned into PGEM T-EZ (Promega, Madison, WI). The identity of all the PCR products was confirmed by DNA sequencing. Each PCR reaction was optimized for annealing temperature and Mg2+ concentration to obtain a PCR efficiency of 90–105%. Each sample was measured in triplicate against a standard curve. All the samples were measured in the same assay to avoid inter-assay variability.
Protein preparation and western blot immunostaining
Pituitary protein was prepared as previously described [10]. Protein lysates (30 µg) were resolved by 8% SDS PAGE electrophoresis and then transferred to nitrocellulose filters. Blots were then immunostained for SF1, DAX1, and SRF (total and phosphorylated). Primary antibodies (rabbit) were obtained from Millipore [Billerica, MA; SF1 (07-618)] Cell Signaling [Beverly, MA; phospho SRF (#4261)] and Santa Cruz Biotech [Santa Cruz, CA; DAX1 (H-300); total SRF (G-20); Glyceraldehyde 3-phosphate dehydrogenase (FL-335)]. Protein loading was standardized by immunostaining for GAPDH. The secondary antibody was horseradish peroxidase (HRP)-conjugated goat anti-rabbit (Millipore, Billerica, MA). Immunoactivity was detected using SuperSignal West Pico chemiluminescent system (Pierce, Rockville, IL), followed by autoradiography. Protein bands were quantified by densitometry using TotalLab Software (Amersham Biosciences, Piscataway, NJ).
Analysis
All the data were examined by ANOVA. Significant differences (P < 0.05) were determined post hoc by Duncan’s Multiple Range test. Before the analyses, all measurements were transformed to the logarithmic scale to attain equal variation among treatments.
Results
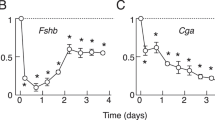
Previously, we have reported that LHβ transcription is maximally stimulated by faster frequency (30 min) and FSHβ by slower (240 min) GnRH pulses [30]. To examine potential links between frequency-dependent regulation of the gene and specific downstream transcriptional mediators, the effects of GnRH pulse frequency in vivo on SF1, DAX1, and SRF PTs and mRNAs were determined (Fig. 1). GnRH pulses every 30 min resulted in a threefold increase in SF1 PT within 1 h (P < 0.05); however, the observed rise was transient and levels returned to basal by 6 h. Similarly, SF1 mRNA was also increased within 1 h (coincident with, but before the peak increase in LHβ PT) [22], which was sustained over 24 h (P < 0.05). In contrast, GnRH pulses every 240 min had no effect on either SF1 PT or mRNA.
Rats received 25 ng GnRH pulses either every 30 min (Fast) or every 240 min (Slow) for 1–24 h (vehicle pulses to controls; n = 5–7/group). Primary transcripts (PT) or steady-state mRNAs were measured by quantitative real-time PCR. Data are expressed as percent 0 h controls ±SE. * Indicates a significant difference (P < 0.05) versus controls (0 h). ** Indicates a significant difference between GnRH pulse frequency paradigms at 24 h
Thirty-minute GnRH pulses transiently increased DAX1 PT (2-fold at 1 h; P < 0.05) and DAX1 mRNA (1.7-fold at 6 h; P < 0.05), whereas GnRH pulses every 240 min had no effect. Similar to SF1 and DAX1, slow frequency GnRH pulses had no effect on SRF PT or mRNA. In contrast, GnRH pulses every 30 min stimulated a transient (2-fold) increase in SRF mRNA after 1 h (P < 0.05). It is of interest that the rise in SRF mRNA was not associated with a corresponding increase in SRF PT, which might suggest a non-transcriptional mechanism for GnRH regulation of SRF gene expression.
To determine the effects of pulse frequency on SF1, DAX1 and SRF protein expressions, rats received 30- or 240-min pulses of GnRH for 8 h. This time point was selected because it followed the rise in mRNA for each transcription factor examined. Protein lysates from αT3 and LβT2 cell lines were included in each immunoblot as internal controls. As shown in Fig. 2, SF1 protein was detectable in rat pituitary cells, but was considerably lower than levels seen in αT3 and LβT2 cells (SF1 protein levels in αT3 and LβT2 cells were >50 fold higher than rat pituitary cells). The differential expression of various regulatory proteins in primary gonadotropes vs. gonadotrope cell lines have been observed previously [22]. In this study, we were unable to demonstrate a SF1-stimulatory response to pulsatile GnRH given every 30 min. The explanation is unclear, but may reflect that 8-h stimulation by GnRH is not adequate to demonstrate an increase in SF1 protein expression in primary rat pituitary cells.
Rats were given pulses of GnRH (25 ng/pulse), either every 30 min or every 240 min for 8 h (vehicle pulses to controls; n = 6–7/group). SF1, DAX1 and phosphorylated SRF (pSRF) protein levels were measured by immunoblot. Cell lysated from αT3 and LβT2 cells were included as internal controls. For pSRF, Hela cell lysates (±PMA) were included as positive controls. A representative immunoblot from two experiments is shown
Similar to a previous report [25], we found that DAX1 protein was abundant in LβT2 cells, and levels were fivefold higher than that seen in αT3 cells. However, we were not able to detect DAX1 protein in pituitary lysates from either control or GnRH pulse-treated rats. In contrast, SRF protein was readily detectable in rat lysates, but there was no observable stimulatory effect of pulsatile GnRH. The inability to show DAX1 or SRF protein responses could relate to the modest and transient nature of the mRNA increases after pulsatile GnRH (Fig. 1).
To address this issue further, we determined whether GnRH pulse frequency regulates SRF activation by measuring phospho-SRF (p-SRF) in response to 30- or 240-min pulses of GnRH. In order to validate the assay, cell lysates from HELA cells (± PMA) were included in each immunoblot as positive controls (cell lysates obtained from Cell Signaling; Beverly, MA), and showed the expected increases in p-SRF. In rat pituitary cells, there was a tendency for p-SRF to increase with 30-min pulses of GnRH; however, the effect was not significant.
Discussion
We have previously shown that GnRH pulses every 30 min maximally increase LHβ PT levels, but only transiently increase FSHβ transcription [30]. In contrast, while GnRH pulses every 240 min are optimal for FSHβ PT, a stimulatory effect on LHβ was not seen [30]. Findings from several studies by our group and others suggest that pulsatile GnRH acts on the gonadotrope both directly and indirectly (i.e., via pituitary follistatin and activin B) to regulate FSHβ transcription [35], but actions on LHβ remain unclear. Egr1 plays a critical role in LHβ transcriptional responses to GnRH [22], and in rats, we found that both fast and slow GnRH pulses increased Egr1 gene expression and protein, but not in a frequency-dependent manner. In light of these results, we examined the regulation of the other transcription factors that bind the LHβ promoter and confer GnRH responsiveness, specifically SF1 (and its corepressor DAX1) and SRF, which likely binds to the CArG box in the distal promoter.
We found that GnRH pulses every 30 min preferentially increased both SF1 PT and steady-state mRNA, whereas GnRH pulses every 240 min had no effect. This is in contrast to data previously reported for either αT3 or LβT2 clonal gonadotrope cells where GnRH had no effect on either SF1 mRNA or protein levels [19, 20, 25]. This difference may be due to in vivo versus in vitro experimental paradigms, as we have previous reported that SF1 mRNA levels increase in vivo in rats under conditions of high GnRH pulse frequency (gonadectomy or exogenous GnRH pulses every 30 min [26]). Alternatively, the difference may be due to basal levels of SF1 gene expression in αT3 and/or LβT2 cells. We observed that basal SF1 protein levels (on a per µg protein level) are much higher in both αT3 and LβT2 cells than in rat pituitary lysates, higher than those that could be expected if only 8–10% of pituitary cells are gonadotropes [36]. We recently made a similar observation of differential expression between rat gonadotropes versus αT3 cells. In the rat pituitary, Egr1 is selectively expressed in gonadotropes [37] and basal Egr1 is readily measurable, whereas it is essentially undetectable in αT3 cells [22]. As a result, GnRH induction of Egr1 gene expression and protein levels in the rat was relatively modest (two to threefold) compared to αT3 cells (150–400-fold). Therefore, if SF1 expression remains elevated in the basal state in clonal gonadotropes, then it is possible that GnRH may have little observed effect.
The issue of how GnRH regulates SF1 gene expression is currently unknown. The SF1 promoter is uncharacteristically short, and the minimal promoter sequence required for tissue specific expression is approximately 80 bp [38, 39]. There are three critical elements for SF1 expression: an E-Box, a CAAT Box, and an SP1-binding site. In αT3 and LβT2 cells, the critical element for SF1 expression is the E-Box which binds the helix loop helix (βHLH) proteins - upstream stimulating factor 1 (USF1) and USF2 [39]. Whether GnRH regulates either USF at the gene expression or the protein synthesis level remains to be determined. Both the α and FSHβ subunit promoters contain E-Box elements that bind USF1/2; inhibition of USF proteins or mutation of the USF-binding site reduce basal α and GnRH-induced FSHβ promoter activities respectively [40, 41].
In addition to regulating SF1 gene expression, GnRH may act upon SF1 post-translationally, as SF1 protein can be acetylated and phosphorylated. SF1 has several consensus acetylation sequences in its N-terminal DNA-binding region, and SF1 has been reported to be acetylated in vivo [42]. Inhibition of deacetylation increases SF1 protein stability and transactivation activity. SF1 interacts with stimulatory co-factors such as CREB-binding protein (CREBBP) and/or p300 that have acetyltransferase activity and may promote SF1 acetylation [43, 44]. SF1 is phosphorylated at Ser-203 in its hinge region, and mutation of this residue (S → A) reduced SF1 transactivation activity by 50% [45]. Ser-203 is phosphorylated by MAPKs, in particular ERK, which are activated by GnRH pulses [1]. PKA activity may also be involved in regulating SF1 phosphorylation, as both increased and decreased SF1 phosphorylation have been reported [46]. This effect may be indirect, via cAMP/PKA induction of MAPK phosphatase 1 (MKP1, aka DUSP1) activity, modulating ERK activity and subsequent cyclic clearance of the SF1 complex(s) from target promoters [47]. At present, there is no information regarding GnRH regulation of either SF1 phosphorylation or acetylation, but it was recently reported that GnRH induced SF1 polyubiquitination and proteasome-mediated degradation [48]. Those investigators hypothesized that proteasome-mediated degradation of SF1 (and Egr1) allows clearing of transcription factors from the LHβ promoter to initiate the next round of transcription.
GnRH may also regulate SF1 bioactivity via induction of DAX1, an endogenous corepressor of SF1. DAX1 is a member of the orphan nuclear receptor family, but unlike other members of this family, it lacks the DNA-binding domain and functions via protein–protein interactions [49]. More specifically, DAX1 binds the SF1 C-terminus and interferes with SF1 interaction with other transcriptional coactivators and/or may recruit other corepressors to promoter regions, rather than suppress SF1–DNA binding [24]. Lawson et al. [25] reported in LβT2 cells that GnRH at slower pulse frequencies (<1 pulse/hour) significantly increased DAX1 mRNA, but did not affect DAX1 protein expression. In primary gonadotropes, we observed that DAX1 gene transcription was increased preferentially, albeit modestly, after GnRH pulses every 30 min. Fast frequency GnRH pulse induction of DAX1 gene expression is consistent with the finding that the DAX1 promoter is regulated in part by SF1. There are two SF1-binding sites in the mouse DAX1 promoter and deletion/mutation of these sites reduced basal DAX1 promoter activity by approximately 50% in αT3 cells [50]. Therefore, fast frequency GnRH induction of pituitary DAX1 gene expression is likely a reflection of increased SF1 and may act as a negative feedback mechanism to regulate SF1-driven LHβ transcription.
We also investigated the regulation of SRF by GnRH pulse frequency. SRF is an immediate early response gene that is best known as a transcription factor involved in muscle physiology, but SRF also regulates other immediate early genes notably cFOS, and is involved in the transcriptional regulation of genes downstream of neurotransmitters that raise intracellular calcium levels, stress agents, and many others [51]. Although a great deal is known about how SRF regulates the transcription of other genes, there is relatively little information about the regulation of SRF transcription. The murine SRF promoter contains two conserved sequences in its promoter [52] a proximal region (approximately the first 300 bp upstream of the transcriptional start site) contains two SRF-binding sites (a GC-Box, and an overlapping SP1/Egr1 site), and an ETS-binding site [51]. In addition, there is a conserved E-Box at approximately −750 bp [52]. However in this study, pulsatile GnRH (either fast or slow) had little or no effect on pituitary SRF transcription. It is of interest that 30-min (but not 240-min) GnRH pulses transiently increased SRF mRNA, suggesting posttranscriptional regulation of mRNA stability. SRF is a phosphoprotein, and phosphorylation has been reported to increase SRF binding to DNA and/or protein–protein interactions with other transcriptional partners [27]. Within the current experimental paradigm in the rat, we did not see a GnRH-induced increase in SRF phosphorylation after 8 h of treatment. However, GnRH activates p90 ribosomal S6 kinase (p90RSK, aka RPS6KA1), which is known to phosphorylate SRF in several cell types, including LβT2 cells [53]. Although SRF binding to the CArG Box in the distal region of the rat LHβ promoter has yet to be demonstrated, SRF does influence LHβ transcription, at least indirectly. The Egr1 promoter contains several SREs, and SRF binds the Egr1 promoter. Also, the mutation of these SREs disrupts GnRH induction of Egr1 [53, 54].
In summary, these data show that fast frequency GnRH pulses preferentially stimulate SF1 transcription and steady-state mRNA. Similarly, DAX1 gene expression is also stimulated by fast frequency pulses, which may limit the duration of SF1 transcriptional responses to GnRH, suggesting a potential negative feedback regulatory action. Thus, an increase in SF1 expression after rapid frequency GnRH pulses may be one of the mechanisms favoring selective LHβ transcription.
References
L.L. Burger, D.J. Haisenleder, A.C. Dalkin, J.C. Marshall, Regulation of gonadotropin subunit gene transcription. J. Mol. Endocrinol. 33, 559–584 (2004)
Z. Naor, Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front. Neuroendocrinol. 30, 10–29 (2009)
S.P. Bliss, A. Miller, A.M. Navratil, J. Xie, S.P. McDonough, P.J. Fisher, G.E. Landreth, M.S. Roberson, ERK signaling in the pituitary is required for female but not male fertility. Mol. Endocrinol. 23, 1092–1101 (2009)
H. Kanasaki, G.Y. Bedecarrats, K.Y. Kam, S. Xu, U.B. Kaiser, Gonadotropin-releasing hormone pulse frequency-dependent activation of extracellular signal-regulated kinase pathways in perifused LbetaT2 cells. Endocrinology 146, 5503–5513 (2005)
L.L. Burger, D.J. Haisenleder, K.W. Aylor, J.C. Marshall, Regulation of intracellular signaling cascades by GNRH pulse frequency in the rat pituitary: roles for CaMK II, ERK, and JNK activation. Biol. Reprod. 79, 947–953 (2008)
D.J. Haisenleder, M.E. Cox, S.J. Parsons, J.C. Marshall, Gonadotropin-releasing hormone pulses are required to maintain activation of mitogen-activated protein kinase: role in stimulation of gonadotrope gene expression. Endocrinology 139, 3104–3111 (1998)
J. Weck, A.C. Anderson, S. Jenkins, P.C. Fallest, M.A. Shupnik, Divergent and composite gonadotropin-releasing hormone-responsive elements in the rat luteinizing hormone subunit genes. Mol. Endocrinol. 14, 472–485 (2000)
T.B. Salisbury, A.K. Binder, J.C. Grammer, J.H. Nilson, Maximal activity of the luteinizing hormone beta-subunit gene requires beta-catenin. Mol. Endocrinol. 21, 963–971 (2007)
C.C. Quirk, K.L. Lozada, R.A. Keri, J.H. Nilson, A single Pitx1 binding site is essential for activity of the LHbeta promoter in transgenic mice. Mol. Endocrinol. 15, 734–746 (2001)
P. Topilko, S. Schneider-Maunoury, G. Levi, A. Trembleau, D. Gourdji, M.A. Driancourt, C.V. Rao, P. Charnay, Multiple pituitary and ovarian defects in Krox-24 (NGFI-A, Egr-1)-targeted mice. Mol. Endocrinol. 12, 107–122 (1998)
S.L. Lee, Y. Sadovsky, A.H. Swirnoff, J.A. Polish, P. Goda, G. Gavrilina, J. Milbrandt, Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science 273, 1219–1221 (1996)
L. Zhao, M. Bakke, K.L. Parker, Pituitary-specific knockout of steroidogenic factor 1. Mol. Cell. Endocrinol. 185, 27–32 (2001)
L. Zhao, M. Bakke, Y. Krimkevich, L.J. Cushman, A.F. Parlow, S.A. Camper, K.L. Parker, Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development 128, 147–154 (2001)
C.D. Horton, L.M. Halvorson, The cAMP signaling system regulates LHbeta gene expression: roles of early growth response protein-1, SP1 and steroidogenic factor-1. J. Mol. Endocrinol. 32, 291–306 (2004)
G.B. Call, M.W. Wolfe, Species differences in GnRH activation of the LHbeta promoter: role of Egr1 and Sp1. Mol. Cell. Endocrinol. 189, 85–96 (2002)
U.B. Kaiser, L.M. Halvorson, M.T. Chen, Sp1, steroidogenic factor 1 (SF-1), and early growth response protein 1 (egr-1) binding sites form a tripartite gonadotropin-releasing hormone response element in the rat luteinizing hormone-beta gene promoter: an integral role for SF-1. Mol. Endocrinol. 14, 1235–1245 (2000)
L.M. Halvorson, M. Ito, J.L. Jameson, W.W. Chin, Steroidogenic factor-1 and early growth response protein 1 act through two composite DNA binding sites to regulate luteinizing hormone beta-subunit gene expression. J. Biol. Chem. 273, 14712–14720 (1998)
J. Fortin, P. Lamba, Y. Wang, D.J. Bernard, Conservation of mechanisms mediating gonadotrophin-releasing hormone 1 stimulation of human luteinizing hormone beta subunit transcription. Mol. Hum. Reprod. 15, 77–87 (2009)
J.J. Tremblay, J. Drouin, Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone beta gene transcription. Mol. Cell. Biol. 19, 2567–2576 (1999)
C. Dorn, Q. Ou, J. Svaren, P.A. Crawford, Y. Sadovsky, Activation of luteinizing hormone beta gene by gonadotropin-releasing hormone requires the synergy of early growth response-1 and steroidogenic factor-1. J Biol Chem 274, 13870–13876 (1999)
M.W. Wolfe, G.B. Call, Early growth response protein 1 binds to the luteinizing hormone-beta promoter and mediates gonadotropin-releasing hormone-stimulated gene expression. Mol. Endocrinol. 13, 752–763 (1999)
L.L. Burger, D.J. Haisenleder, K.W. Aylor, J.C. Marshall, Regulation of Lhb and Egr1 gene expression by GNRH pulses in rat pituitaries is both c-Jun N-terminal kinase (JNK)- and extracellular signal-regulated kinase (ERK)-dependent. Biol. Reprod. 81, 1206–1215 (2009)
R.A. Keri, J.H. Nilson, A steroidogenic factor-1 binding site is required for activity of the luteinizing hormone beta subunit promoter in gonadotropes of transgenic mice. J. Biol. Chem. 271, 10782–10785 (1996)
B.P. Schimmer, P.C. White, Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol. Endocrinol. 24, 1322–1337 (2010)
M.A. Lawson, R. Tsutsumi, H. Zhang, I. Talukdar, B.K. Butler, S.J. Santos, P.L. Mellon, N.J. Webster, Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol. Endocrinol. 21, 1175–1191 (2007)
D.J. Haisenleder, M. Yasin, A.C. Dalkin, J. Gilrain, J.C. Marshall, GnRH regulates steroidogenic factor-1 (SF-1) gene expression in the rat pituitary. Endocrinology 137, 5719–5722 (1996)
J.M. Miano, Serum response factor: toggling between disparate programs of gene expression. J. Mol. Cell. Cardiol. 35, 577–593 (2003)
S. Mutiara, H. Kanasaki, T. Harada, A. Oride, K. Miyazaki, The involvement of phosphatidylinositol 3-kinase in gonadotropin-releasing hormone-induced gonadotropin alpha- and FSHbeta-subunit genes expression in clonal gonadotroph LbetaT2 cells. Mol. Cell. Endocrinol. 283, 1–11 (2008)
T. Harada, H. Kanasaki, S. Mutiara, A. Oride, K. Miyazaki, Cyclic adenosine 3′, 5′monophosphate/protein kinase A and mitogen-activated protein kinase 3/1 pathways are involved in adenylate cyclase-activating polypeptide 1-induced common alpha-glycoprotein subunit gene (Cga) expression in mouse pituitary gonadotroph LbetaT2 cells. Biol. Reprod. 77, 707–716 (2007)
L.L. Burger, A.C. Dalkin, K.W. Aylor, D.J. Haisenleder, J.C. Marshall, GnRH pulse frequency modulation of gonadotropin subunit gene transcription in normal gonadotropes–assessment by primary transcript assay provides evidence for roles of GnRH and Follistatin. Endocrinology 143, 3243–3249 (2002)
A.C. Dalkin, L.L. Burger, K.W. Aylor, D.J. Haisenleder, L.J. Workman, S. Cho, J.C. Marshall, Regulation of gonadotropin subunit gene transcription by gonadotropin-releasing hormone: measurement of primary transcript ribonucleic acids by quantitative reverse transcription-polymerase chain reaction assays. Endocrinology 142, 139–146 (2001)
D.J. Haisenleder, L.L. Burger, H.E. Walsh, J. Stevens, K.W. Aylor, M.A. Shupnik, J.C. Marshall, Pulsatile gonadotropin-releasing hormone stimulation of gonadotropin subunit transcription in rat pituitaries: evidence for the involvement of Jun N-terminal kinase but not p38. Endocrinology 149, 139–145 (2008)
P. Chomczynski, N. Sacchi, Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159 (1987)
L.L. Burger, D.J. Haisenleder, G.M. Wotton, K.W. Aylor, A.C. Dalkin, J.C. Marshall, The regulation of FSHbeta transcription by gonadal steroids: testosterone and estradiol modulation of the activin intracellular signaling pathway. Am. J. Physiol. Endocrinol. Metab. 293, E277–E285 (2007)
L.L. Burger, D.J. Haisenleder, K.W. Aylor, A.C. Dalkin, K.A. Prendergast, J.C. Marshall, Regulation of luteinizing hormone-{beta} and follicle-stimulating hormone (fsh)-{beta} gene transcription by androgens: testosterone directly stimulates fsh-{beta} transcription independent from its role on follistatin gene expression. Endocrinology 145, 71–78 (2004)
S.N. Ibrahim, S.M. Moussa, G.V. Childs, Morphometric studies of rat anterior pituitary cells after gonadectomy: correlation of changes in gonadotropes with the serum levels of gonadotropins. Endocrinology 119, 629–637 (1986)
C. Knight, J.P. Slade, D. Carter, The nuclear, 75 kDa form of early growth response protein-1/nerve growth factor-induced A protein is primarily restricted to LH beta-subunit-expressing cells in rat anterior pituitary. Eur. J. Endocrinol. 143, 817–821 (2000)
K.G. Woodson, P.A. Crawford, Y. Sadovsky, J. Milbrandt, Characterization of the promoter of SF-1, an orphan nuclear receptor required for adrenal and gonadal development. Mol. Endocrinol. 11, 117–126 (1997)
A.N. Harris, P.L. Mellon, The basic helix-loop-helix, leucine zipper transcription factor, USF (upstream stimulatory factor), is a key regulator of SF-1 (steroidogenic factor-1) gene expression in pituitary gonadotrope and steroidogenic cells. Mol. Endocrinol. 12, 714–726 (1998)
S.M. Jackson, A. Gutierrez-Hartmann, J.P. Hoeffler, Upstream stimulatory factor, a basic-helix-loop-helix-zipper protein, regulates the activity of the alpha-glycoprotein hormone subunit gene in pituitary cells. Mol. Endocrinol. 9, 278–291 (1995)
N.A. Ciccone, C.T. Lacza, M.Y. Hou, S.J. Gregory, K.Y. Kam, S. Xu, U.B. Kaiser, A composite element that binds basic helix loop helix and basic leucine zipper transcription factors is important for gonadotropin-releasing hormone regulation of the follicle-stimulating hormone beta gene. Mol. Endocrinol. 22, 1908–1923 (2008)
A.L. Jacob, J. Lund, P. Martinez, L. Hedin, Acetylation of steroidogenic factor 1 protein regulates its transcriptional activity and recruits the coactivator GCN5. J. Biol. Chem. 276, 37659–37664 (2001)
W.Y. Chen, L.J. Juan, B.C. Chung, SF-1 (nuclear receptor 5A1) activity is activated by cyclic AMP via p300-mediated recruitment to active foci, acetylation, and increased DNA binding. Mol. Cell. Biol. 25, 10442–10453 (2005)
W.Y. Chen, J.H. Weng, C.C. Huang, B.C. Chung, Histone deacetylase inhibitors reduce steroidogenesis through SCF-mediated ubiquitination and degradation of steroidogenic factor 1 (NR5A1). Mol. Cell Biol. 27, 7284–7290 (2007)
G.D. Hammer, I. Krylova, Y. Zhang, B.D. Darimont, K. Simpson, N.L. Weigel, H.A. Ingraham, Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol. Cell 3, 521–526 (1999)
R.C. Fowkes, J.M. Burrin, Steroidogenic factor-1: a key regulator of gonadotroph gene expression. J. Endocrinol. 177, 345–350 (2003)
J.N. Winnay, G.D. Hammer, Adrenocorticotropic hormone-mediated signaling cascades coordinate a cyclic pattern of steroidogenic factor 1-dependent transcriptional activation. Mol. Endocrinol. 20, 147–166 (2006)
H.E. Walsh, M.A. Shupnik, Proteasome regulation of dynamic transcription factor occupancy on the GnRH-stimulated luteinizing hormone beta-subunit promoter. Mol. Endocrinol. 23, 237–250 (2009)
K.L. Parker, B.P. Schimmer, A. Schedl, Genes essential for early events in gonadal development. Cell Mol. Life Sci. 55, 831–838 (1999)
R.N. Yu, J.C. Achermann, M. Ito, J.L. Jameson, The Role of DAX-1 in Reproduction. Trends Endocrinol. Metab. 9, 169–175 (1998)
J.A. Spencer, R.P. Misra, Expression of the SRF gene occurs through a Ras/Sp/SRF-mediated-mechanism in response to serum growth signals. Oncogene 18, 7319–7327 (1999)
T.J. Nelson, S.A. Duncan, R.P. Misra, Conserved enhancer in the serum response factor promoter controls expression during early coronary vasculogenesis. Circ. Res. 94, 1059–1066 (2004)
W.R. Duan, M. Ito, Y. Park, E.T. Maizels, M. Hunzicker-Dunn, J.L. Jameson, GnRH regulates early growth response protein 1 transcription through multiple promoter elements. Mol. Endocrinol. 16, 221–233 (2002)
S.I. Mayer, G.B. Willars, E. Nishida, G. Thiel, Elk-1, CREB, and MKP-1 regulate Egr-1 expression in gonadotropin-releasing hormone stimulated gonadotrophs. J. Cell. Biochem. 105, 1267–1278 (2008)
Acknowledgment
This research was supported by USPHS Grant HD-33039 (to JCM) and NICHD/NIH through a cooperative agreement (U54-HD28934, Ligand Assay and Analysis Core) as part of the Specialized Cooperative Centers Program in Reproductive Research (JCM, DJH).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burger, L.L., Haisenleder, D.J. & Marshall, J.C. GnRH pulse frequency differentially regulates steroidogenic factor 1 (SF1), dosage-sensitive sex reversal-AHC critical region on the X chromosome gene 1 (DAX1), and serum response factor (SRF): potential mechanism for GnRH pulse frequency regulation of LH beta transcription in the rat. Endocr 39, 212–219 (2011). https://doi.org/10.1007/s12020-011-9440-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-011-9440-y