Abstract
Muscle and bone are in constant interaction. With aging, there is a progressive decline in muscle mass, known as sarcopenia, as well as in bone mass, which is known as osteopenia/osteoporosis. Sarcopenia and osteoporosis increase the risk of suffering falls and fractures, respectively. In fact, the simultaneous occurrence of osteoporosis and sarcopenia has been observed in a subset of frailer individuals at higher risk of disability, falls and fractures. However, the particular clinical outcomes that are unique to the sarco-osteoporotic patients remain unknown. In this review, we propose a common mechanism of sarco-osteoporosis and summarize those clinical and biochemical features that are prevalent in sarco-osteoporotic subjects. We expect that by describing a set of biological, clinical and functional characteristics that are associated with sarco-osteoporosis, this information could be used to inform the design of future trials and to develop interventions for this particular syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Falls and fractures affect older adults around the world and are associated with devastating complications that impair their well-being and quality of life. Falls have been associated with multiple risk factors including sarcopenia [1, 2], which is defined as the “age-associated loss of skeletal muscle mass and function” [3] and is considered as one of the most important determinants of fall risk in older persons. In contrast, among the risk factors for fractures, age and low bone mineral density (BMD) are considered the major determinants of fracture risk in older persons [4]. Interestingly, both falls and fractures share several risk factors (Table 1), thus indicating that these two entities are not only age-related but also share similar pathophysiology and possibly related diagnostic and therapeutic approach.
In fact, analysis of the pathophysiological pathways of sarcopenia and osteoporosis reveals several similarities [1, 5, 6]. Both conditions are age-related, are multifactorial and are characterized by progressive loss of tissue mass. Additionally, physical inactivity and poor nutrition accelerate the progression of both conditions [1, 3]. Despite these similarities, most diagnostic and therapeutic interventions to date target these conditions separately.
In this review, we will summarize the current state of knowledge about common pathophysiology and clinical outcomes in sarcopenia and osteoporosis. Additionally, we will discuss the benefits and limitations of potential diagnostic methods for both conditions. Finally, we will discuss diagnostic and therapeutic interventions that pose promising opportunities to improve clinical outcomes for both conditions. We expect that by linking osteoporosis and sarcopenia, the clinicians will be able to effectively identify patients at high risk and thus prevent both falls and fractures in our increasingly aging population.
Sarcopenia, Osteoporosis and Sarco-osteoporosis: Different entities or Just a Continuum?
Sarcopenia
Although sarcopenia was originally defined as an age-related reduction in muscle mass, strength and power [7]-specific clinical criteria for sarcopenia remained undefined. Recently, the European Working Group on Sarcopenia in Older People (EWGSOP) defined it as “a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcomes” [3]. This clinical definition involves not only the assessment of muscle mass but also the assessment of strength and functional performance, which are described in Fig. 1.
Suggested algorithm for the diagnosis of sarcopenia (adapted from Cruz-Jentoff et al. [3])
In terms of its pathophysiology, sarcopenia has been associated with accelerated loss of fast motor units, loss and atrophy of type II fibers and adaptive conversion of fiber II into fiber I [8]. In addition, sarcopenic patients show a significant amount of inter- and intra-fiber fat infiltration [6, 9] and high levels of circulating inflammatory cytokines [10].
Osteopenia/Osteoporosis
Osteopenia and osteoporosis are skeletal conditions associated with progressive bone loss induced by high levels of bone resorption by the osteoclasts and/or low levels of bone formation by the osteoblasts [11]. The differentiation between osteopenia and osteoporosis was established by the World Health Organization (WHO) densitometry criteria [12] in which a BMD between −1.0 and −2.5 SD is considered as osteopenia, whereas a bone density <−2.5 SD is diagnostic of osteoporosis.
Although BMD by dual-energy X-ray absorptiometry (DXA) is still considered as the gold standard for the diagnosis of osteopenia and osteoporosis, its predictive value for fracture risk in normal BMD and osteopenic patients is less accurate than in osteoporotic patients. Therefore, it has been proposed that in order to identify fracture risk, fracture risk assessment tools such as the fracture risk assessment tool (FRAX) [13] and the Garvan fracture risk assessment tool [14] could have a better accuracy in predicting fractures than an isolated BMD by DXA [15].
In terms of its pathophysiology, increasing levels of bone resorption could be induced by several risk factors including menopause, use of corticosteroids, hyperparathyroidism or inflammatory arthritis [11]. In contrast, although low bone formation could also be induced by corticosteroids, other factors that affect bone formation include vitamin D deficiency and increasing fat infiltration within the bone marrow [16]. As in sarcopenia, osteoporotic patients have increasing serum concentrations of inflammatory cytokines, predominantly interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFα) [17].
Sarco-Osteoporosis
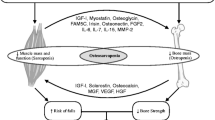
Recently, it has been proposed that there is a subgroup of older persons presenting with the concurrent clinical criteria for both sarcopenia and osteopenia/osteoporosis. These patients could be at a higher risk for poor outcomes such as falls, fractures, hospitalization, frailty and death [18]. Although the clinical and biological characteristics of these patients remain unknown, there is enough evidence to suggest that patients presenting with both entities would have a combination of risk factors, pathophysiology and clinical outcomes that predispose them to higher risk of falls and fractures.
In terms of a common pathophysiology, apart from age, there are risk factors associated with both sarcopenia and osteoporosis including fat infiltration of muscle and bone, which was demonstrated in an animal model of sarco-osteopenia [6]. Other risk factors include low levels of vitamin D associated with high levels of parathyroid hormone (PTH), low levels of anabolic hormones in men and menopause in women, use of corticosteroids, malnutrition, disuse and frailty [19]. In addition, from the biological point of view, both entities are associated with high levels of circulating inflammatory cytokines, which are most likely secreted by both marrow and body fat in a process known as lipotoxicity [17].
In fact, sarco-osteoporotic patients could be classified as a subgroup of high-risk patients that share risk factors and biological features for both sarcopenia and osteoporosis (Table 2). Whether patients start their decline as sarcopenic and then become osteoporotic or vice versa still remains unknown. In addition, since it is expected that poor outcomes are more frequent in this subgroup, appropriate identification of clinical outcomes and development of reliable and robust biomarkers are highly needed in this particular population.
Assessment for Sarco-Osteoporosis and Sarco-Osteopenia: A Proposed Framework (Table 3)
Image Analysis of Muscle and Bone Mass
According to EWGSOP (Fig. 1) [3], sarcopenia could be diagnosed based on three measures: (1) loss of muscle mass combined with, (2) decreased strength and/or (3) poor physical performance. Since muscle strength and physical performance are variables that only relate to muscle and not to bone mass, we will focus this section on the quantification of tissue mass by image analysis as the most feasible and reliable methods of diagnosing sarco-osteoporosis.
Muscle mass could be indirectly measured by DXA, bioelectrical impedance, magnetic resonance imaging (MRI) and CT scan [20]. Each of these methods has its strengths and limitations, which have been reviewed elsewhere [21] and are also described in detail in another chapter of this special issue. In terms of practicality, DXA seems to be the most widely accepted due to its low cost, low radiation levels and easy interpretation of the results. However, body composition analysis by DXA has major limitations since it remains unclear what would be the best region of interest (ROI) that should be used when assessing patients for sarcopenia and also because the results could vary according to body water content and positioning issues [21].
When comparing these indirect methods to measure muscle mass, and in the context of sarco-osteoporosis and sarco-osteopenia, the optimal method should allow us to accurately measure muscle and bone mass together with the quantification of fat infiltration within these tissues. In the case of DXA, it measures fat and lean mass and bone mineral content but is unable to accurately calculate the levels of fat infiltration within. Other methods have similar limitations; For instance, although CT scan measures bone mass, its accuracy to measure fat infiltration is limited and requires the use of some complex algorithms [22]. MRI measures bone density and is considered the gold standard to quantify marrow fat composition but has multiple contraindications and is less accurate when measuring fat infiltration within the muscle fibers [23]. Overall, although all these imaging methods have been validated to measure bone mass, their reliability and validity to measure marrow and muscle fat remain unexplored in well-designed human studies; therefore, their use in the diagnosis of sarco-osteoporosis is expected to be limited by cost, radiation and particular methodological issues.
Recently, we reported a new reliable method to quantify levels of fat infiltration in muscle and bone. This method, which was initially validated in rats [24], could be used to measure bone and fat volumes in small ROI of CT scan images of proximal femur and mid-thigh [6, 24] (Fig. 2). Although further human studies are still required, our evidence suggests that this is a reliable and safe method to perform image analysis of muscle and bone including quantification of their fat volumes, which is expected to have some importance in clinical practice.
Noninvasive quantification of fat infiltration in muscle and bone in a mouse model of sarco-osteopenia. Muscle, bone, marrow fat and inter-myofiber fat volumes were highlighted (upper panels) and quantified (lower panels) in micro-CT sections of the contralateral mid-thigh using Slice-O-Matic image analysis software. In Lmna −/− mice, there is progressive thinning of cortical bone (blue), muscle atrophy (red) and “marbling” of the muscle, with significant infiltration of inter-myofiber fat (yellow). Note the comparable red bone marrow volume (green) in the three groups. (*p < 0.001, Lmna +/− vs. Lmna +/+ mice; # p < 0.001, Lmna −−- vs. Lmna +/− mice) (adapted from Tong et al. [6]) (Color figure online)
Serological Assessment of Sarco-Osteopenic Patients
In the absence of a robust biomarker for sarco-osteoporosis, we propose using a set of indirect serum parameters to predict poor outcomes and to identify therapeutic response in both muscle and bone (Table 3). However, evidence supporting the use of these biochemical markers in sarco-osteoporosis is restricted to the research settings with their applicability in clinical settings still untested.
Since vitamin D deficiency is associated with both sarcopenia and osteoporosis and with high levels of serum PTH being associated with higher risk of falls and fractures [25], it would be expected that poor outcomes in sarco-osteopenic patients could be prevented after normalization of their serum concentrations of vitamin D. Studies have shown that falls and fractures could be prevented by normalization of serum vitamin D in older patients. This effect has been explained by an increase in calcium absorption and improvement in muscle function. In terms of PTH, although high levels of this hormone are associated with low bone and muscle mass, no studies have looked at the effect of normalizing PTH on falls and fractures. Further studies looking at the role of PTH in the pathogenesis of sarco-osteoporosis are still pending.
In addition, considering that high levels of inflammatory cytokines such as IL-6 and TNFα are associated with both osteoporosis and sarcopenia [25], it would be expected that patients suffering from both entities should show much higher levels of these inflammatory cytokines in their serum. In fact, very few studies have looked at serum levels of inflammatory cytokines in the particular subset of sarco-osteoporotic patients, and thus, the evidence is still scarce. Finally, other biomarkers of sarcopenia (alpha chymotrypsin, C-creatine dilution, etc.) [26], although are mostly related to muscle biology, still deserve some exploration in the osteoporosis field and could constitute the missing biomarker that is so highly needed.
Identification of Therapeutic Response in Sarco-Osteoporotic Patients
Therapeutic response in sarcopenic patients is demonstrated by either an improvement in muscle strength with better grip strength and leg extension or an increase in muscle mass demonstrated by DXA. As the main outcomes, this improvement is expected to correlate with higher gait velocity and with a reduction in falls risk in the treated patient. In contrast, patients treated for osteopenia or osteoporosis are expected to increase their BMD and/or show a reduction in the incidence of minimal trauma fractures.
Based on these expected outcomes, therapeutic response to interventions targeting both osteoporosis and sarcopenia should show improvement in both muscle and bone mass together with a better muscle strength and a significant reduction in the incidence of falls and fractures. Other secondary outcomes should include a lower incidence of disability and frailty and lower mortality. Although several studies have demonstrated the effect of vitamin D on these parameters, no studies have been performed treating a particular subpopulation of sarco-osteoporotic patients with vitamin D.
In contrast, exercise has demonstrated to improve both muscle and bone mass while increasing muscle strength, power and mass [25, 27]. Although the evidence is variable, there is some consensus that a regular strength and balance exercise—at least three times a week for at least 20 min in duration—could significantly improve both muscle and bone parameters and prevent falls and fractures in older persons [27]. The mechanisms that explain this concurrent muscle and bone response to exercise remain unclear, but it has been suggested that both tissues improve due to the mechanical loading exerted by the muscle on the bone tissue and by stimulating the differentiation of mesenchymal stem cells into osteoblasts at the expense of adipocytes [28].
Increased protein intake is another intervention that could have a potential therapeutic effect on both sarcopenic and osteoporotic patients [29]. This effect on muscle and bone is associated not only with the quantity but also with the quality of the protein intake. For instance, a large cohort of elderly women and men were exposed to several protein regimes and were prospectively followed over 4 years as part of the Framingham Osteoporosis Study [30]. It was observed that the highest levels of protein intake (84–152 g/day) were correlated with protection against spinal and femoral bone loss in both genders when compared to the lowest quartile (17–51 g/day). In terms of the quality of protein intake and its effect on bone mass, studies have observed that animal protein intake (meat and dairy products) is associated with higher BMD and also a better effect on bone and calcium metabolism, while vegetable protein intake (soy food) has not been linked with an effect on BMD.
In addition, the beneficial effect of protein intake on muscle mass and function has been already demonstrated with general agreement that protein intake beyond 0.8 mg/Kg/d of animal proteins rich in essential amino acids enhances muscle protein anabolism and protects against age-related muscle loss [29]. Taken together, there is sufficient evidence to suggest that increased protein intake—at levels > 0.8 mg/Kg/d—is expected to increase muscle and bone mass. However, the effect of protein supplementation on falls and fractures prevention in a subset of sarco-osteopenic patients remains to be elucidated.
Finally, myostatin antibodies have been proposed as a new therapeutic approach to both osteoporosis and sarcopenia [31]. Although myostatin antibodies are still in their experimental phase, evidence obtained from animal models suggests that treatment with these antibodies induces a gain in both muscle and bone mass in the treated groups [32]; however, the significance of these findings and the impact of this treatment on the prevention of falls and fractures will only be determined after the completion of several ongoing clinical trials.
Conclusion
We have focused this review on the clinical outcomes of impaired bone and muscle interaction in older persons, which could be defined as sarco-osteoporosis. Although a clear definition of sarco-osteoporosis is still lacking, there is solid evidence demonstrating that this syndrome may exist as a single entity, and as such, it should have its own pathophysiology, clinical features and therapeutic approach. In terms of clinical outcomes, a combination of clinical criteria for sarcopenia and the identification of risk factors for sarcopenia and osteoporosis together with imaging analysis of bone and muscle mass—including the quantification of levels of fat infiltration within these tissues—seems to be the most reasonable approach to identify and treat sarco-osteoporotic patients in the future (Table 3).
In conclusion, sarco-osteoporosis is a new syndrome that should be diagnosed using clinical outcomes and image analysis. These clinical outcomes should be clearly defined in order to identify those patients at higher risk of falls, fractures, frailty and disability and to determine whether interventions that simultaneously improve muscle and bone mass have a significant effect in reducing these poor outcomes, which are highly prevalent in our aging population.
References
Cederholm T, Cruz-Jentoft AJ, Maggi S. Sarcopenia and fragility fractures. Eur J Phys Rehabil Med. 2013;49:111–7.
Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med. 2011;27:387–99.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23.
Krege JH, Wan X, Lentle BC, Berger C, Langsetmo L, Adachi JD, Prior JC, Tenenhouse A, Brown JP, Kreiger N, Olszynski WP, Josse RG, Goltzman D; CaMos Research Group. Fracture risk prediction: importance of age, BMD and spine fracture status. Bonekey Rep 2013;2:404.
DiGirolamo DJ, Kiel DP, Esser KA. Bone and skeletal muscle: neighbors with close ties. J Bone Miner Res. 2013;28:1509–18.
Tong J, Li W, Vidal C, Yeo LS, Fatkin D, Duque G. Lamin A/C deficiency is associated with fat infiltration of muscle and bone. Mech Ageing Dev. 2011;132:552–9.
Carla Task Force on Sarcopenia: propositions for clinical trials. Abellan van Kan G, André E, Bischoff Ferrari HA, Boirie Y, Onder G, Pahor M, Ritz P, Rolland Y, Sampaio C, Studenski S, Visser M, Vellas B. J Nutr Health Aging. 2009;1:700–7.
Sayer AA, Robinson SM, Patel HP, Shavlakadze T, Cooper C, Grounds MD. New horizons in the pathogenesis, diagnosis and management of sarcopenia. Age Ageing. 2013;42:145–50.
Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH. Health, aging, and body. longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–85.
Bucci L, Yani SL, Fabbri C, Bijlsma AY, Maier AB, Meskers CG, Narici MV, Jones DA, McPhee JS, Seppet E, Gapeyeva H, Pääsuke M, Sipilä S, Kovanen V, Stenroth L, Musarò A, Hogrel JY, Barnouin Y, Butler-Browne G, Capri M, Franceschi C, Salvioli S. Circulating levels of adipokines and IGF-1 are associated with skeletal muscle strength of young and old healthy subjects. Biogerontology. 2013;14:261–72.
Demontiero O, Vidal C, Duque G. Aging and bone loss: new insights for the clinician. Ther Adv Musculoskelet Dis. 2012;4:61–76.
Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. Geneva, World Health Organization, 1994 (WHO Technical Report Series, No. 843).
McCloskey E, Johansson H, Oden A, Kanis JA. Fracture risk assessment. Clin Biochem. 2012;45:887–93.
Nguyen TV, Center JR, Eisman JA. Individualized fracture risk assessment: progresses and challenges. Curr Opin Rheumatol. 2013;25:532–41.
Leslie WD, Lix LM. Comparison between various fracture risk assessment tools. Osteoporos Int. 2013 Jun 25.
Ng A, Duque G. Osteoporosis as a lipotoxic disease. Bonekey Rep. 2010;7:108–23.
Maugeri D, Russo MS, Franzé C, Motta V, Motta M, Destro G, Speciale S, Santangelo A, Panebianco P, Malaguarnera M. Correlations between C-reactive protein, interleukin-6, tumor necrosis factor-alpha and body mass index during senile osteoporosis. Arch Gerontol Geriatr. 1998;27:159–63.
Binkley N, Buehring B. Beyond FRAX: it’s time to consider “sarco-osteopenia”. J Clin Densitom. 2009;12:413–6.
Kull M, Kallikorm R, Lember M. Impact of a new sarco-osteopenia definition on health-related quality of life in a population-based cohort in Northern Europe. J Clin Densitom. 2012;15:32–8.
Walston JD. Sarcopenia in older adults. Curr Opin Rheumatol. 2012;24:623–7.
Mijnarends DM, Meijers JM, Halfens RJ, ter Borg S, Luiking YC, Verlaan S, Schoberer D, Cruz Jentoft AJ, van Loon LJ, Schols JM. Validity and reliability of tools to measure muscle mass, strength, and physical performance in community-dwelling older people: a systematic review. J Am Med Dir Assoc. 2013;14:170–8.
Patsch JM, Li X, Baum T, Yap SP, Karampinos DC, Schwartz AV, Link TM. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res. 2013;28:1721–8.
Shen W, Gong X, Weiss J, Jin Y. Comparison among T1-weighted magnetic resonance imaging, modified Dixon method, and magnetic resonance spectroscopy in measuring bone marrow fat. J Obes. 2013. doi:10.1155/2013/298675.
Demontiero O, Li W, Thembani E, Duque G. Validation of noninvasive quantification of bone marrow fat volume with microCT in aging rats. Exp Gerontol. 2011;46:435–40.
Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care. 2013;16:272–7.
Sirola J, Kröger H. Similarities in acquired factors related to postmenopausal osteoporosis and sarcopenia. J Osteoporos. 2011;2011:536735.
Gianoudis J, Bailey CA, Sanders KM, Nowson CA, Hill K, Ebeling PR, Daly RM. Osteo-cise: strong bones for life: protocol for a community-based randomised controlled trial of a multi-modal exercise and osteoporosis education program for older adults at risk of falls and fractures. BMC Musculoskelet Disord. 2012;13:78.
Ozcivici E, Luu YK, Adler B, Qin YX, Rubin J, Judex S, Rubin CT. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6:50–9.
Genaro Pde S, Martini LA. Effect of protein intake on bone and muscle mass in the elderly. Nutr Rev. 2010;68:616–23.
Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:2504–12.
Buehring B, Binkley N. Myostatin—the holy grail for muscle, bone, and fat? Curr Osteoporos Rep. 2013;11:407–14.
Bradley L, Yaworsky PJ, Walsh FS. Myostatin as a therapeutic target for musculoskeletal disease. Cell Mol Life Sci. 2008;65:2119–24.
Acknowledgments
The authors’ research cited in this review has been funded by project grants from the National Health and Medical Research Council (NHMRC) of Australia (Grants 632766 and 632767) and the Nepean Medical Research Foundation.
Disclosures
Conflict of interest
Oddom Demontiero, Derek Boersma, Pushpa Suriyaarachchi and Gustavo Duque declare that they have no conflict of interest.
Animal/Human Studies
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Demontiero, O., Boersma, D., Suriyaarachchi, P. et al. Clinical Outcomes of Impaired Muscle and Bone Interactions. Clinic Rev Bone Miner Metab 12, 86–92 (2014). https://doi.org/10.1007/s12018-014-9164-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12018-014-9164-7