Abstract
Circulating endothelial progenitor cells (EPCs) play an important role in the development and progression of diabetic vascular complications. The aim of this study was to investigate the effects of gliclazide plus metformin (GLIMET) compared with metformin alone (MET) on number and function of circulating EPCs in T2DM patients. Patients with newly diagnosed T2DM were randomly divided into two groups, receiving the following treatments for 16 weeks: MET group (assuming metformin 500–2500 mg/day, n = 24) and GLIMET group [assuming gliclazide (modified release, 30–60 mg/day) + metformin (250–1000 mg/day), n = 23]. Circulating EPCs were quantified by flow cytometry, and the ability to uptake LDL and stain for lectin were used as another method of characterizing EPCs ex vivo. The functions of circulating EPCs were evaluated by colony-forming units (CFU) and migration. The status of oxidative stress was analyzed by serum-free malonaldehyde (MDA) and superoxide dismutase (SOD). There were no significant differences in clinical characteristics and number and function of circulating EPCs between two groups at baseline. Glycemic responses were similar after treatments. Compared with MET group, GLIMET group was associated with an increase in circulating EPCs number, DiLDL–lectin-positive EPCs, and migration. The mean improvements in MDA and SOD of GLIMET group were more strongly upregulated than those of MET group. This study demonstrated that both metformin mono-treatment and metformin plus gliclazide combination treatment provided with improvements in number and function of circulating EPCs. Compared with metformin mono-treatment, early use of combination therapy with gliclazide plus metformin made more effective improvements in circulating EPCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The main etiology for mortality and a great percent of morbidity in patients with type 2 diabetes mellitus (T2DM) is vascular complications. A growing amount of data has become available indicating that alterations in circulating endothelial progenitor cells (EPCs) may have a causative role in the development and progression of all diabetes complications [1, 2]. Derived from bone marrow, EPCs are circulating immature cells that contribute to vascular homeostasis and compensatory angiogenesis. Many studies have demonstrated that the number of circulating EPCs is decreased in T2DM and its vascular complications [3]. Moreover, diabetic EPCs display the impaired function, such as reduced proliferation, adhesion, and migration into tubular structures [4]. Recent data have shown that number of circulating EPCs may be a marker of diabetic vasculopathy [5]. Given the comprehensive role of circulating EPCs alterations in diabetes vascular complications, modulation of the number and function of EPCs may be considered as a potential therapeutic strategy for diabetic patients.
Although some clinical studies of oral antidiabetic agents on cardiovascular effects have been reported [6, 7], the cardiovascular protective effects of metformin or sulfonylureas treatment remained controversial. In UKPDS, metformin was found to decrease the macrovascular morbidity and mortality only in overweight T2DM patients [8]. In ADVANCE trial, there were no significant effects of gliclazide treatment on major macrovascular events or death from cardiovascular causes in T2DM patients [9]. Since metformin mono-treatment and gliclazide plus metformin combination treatment are the commonly prescribed medications for T2DM and the medical therapies of the ADA recommendation in diabetes standards [10], it is necessary to identify the effects of these two kinds of treatment on circulating EPCs, which would be good for proving the evidence of cardiovascular protective effects and supplying a reasonable clinical choice of drugs.
A common approach for the prevention and treatment of diabetes vascular complications relies on the understanding of their complex pathophysiology. Besides circulating EPCs, a unifying biochemical theory suggests that oxidative stress underlies subcellular damage pathways, which leads to diabetes complications [11]. It is interesting to point out that a close correlation between functional capacity of EPCs and oxidative stress status has been proposed [12]. In addition, metformin and gliclazide have been shown to exert anti-oxidative effects on vascular beds [13, 14]. Therefore, it is valuable to observe the role of oxidative stress-mediated pathway in circulating EPCs modulation by metformin or gliclazide.
With this background, we investigated the effects of 16 weeks metformin mono-treatment and gliclazide plus metformin combination treatment on the number and function of circulating EPCs in newly diagnosed patients with T2DM. Furthermore, we evaluated the oxidative stress status simultaneously so that the role of oxidative stress-mediated pathway in circulating EPCs modulation by metformin mono-treatment and metformin plus gliclazide combination treatment can be discussed.
Materials and methods
Study subjects
This study was approved from the local ethics committee and informed consent was obtained from all subjects. From February 2008 to January 2009, a total of 47 Chinese newly diagnosed T2DM patients (25 men and 22 women) were enrolled. T2DM patients were recruited from the outpatients and inpatients of the Endocrinology Department in Wuhan Union Hospital. The diagnosis of T2DM was based on the guidelines in the recent Expert Committee Report of the American Diabetes Association [15]. In order to avoid the drug interferences in the clinical characteristics, all T2DM patients were newly diagnosed without any drug treatments for at least 3 months before the beginning of this study [with fasting blood glucose (FBG) ≥7.0 mmol/l and ≤10.0 mmol/l; plasma glycosylated hemoglobin (HbA1c) ≥6.0% and ≤8.0%]. Predefined exclusion criteria were subject’s refusal, age over seventy or less than thirty, acute illness or infection, neoplasm, recent surgery or vascular intervention, hemodialysis, immunosuppression, and immunological diseases. Females during the examination were not in menstrual phase in order to avoid the intervention on circulating EPCs number and function.
All the subjects were randomized into two groups: MET group (assuming metformin 500–2500 mg/day, n = 24) and GLIMET group [assuming gliclazide (modified release, 30–60 mg/day) + metformin (250–1000 mg/day), n = 23] in an open-label fashion for 16 weeks. Metformin was started at the dose of 500 mg/day while gliclazide was started at the dose of 30 mg/day. During the treatment, all the patients were instructed to follow a moderately hypocaloric diet (−20% of estimated daily energy requirement) and to perform home blood glucose monitoring. Patients returned to visit in the clinic every 2 weeks during the first month of treatment, and monthly thereafter. At each visit, FBG and 2-h blood glucose (2hBG) were assessed. Drugs were up-titrated as needed on the basis of home-recorded blood glucose profiles, FBG and 2hBG levels, so as to achieve good glycemic control (FBG <6.1 mmol/l and 2hBG <7.8 mmol/l). None of these patients were taking other drugs during the observation period.
Anthropometric and biochemical measures
Anthropometric and biochemical measures were performed in all subjects before and after treatment. Height and weight were recorded without shoes in light clothing. Taken after 15 min rest, blood pressure (SBP, DBP) were recorded as the mean of three times. After a 10-h fasting, a total of 30 ml of peripheral venous blood in sodium heparin was collected from each subject. Four milliliter was used for enumeration of circulating EPCs number. One milliliter was used for identifying the absolute count of lymphocytes per 1 ml whole blood simultaneously. Another 20 ml was used for cell culture of circulating EPCs. Rest of the blood sample was used for biochemical assays. Measurement of FBG, 2hBG, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were performed with an autoanalyzer (Hitachi, Japan). Measurement of HbA1c was performed with another autoanalyzer (Biorad, American, California).
Quantification of circulating EPCs by flow cytometry
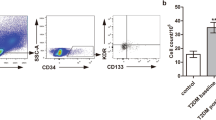
Circulating EPCs were defined by the surface expression of CD45low/CD34+/VEGFR2+ as previously described [16–18]. Peripheral blood mononuclear cells (MNCs) were separated from other components of peripheral blood by centrifugation on density gradient media, washed twice and solved in 0.2 ml of phosphate buffered saline (PBS). The solution was then labeled with a 10 μl panel of, Peridinin–chlorophyll–protein complex (PerCP), fluorescein isothiocyanate (FITC), and R-phycoerythrin (R-PE) conjugated antibodies anti-CD45 (Becton Dickinson, American, New Jersey), anti-CD34 (Becton Dickinson), and anti-VEGFR2(R&D system), for 20 min at room temperature away from light. After conjugation, red blood cells were lysed by incubating in FACS lysing solution (Becton Dickinson) for 15 min. Cells stained with isotypic controls for IgG1-FITC or R-PE were used as negative controls. After appropriate gating with lymphocytes, CD45low/CD34+/VEGFR2+ cells were identified by the dual expression of CD34+ and VEGFR2+ in the CD45low gates. The cytometer was set to acquire 100,000 events, and analyses were performed within the lymphocyte gate, in accordance with a technique used by other investigators [19, 20]. Data were processed using the Macintosh CellQuest software program (Becton Dickinson). The absolute count of individual human peripheral blood lymphocytes was performed by 5DF blood cell analysis apparatus testing. On the basis of the lymphocytes count of the whole blood, the absolute number of circulating EPCs was converted and expressed as the number of cells per 1 ml of whole blood. Those assessments were executed in all subjects by same well-trained operator who was unaware of the patients’ clinical status (Fig. 1a).
a (A) Showing the gate used to label lymphocyte cells, (B) showing the gate used to label CD45low cells, (C) showing the R1 and R2 gate used to identify the CD45low/CD34+/VEGFR2+ cells. Mononuclear cells were separated from other components of peripheral blood by centrifugation on density gradient media. The cytometer was set to acquire 100,000 events, and analyses were performed within the lymphocyte gate. On the basis of the lymphocyte counts, the absolute number of circulating EPCs/ml was calculated. b MET and GLIMET groups were comparable with respect to circulating EPCs number at baseline. At week 16, there was an increase from baseline both in MET group and in GLIMET group (P < 0.01). After adjusted for the baseline values, the mean improvements in circulating EPCs number of GLIMET group were significantly more obvious than those of MET group (P = 0.003)
Cell culture and characterization of circulating EPCs
Endothelial progenitor cells (EPCs) were cultured according to previously described techniques [16]. Briefly, MNCs were isolated by ficoll density gradient centrifugation. Recovered cells were washed once with PBS and once in growth medium consisting of Medium 199 (GIBCO BRL Life Technologies, American, New York) supplemented with 10% fetal-calf serum (FCS), penicillin (100 U per milliliter), and streptomycin (100 μg per milliliter). 1 × 107 MNCs were plated onto fibronectin (Biocoat, Becton Dickinson Labware)-coated 6-well plates. Cells were cultured in Medium 199 supplemented with 10% FCS, vascular endothelial growth factors (VEGF), fibroblast growth factor-2, epidermal growth factor, and ascorbic acid. After 48 h, 1 × 106 non-adherent cells in the supernatant were transferred into 24-well plates to avoid contamination with mature endothelial cells and non-progenitor cells. Growth medium was changed every 2 or 3 days. At 7th day in culture, adherent cells were incubated with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labeled acetylated LDL (DiLDL, 2.4 μg/ml, Vector) and stained using FITC labeled Ulex europaeus agglutinin I (lectin, 10 μg/ml, Sigma). Double-positive cells were counted manually by two independent observers blinded to the study (Olimpus, FV-500) (Fig. 2a).
a, b Before treatment, the number of DiLDL/lectin double-positive cells per microscopic field was similar in both groups (MET, 8 ± 2; GLIMET, 8 ± 2). After treatment, double-positive cells per microscopic field of GLIMET group were significantly improved in two groups (MET, 11 ± 3, P = 0.001; METGLI, 17 ± 4, P < 0.001). After adjusted for the baseline values, the mean improvements in circulating EPCs number of GLIMET group were significantly more obvious than those of MET group (6 ± 2, P = 0.003)
Colony-forming units of circulating EPCs
Another 1 × 107 MNCs were plated in a fibronectin-coated 6-well plate for 48 h, then, 1 × 106 non-adherent cells in the supernatant were transferred into 24-well plates. After 7 days ex vivo, EPC colonies were counted by two independent investigators. A colony of EPCs consisted of multiple thin, flat cells emanating from a central cluster of rounded cells. A central cluster alone without associated emerging cells was not counted as a colony. CFU are expressed as absolute numbers of colonies per well (in triplicate) (Fig. 3a).
a, b At baseline, the number of CFU in MET and GLIMET groups were 49 ± 6 per well and 50 ± 6 per well, respectively. After 16 weeks of treatment, the development of CFUs per number of EPC was found both in MET group (12 ± 4, P = 0.008) and in GLIMET group (15 ± 5, P = 0.003). After adjusted for the baseline values, the mean improvements of CFU in two groups showed no significant differences (3 ± 2, P = 0.067)
Migration assay
Polyvinylpyrrolidone-free polycarbonate filter Transwell inserts (BD Biosciences, 8 μm pore size, in triplicate) were incubated with 0.1% fibronectin and dried. Inserts were placed in a 24-well plate containing 500 μl growth medium with 50 ng/ml of VEGF as prepared above. After 7 days in culture, isolated EPCs (1 × 105) were added to the upper chamber of the inserts in the medium prepared above. Cells were allowed to migrate from the upper to the lower chamber for 24 h at 37°C. Cells that had migrated to the lower side of the Transwell insert were fixed for 15 min with 2% paraformaldehyde and incubated with 4,6-diamino-2-phenylindole (DAPI). The number of cells that had migrated was counted in three different representative high power (×200) fields per insert. The data are presented as number of cells migrating ± standard error (SE) of the mean (Fig. 4a).
a, b Both two groups had similar baseline of migration capacity (20 ± 6 cell/field in MET group; 20 ± 5cell/field in GLIMET group). At week 16, there was an increase from baseline both in MET group (15 ± 4 cell/field, P = 0.005) and in GLIMET group (28 ± 6 cell/field, P = 0.001). After adjusted for the baseline values, the mean improvements in migratory capacity of GLIMET group were more obvious in GLIMET group than in MET group (12 ± 3 cell/field, P = 0.001)
Measurement of oxidative stress status in plasma
Fasting plasma-free malonaldehyde (MDA) and superoxide dismutase (SOD) were used for analyzing the status of oxidative stress. Plasma-free MDA was assayed by thiobarbituric acid adduct with colorimetric method. The activity of SOD was measured by xanthine oxidase with colorimetric method. Analyses above were performed with corresponding assay kits (Nanjing Jiancheng Biochemical Reagent Co., China) according to the manufacturer’s instructions.
Statistical analysis
Data were expressed as means ± SE. All results from flow cytometry were expressed as number of cell/ml. Values of lipid profile were log transformed before statistical analysis. Baseline data for the MET and GLIMET groups were compared using 2-tailed Student t test. The Chi-square test was used for dichotomous variables. Within-treatment comparisons of the mean changes in variables from baseline to week 16 were performed using a paired t-test. The variables after treatment between two groups were compared by the analysis of covariance (ANCOVA) test with adjustment for baseline values. All the data analysis was performed with the Statistical Package for the Social Sciences (SPSS) 13.0 software package. P values less than 0.05 were considered significant.
Results
General clinical characteristics
The clinical characteristics of study participants in this study before and after treatment were shown in Table 1. There were no significant differences in baseline characteristics between the two groups. Glycemic responses were similar after 16 weeks of treatment: plasma HbA1c was 6.1 ± 0.5 mmol/l in MET group versus 6.0 ± 0.6 mmol/l in GLIMET group (P = 0.792); FBG was 6.1 ± 1.0 mmol/l in MET group versus 6.0 ± 1.1 mmol/l in GLIMET group (P = 0.417); 2hBG was 7.4 ± 1.5 mmol/l in MET group versus 7.2 ± 1.4 mmol/l in GLIMET group (P = 0.664). Serum TC decreased significantly (5.17 ± 0.83 before treatment versus 4.28 ± 0.63 mmol/l after treatment, P = 0.01) in the GLIMET group but remained unchanged in the MET group. There were no significant changes in BMI, TG, HDL, LDL, SBP, and DBP neither in MET nor in GLIMET group (P > 0.05).
Circulating EPCs number
MET and GLIMET groups were comparable with respect to circulating EPCs number at baseline (P > 0.05). At week 16, there was an increase from baseline both in MET group (from 932 ± 96 cell/ml to 1449 ± 105 cell/ml, P = 0.001) and in GLIMET group (from 919 ± 97 cell/ml to 1581 ± 106 cell/ml, P < 0.001). After adjusted for the baseline values, the mean improvements in circulating EPCs number of GLIMET group were significantly more obvious than those of MET group (151 ± 21 cell/ml, P = 0.003) (Fig. 1b).
Number of cultured DiLDL–lectin-positive EPCs
Before treatment, the number of DiLDL/lectin double-positive cells per microscopic field was similar in both groups (8 ± 2 cell/field in MET group; 8 ± 2cell/field in GLIMET group). After treatment, double-positive cells per microscopic field of GLIMET group were significantly improved in two groups (MET, 11 ± 3 cell/field, P = 0.001; METGLI, 17 ± 4 cell/field, P < 0.001). After adjusted for the baseline values, the mean improvements in circulating EPCs number of GLIMET group were more strongly upregulated than those of MET group (6 ± 2 cell/field, P = 0.003) (Fig. 2b).
Counts of EPC colonies
At baseline, the number of CFU in MET and GLIMET groups was 49 ± 6 unit/well and 50 ± 6 unit/well, respectively, (P > 0.05). After 16 weeks treatment, the development of CFUs were found both in MET group (12 ± 4 unit/well, P = 0.008) and in GLIMET group (15 ± 5 unit/well, P = 0.003). After adjusted for the baseline values, the mean improvements of CFU in two groups showed no significant differences (3 ± 2 unit/well, P = 0.067) (Fig. 3b).
Migratory capacity
Both two groups had similar baseline of migration capacity (20 ± 6 cell/field in MET group; 20 ± 5 cell/field in GLIMET group). At week 16, there was an increase from baseline both in MET group (15 ± 4 cell/field, P = 0.005) and in GLIMET group (28 ± 6 cell/field, P = 0.001). After adjusted for the baseline values, the mean improvements in migratory capacity of GLIMET group were more obvious in GLIMET group than in MET group (12 ± 3 cell/field, P = 0.001) (Fig. 4b).
Plasma markers of oxidative stress
Gliclazide plus metformin-treated patients exhibited a significant decrease in the plasma concentration of MDA (from 3.97 ± 0.60 to 2.35 ± 0.51 mmol/l, P < 0.001) and an increase in SOD (25.80 ± 7.04 to 38.68 ± 7.42U/ml, P < 0.001) from the baseline measurements to those at week 16. Metformin-treated patients show a significant decrease in the plasma concentration of MDA (from 3.86 ± 0.61 to 3.29 ± 0.58 mmol/l, P = 0.042), but no increase in SOD (24.21 ± 6.98 to 28.07 ± 7.25 U/ml, P = 0.078) from the baseline measurements to those at week 16. After adjusted for the baseline values, the mean improvements in MDA and SOD of GLIMET group were more strongly upregulated than those of MET group (−1.01 ± 0.24, P = 0.002; 9.06 ± 2.02, P = 0.003) (Table 1).
Discussion
The bone marrow-derived EPCs circulate in the blood and play an important role in the formation of new blood vessels as well as contribute to vascular homeostasis [21]. In this study, two independent methods were adopted to study EPCs: flow cytometry for quantification and ex vivo culture for functional testing. CD45low/CD34+/VEGFR2+ cells were quantified by FACS because CD34+/VEGFR2+ cells have been shown to predict cardiovascular outcomes in patients with coronary artery disease [20] and CD45low was used to exclude hematopoietic cells [16–18]. In addition to FACS analysis, the ability to uptake LDL and stain for lectin were used as another established method of characterizing EPCs ex vivo [14]. The functional properties of EPCs were evaluated by migration and the ability of CFU. Migration of EPCs through the extracellular matrix is crucial for the growth of new vessels. And EPCs should proliferate (form colonies) in response to growth factors released locally after vascular damage or tissue ischemia.
Some large-scale clinical researches such as UKPDS, PRESTO [22] have shown the beneficial effects of metformin on cardiovascular diseases in diabetic patients. However, the mechanism underlying its cardiovascular protective effects remains unclear. In addition, observational studies on the association between combination therapy of sulfonylureas and metformin and the risk of cardiovascular diseases have shown conflicting results. Some studies have reported that this combination therapy decreased the risk of mortality from all causes and cardiovascular disease [23], while others have reported no association [24, 25] or an increased risk of all-cause and cardiovascular disease mortality [26]. In this study, the change of circulating EPC function was nearly the same as the change of circulating number. Furthermore, all quantification and functional testing showed the improvements in patients taking both metformin mono-treatment and gliclazide plus metformin combination treatment. Considering the fact that the number of circulating EPCs has been considered as a surrogate biologic marker for vascular endothelial function [16] and endothelial dysfunction is a reflection of overall vascular health, the results above indicated that for newly diagnosed patients with T2DM, short-term hypoglycemic treatment (including metformin mono-treatment and gliclazide plus metformin combination treatment) can bring the early cardiovascular benefit. Similarly, several drugs showing beneficial effects on cardiovascular risk factors, such as glitazones and statins, have also been confirmed to stimulate circulating EPCs level [27, 28]. Interestingly, it has been demonstrated that early intensive blood glucose control by metformin or sulfonylurea could bring a long-term cardiovascular benefit after 10 years [29]. Therefore, it is reasonable to choose these two kinds of treatments as the most commonly prescribed medications for newly diagnosed patients with T2DM.
In this study, the change of average blood glucose was an independent determinant of circulating EPCs in both two kinds of treatment. Several studies have showed that inhibition of nitric oxide (NO) biology availability plays an important role in EPCs mobilization and migration from bone marrow in diabetes [30]. Meanwhile, several studies suggested that high glucose might impair EPCs by modifying NO-related mechanisms [31]. Therefore, the improvement of circulating EPCs with some hypoglycemic treatments could be partly explained by the improved glucose control. Besides this, it has been widely accepted that therapeutic effects of metformin might be mediated by its activation on AMP-activated protein kinase (AMPK) [32, 33]. Recent studies demonstrated that AMPK signaling in endothelial cells is essential for angiogenesis, as activation of its activity in endothelial cells stimulate endothelial nitric oxide synthesis (eNOS) and nitric oxide (NO) production [22]. Thus, it may be assumed that activation of the AMPK by metformin stimulates eNOS and NO production which in turn increases NO-mediated EPC mobilization and improves its biological function.
However, in this study, metformin plus gliclazide combination treatment was found more effective than metformin mono-treatment in improving number and function of EPCs. It is obvious that only glucose control and metformin treatment cannot explain the result above, as the improvement of glucose control was similar and metformin treatment participated in both two groups. The advantages of early combination therapy may present some implications, including reduction in side effects with the submaximal doses of drugs, more rapid achievement of therapeutic target, and the opportunity to combine oral antiglycemic drugs with complementary modes of action. For example, some studies have shown that gliclazide not only lowers blood glucose but also confers beneficial effects on diabetic vascular disease. Clinically, gliclazide can improve endothelial function and ameliorate oxidative stress [34]. As the causal role of oxidative stress in NO metabolism in T2DM [35], gliclazide treatment might increase the NO production by ameliorating oxidative stress and subsequently increases NO-mediated EPC mobilization and its biological function. In this study, the findings that metformin and gliclazide-treated patients exhibited a greater decrease in the plasma concentration of MDA and a greater increase in SOD than in metformin mono-treatment have confirmed this assumption. It should be mentioned that although both metformin and gliclazide have benefit effects on circulating EPCs, the mechanisms underlying those effects may be completely different. The free radical scavenging ability of gliclazide was related to the unique aminoazabicyclo-octane ring grafted onto the sulfonylurea [34]. Meanwhile, the benefit effects of metformin might mainly be mediated by its activation on AMPK. Therefore, it is reasonable to show that metformin plus gliclazide combination treatment has more significant improvements on circulating EPCs than metformin mono-treatment. In addition, gliclazide plus metformin combination treatment provided with greater number of improved cardiovascular risk factors than metformin mono-treatment might offer another important reason. The level of EPCs has been reported to be inversely correlated with the number of cardiovascular risk factors and might be a surrogate biologic marker for cumulative cardiovascular risk. In this study, it is conspicuous that with the similar short-term glycemic control to metformin monotherapy, the combined treatment could produce greater beneficial effects on lipid metabolism and oxidative stress.
There were several limitations in this study. The first limitation is represented by the relatively short term of the study duration. Although reductions in HbA1c were maintained in this study for 16 weeks, it is unknown whether the effects on circulating EPCs would be maintained after long antidiabetic drugs treatment. The second limitation derives from the limitations of research conditions that direct effects of metformin or gliclazide on EPCs ex vivo were not investigated. Finally, due to the financial limitation, this manuscript details a rather small clinical study. It remains undefined that the data might be misleading. Therefore, further penetrating investigations with longer and larger study are necessarily required.
In summary, this study provided new evidence of the vascular protective function of metformin and gliclazide for newly diagnosed patients with T2DM. Furthermore, compared with metformin mono-treatment, early use of combination therapy with gliclazide plus metformin was more effective in improving number and function of circulating EPCs.
References
G.P. Fadini, C. Agostini, A. Avogaro, Endothelial progenitor cells and vascular biology in diabetes mellitus: current knowledge and future perspectives. Curr. Diabetes Rev. 1, 41–58 (2005)
G.P. Fadini, S. Sartore, C. Agostini, A. Avogaro, Significance of endothelial progenitor cells in subjects with diabetes. Diabetes Care 30, 1305–1313 (2007)
G.P. Fadini, M. Miorin, M. Facco, S. Bonamico, I. Baesso, F. Grego, M. Menegolo, S.V. de Kreutzenberg, A. Tiengo, C. Agostini, A. Avogaro, Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J. Am. Coll. Cardiol. 45, 1449–1457 (2005)
O.M. Tepper, R.D. Galiano, J.M. Capla, C. Kalka, P.J. Gagne, G.R. Jacobowitz, J.P. Levine, G.C. Gurtner, Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 106, 2781–2786 (2002)
G.P. Fadini, S. Sartore, M. Albiero, I. Baesso, E. Murphy, M. Menegolo, F. Grego, S. Vigili de Kreutzenberg, A. Tiengo, C. Agostini, A. Avogaro, Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler. Thromb. Vasc. Biol. 26, 2140–2146 (2006)
D.P. Macfarlane, K.R. Paterson, M. Fisher, Oral antidiabetic agents as cardiovascular drugs. Diabetes Obes. Metab. 9, 23–30 (2007)
Y. Sahin, K. Unluhizarci, A. Yilmazsoy, A. Yikilmaz, E. Aygen, F. Kelestimur, The effects of metformin on metabolic and cardiovascular risk factors in nonobese women with polycystic ovary syndrome. Clin. Endocrinol. (Oxf) 67, 904–908 (2007)
UK Prospective Diabetes Study UKPDS Group, Effect of intensive blood glucose control with metformin on complications in overnight patients with type 2 diabetes (UKPDS 34). Lancet 352, 854–865 (1998)
ADVANCE Collaborative Group, A. Patel, S. MacMahon, J. Chalmers, B. Neal, L. Billot, M. Woodward, M. Marre, M. Cooper, P. Glasziou, D. Grobbee, P. Hamet, S. Harrap, S. Heller, L. Liu, G. Mancia, C.E. Mogensen, C. Pan, N. Poulter, A. Rodgers, B. Williams, S. Bompoint, B.E. de Galan, R. Joshi, F. Travert, Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358, 2560–2572 (2008)
American Diabetes Association, Standards of Medical Care in Diabetes-2008. Diabetes Care 31, S12–54S (2008)
M. Brownlee, The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54, 1615–1625 (2005)
J. Haendeler, S. Dimmeler, Inseparably tied: functional and antioxidative capacity of endothelial progenitor cells. Circ. Res. 98, 157–158 (2006)
G. Formoso, E.A. De Filippis, N. Michetti, P. Di Fulvio, A. Pandolfi, T. Bucciarelli, G. Ciabattoni, A. Nicolucci, G. Davì, A. Consoli, Decreased in vivo oxidative stress and decreased platelet activation following metformin treatment in newly diagnosed type 2 diabetic subjects. Diabetes Metab. Res. Rev. 24, 231–237 (2008)
C.M. Sena, T. Louro, P. Matafome, E. Nunes, P. Monteiro, R. Seiça, Antioxidant and vascular effects of gliclazide in type 2 diabetic rats fed high fat diet. Physiol. Res. 58, 203–209 (2009)
The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20, 1183–1197 (1997)
J.M. Hill, G. Zalos, J.P. Halcox, W.H. Schenke, M.A. Waclawiw, A.A. Quyyumi, T. Finkel, Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 348, 593–600 (2003)
W. Kim, M.H. Jeong, S.H. Cho, J.H. Yun, H.J. Chae, Y.K. Ahn, M.C. Lee, X. Cheng, T. Kondo, T. Murohara, J.C. Kang, Effect of green tea consumption on endothelial function and circulating endothelial progenitor cells in chronic smokers. Circ. J 70, 1052–1057 (2006)
Y. Numaguchi, T. Sone, K. Okumura, M. Ishii, Y. Morita, R. Kubota, K. Yokouchi, H. Imai, M. Harada, H. Osanai, T. Kondo, T. Murohara, The impact of the capability of circulating progenitor cell to differentiate on myocardial salvage in patients with primary acute myocardial infarction. Circulation 114, I114–I119 (2006)
C. Murphy, G.S. Kanaganayagam, B. Jiang, P.J. Chowienczyk, R. Zbinden, M. Saha, S. Rahman, A.M. Shah, M.S. Marber, M.T. Kearney, Vascular dysfunction and reduced circulating endothelial progenitor cells in young healthy UK south Asian men. Arterioscler. Thromb. Vasc. Biol. 27, 936–942 (2007)
N. Werner, S. Kosiol, T. Schiegl, P. Ahlers, K. Walenta, A. Link, M. Böhm, G. Nickenig, Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 353, 999–1007 (2005)
S. Rafii, D. Lyden, Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 9, 702–712 (2003)
J. Kao, J. Tobis, R.L. McClelland, M.R. Heaton, B.R. Davis, D.R. Holmes, J.W. Currier, Relation of metformin treatment to clinical events in diabetic patients undergoing percutaneous intervention. Am. J. Cardiol. 93, 1347–1350 (2004)
J.A. Johnson, S.R. Majumdar, S.H. Simpson, E.L. Toth, Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care 25, 2244–2248 (2002)
M. Gulliford, R. Latinovic, Mortality in type 2 diabetic subjects prescribed metformin and sulphonylurea drugs in combination: cohort study. Diabetes Metab. Res. Rev. 20, 239–245 (2004)
K.H. Kahler, M. Rajan, G.G. Rhoads, M.M. Safford, K. Demissie, S.E. Lu, Impact of oral antihyperglycemic therapy on all-cause mortality among patients with diabetes in the veterans health administration. Diabetes Care 30, 1689–1693 (2007)
J.M. Evans, S.A. Ogston, A. Emslie-Smith, A.D. Morris, Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia 49, 930–936 (2006)
C. Werner, C.H. Kamani, C. Gensch, M. Böhm, U. Laufs, The peroxisome proliferator-activated receptor-gamma agonist pioglitazone increases number and function of endothelial progenitor cells in patients with coronary artery disease and normal glucose tolerance. Diabetes 56, 2609–2615 (2007)
M. Hristov, C. Fach, C. Becker, N. Heussen, E.A. Liehn, R. Blindt, P. Hanrath, C. Weber, Reduced numbers of circulating endothelial progenitor cells in patients with coronary artery disease associated with long-term statin treatment. Atherosclerosis 192, 413–420 (2007)
R.R. Holman, S.K. Paul, M.A. Bethel, D.R. Matthews, H.A. Neil, 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359, 1577–1589 (2008)
T. Thum, D. Fraccarollo, M. Schultheiss, S. Froese, P. Galuppo, J.D. Widder, D. Tsikas, G. Ertl, J. Bauersachs, Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes 56, 666–674 (2007)
Y.H. Chen, S.J. Lin, F.Y. Lin, T.C. Wu, C.R. Tsao, P.H. Huang, P.L. Liu, Y.L. Chen, J.W. Chen, High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes 56, 1559–1568 (2007)
G. Zhou, R. Myers, Y. Li, Y. Chen, X. Shen, J. Fenyk-Melody, M. Wu, J. Ventre, T. Doebber, N. Fujii, N. Musi, M.F. Hirshman, L.J. Goodyear, D.E. Moller, Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 (2001)
N. Musi, M.F. Hirshman, J. Nygren, M. Svanfeldt, P. Bavenholm, O. Rooyackers, G. Zhou, J.M. Williamson, O. Ljunqvist, S. Efendic, D.E. Moller, A. Thorell, L.J. Goodyear, Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 51, 2074–2081 (2002)
P.E. Jennings, Vascular benefits of gliclazide beyond glycemic control. Metabolism 49, 17–20 (2000)
K. Ukinc, S. Eminagaoglu, H.O. Ersoz, C. Erem, C. Karahan, A.B. Hacihasanoglu, M. Kocak, A novel indicator of widespread endothelial damage and ischemia in diabetic patients: ischemia-modified albumin. Endocrine 26, (2009) [Epub ahead of print]
Acknowledgments
This study was supported by grants from scientific and technological project of Hubei province (No. 2007AA302B04) and the scientific research foundation from health department of Hubei province (No. JX3A04).
Disclosure
The authors declare that there is no conflict of interest that would prejudice its impartiality.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lu-lu Chen and Yun-fei Liao contributed equally to this study.
Rights and permissions
About this article
Cite this article
Chen, Ll., Liao, Yf., Zeng, Ts. et al. Effects of metformin plus gliclazide compared with metformin alone on circulating endothelial progenitor cell in type 2 diabetic patients. Endocr 38, 266–275 (2010). https://doi.org/10.1007/s12020-010-9383-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-010-9383-8