Abstract
Adult hippocampal neurogenesis is a dynamic process involved in cognitive functions, like learning and memory. Numerous intrinsic and extrinsic factors regulate and affect hippocampal neurogenesis. An exceptionally beneficial external factor is physical exercise due to the impact of the lactate accumulated during physical effort on neural plasticity. Lactate has recently emerged as one of the most interesting and potent factors in health and disease due to its involvement in the metabolism and signaling of most, if not all, of the cells in the CNS. Herein, we illustrate the effects induced by lactate on the different cell types within the neurogenic niche, in light of their described roles in regulating adult hippocampal neurogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adult hippocampal neurogenesis is a dynamic process important to cognitive function, such as learning and memory. Numerous intrinsic and extrinsic factors regulate neurogenesis, including environmental enrichment, stress, learning, dietary interventions, and one exceptionally potent– physical exercise. During exercise, the metabolite lactate accumulates due to enhanced glycolysis in muscle cells. It is then transported to various organs, including the brain. In neurons, lactate is preferred over glucose as a metabolic substrate to sustain neuronal activity, particularly under high energy requirements (Matsui et al. 2017).
Lactate modulates molecular pathways interconnected with neurogenesis, such as angiogenesis (Morland et al. 2017), neuronal excitability, and plasticity, all of which promote the survival of newly formed neurons (Álvarez et al. 2014; Lev-Vachnish et al. 2019; Zhou et al. 2018). Lactate also modulates metabolic and signaling pathways of non-neural cells in the neurogenic niche, including astrocytes, endothelial cells, oligodendrocytes, and microglia, which cue the process of neurogenesis.
Herein, we illustrate the effects induced by lactate on the cells within the neurogenic niche, with respect to their roles in regulating adult hippocampal neurogenesis.
Endothelial Cells
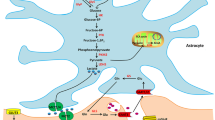
Blood vessels within the neurogenic niche shuttle, from the periphery to the CNS, factors that regulate adult neurogenesis, namely: brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF), a strong inducer of adult hippocampal neurogenesis (Fabel et al. 2003) and angiogenesis. Production of VEGF is mediated by activation of the lactate receptor Hydroxycarboxylic Acid Receptor 1 (HCAR1), highly expressed in pial fibroblast-like cells that wrap endothelial cells and in pericyte-like cells along micro-vessels (Morland et al. 2017) (Fig. 1).
Lactate involvement in metabolic and plasticity events in cells of the neurogenic niche. In endothelial cells, the PI3K/AKT pathway regulates monocarboxylate transporter (MCT)-1 expression and subsequently lactate transport. Lactate activates HCAR1 on pial astrocytes to regulate VEGF levels, which enhance angiogenesis and promote neurogenesis. Microglial polarization to either homeostatic or pro-inflammatory phenotype regulates lactate synthesis and secretion, which can be modulated by phagocytosis of newborn neurons. Oligodendrocytes can actively participate in supplying neurons with lactate and promote axonal integrity. Astrocytes can export lactate to neurons as a result of recycling glutamate from the synaptic cleft as part of the astrocyte-neuron lactate shuttle hypothesis, to activate NMDARs and promote immediate-early gene transcription, neuronal survival, axonal integrity, LTP, and spine stabilization
Endothelial cells also regulate lactate homeostasis in the neurogenic niche, affecting the neurogenic lineage by activating the Phosphatidylinositol-4, 5-Bisphosphate 3-Kinase (PI3K)/AKT pathway, which is central to cell proliferation. Knockout of Phosphatase and Tensin Homolog (PTEN) imbalances this homeostasis and increases neural stem cell (NSC) proliferation in young mice. Lack of PTEN, which typically inhibits AKT, increases AKT activation and decreases the expression of the lactate transporter monocarboxylate transporter 1 (MCT1), leading to intracellular lactate accumulation. When PTEN knockout mice get older, NSC proliferation and differentiation decrease, possibly due to exhaustion of the NSC pool. The same phenomenon also occurs following intrahippocampal 10 mM lactate supplementation; an effect that is rescued following MCT1 overexpression (Fig. 1). Thus, abnormal lactate accumulation in the hippocampus disrupts adult hippocampal neurogenesis and impairs memory (Scandella & Knobloch, 2019; Wang et al. 2019). These observations contradict studies that linked lactate to enhanced neurogenesis (Lev-Vachnish et al. 2019), angiogenesis (Morland et al. 2017), and memory function (El Hayek et al. 2019). The differences may stem from variations in experimental settings, as the injected 10 mM lactate to the hippocampus is much higher than typical lactate levels under normal physiological state. Nevertheless, these observations emphasize the importance of a balanced metabolic environment orchestrated by endothelial cells to support neurogenesis by shuttling metabolites, including lactate, to the brain parenchyma.
Astrocytic-Neuronal Lactate Coupling
According to the astrocytes-neuron lactate shuttle (ANLS) hypothesis, astrocytes and neurons are metabolically coupled. Astrocytes recycle glutamate from the synaptic cleft and convert it into glutamine. The glutamine enhances glucose consumption or glycogen breakup, which elevates lactate production (Pellerin & Magistretti, 1994). When energy demand increases during intense synaptic activity, astrocytes supply lactate to neurons via MCTs, supporting neuronal function (Fig. 1). Receiving signals and forming synapses with other cells are crucial for the survival and integration of immature neurons in the circuit. Astrocytes are involved in synapse formation, as blocking exocytosis from astrocytes disrupts neuronal NMDA receptor activity and reduces dendritic spine formation on adult-born neurons (Sultan et al. 2015). Lactate was shown to induce structural stabilization of newly formed spines following learning (Vezzoli et al. 2020) and enhance LTP in neurons (Suzuki et al. 2011), which could be the mechanism by which lactate promotes the survival of immature neurons (Lev-Vachnish et al. 2019) (Fig. 1). Also, lactate shuttle from astrocytes to neurons facilitates calcium influx through NMDARs, thereby increasing the expression of synaptic plasticity-related genes, like Arc, cFOS, and BDNF (Yang et al. 2014) (Fig. 1). Whether lactate mediates newborn neurons’ survival and whether this is mediated via NMDAR facilitation and synaptogenesis remains to be seen.
Oligodendrocytes
Myelin production is the central function of oligodendrocytes. Lactate is imported to oligodendrocytes through MCTs to support myelination by promoting differentiation of oligodendrocytes progenitor cells (OPCs) (Ichihara et al. 2017); moreover, lactate is used as a precursor of lipids which mediates myelin synthesis (Sánchez-Abarca et al. 2001). Although mossy fibers which stem from granule cells in the dentate gyrus are unmyelinated, a recent study showed that hippocampal demyelination diminishes adult neurogenesis, reduces dendritic length and spine density of adult-born neurons. This indicates that myelination and OPCs differentiation can have an indirect effect on neurogenesis, possibly via inputs received from the myelinated axons that originate in the entorhinal cortex (Zhang et al. 2020). Beyond myelination, oligodendrocytes support axonal survival independently of myelin synthesis (Lee et al. 2012). Like astrocytes, oligodendrocytes are mainly glycolytic and can shuttle lactate to neurons through MCTs to supply local energy and promote axonal integrity and neuronal survival. MCT1 deficiency induces myelination-independent axonal degeneration, an effect that can be rescued by exogenous lactate supplementation (Lee et al. 2012) (Fig. 1). Whether survival of young neurons is impaired due to reduced lactate transfer from oligodendrocyte remains to be seen.
Microglia
As brain-resident immune cells, microglia conduct brain surveillance by eliminating and remodeling synapses and activating inflammatory responses. Metabolic shift toward enhanced glycolysis and accumulation of lactate occurs when microglial cells are polarized toward a pro-inflammatory state. Blocking MCT1 during inflammation or balancing lactate levels outside the cells reduces microglial glycolysis rate, cell activation, and pro-inflammatory response (Kong et al. 2019). Microglia eliminates apoptotic cells, including newborn neurons, through phagocytosis. This is essential for balanced neurogenesis, as chronic inhibition of phagocytosis impairs neurogenesis, while acute inhibition transiently enhances neurogenesis. Following phagocytosis of apoptotic NSCs, microglial cells secrete molecules that alter the production of new neurons and balance between proliferation and survival of newborn neurons; the secretome of phagocytotic microglia reduces neuronal differentiation in vivo and in vitro (Diaz-Aparicio et al. 2020). Moreover, phagocytosis of apoptotic cells triggers transcriptomic changes that include metabolic reprogramming (Diaz-Aparicio et al. 2020). This is similar to macrophages, in which phagocytosis of apoptotic cells induct aerobic glycolysis and suppress oxidative phosphorylation, coupled with MCT1 upregulation and increased lactate release (Morioka et al. 2018). It is intriguing to investigate whether the elimination of newborn neurons by microglia leads to lactate release in the neurogenic niche due to the upregulation of aerobic glycolysis and how this metabolic shift affects adult neurogenesis.
Summary and Prospective
As the metabolic and molecular effects of lactate on neurogenesis begin to unravel, we overview the mechanism in which astrocytes and oligodendrocytes sense neuronal activity by tracking glutamate release from neurons and reacting rapidly via glycolysis to provide neurons with the energy needed in the form of lactate. This energy supply chain may begin in endothelial cells that lay in proximity, as astrocytes and oligodendrocytes increase glucose uptake from the blood via glucose transporters. Maintaining lactate levels in the neurogenic niche can be directly exerted by endothelial cells, thereby affecting the proliferation and maturation of newborn neurons.
References
Álvarez, Z., Castaño, O., Castells, A. A., Mateos-Timoneda, M. A., Planell, J. A., Engel, E., et al. (2014). Neurogenesis and vascularization of the damaged brain using a lactate-releasing biomimetic scaffold. Biomaterials, 35(17), 4769–4781. https://doi.org/10.1016/j.biomaterials.2014.02.051.
Diaz-Aparicio, I., Paris, I., Sierra-Torre, V., Plaza-Zabala, A., Rodríguez-Iglesias, N., Márquez-Ropero, M., et al. (2020). Microglia Actively Remodel Adult Hippocampal Neurogenesis through the Phagocytosis Secretome. Journal of Neuroscience, 40(7), 1453–1482. https://doi.org/10.1523/JNEUROSCI.0993-19.2019.
El Hayek, L., Khalifeh, M., Zibara, V., Abi Assaad, R., Emmanuel, N., Karnib, N., et al. (2019). Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). Journal of Neuroscience, 39(13), 2369–2382. https://doi.org/10.1523/JNEUROSCI.1661-18.2019.
Fabel, K., Fabel, K., Tam, B., Kaufer, D., Baiker, A., Simmons, N., et al. (2003). VEGF is necessary for exercise-induced adult hippocampal neurogenesis. European Journal of Neuroscience, 18(10), 2803–2812. https://doi.org/10.1111/j.1460-9568.2003.03041.x.
Ichihara, Y., Doi, T., Ryu, Y., Nagao, M., Sawada, Y., & Ogata, T. (2017). Oligodendrocyte progenitor cells directly utilize lactate for promoting cell cycling and differentiation. Journal of Cellular Physiology, 232(5), 986–995. https://doi.org/10.1002/jcp.25690.
Kong, L., Wang, Z., Liang, X., Wang, Y., Gao, L., & Ma, C. (2019). Monocarboxylate transporter 1 promotes classical microglial activation and pro-inflammatory effect via 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3. Journal of Neuroinflammation, 16(1), 240. https://doi.org/10.1186/s12974-019-1648-4.
Lee, Y., Morrison, B. M., Li, Y., Lengacher, S., Farah, M. H., Hoffman, P. N., et al. (2012). Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature, 487(7408), 443–448. https://doi.org/10.1038/nature11314.
Lev-Vachnish, Y., Cadury, S., Rotter-Maskowitz, A., Feldman, N., Roichman, A., Illouz, T., et al. (2019). L-lactate promotes adult hippocampal neurogenesis. Frontiers in Neuroscience, 13, 403. https://doi.org/10.3389/fnins.2019.00403.
Matsui, T., Omuro, H., Liu, Y. F., Soya, M., Shima, T., McEwen, B. S., et al. (2017). Astrocytic glycogen-derived lactate fuels the brain during exhaustive exercise to maintain endurance capacity. Proceedings of the National Academy of Sciences USA, 114(24), 6358–6363. https://doi.org/10.1073/pnas.1702739114.
Morioka, S., Perry, J. S. A., Raymond, M. H., Medina, C. B., Zhu, Y., Zhao, L., et al. (2018). Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature, 563(7733), 714–718. https://doi.org/10.1038/s41586-018-0735-5.
Morland, C., Andersson, K. A., Haugen, Ø., Hadzic, A., Kleppa, L., Gille, A., et al. (2017). Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nature Communications, 8, 15557. https://doi.org/10.1038/ncomms15557.
Pellerin, L., & Magistretti, P. J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A, 91(22), 10625–10629.
Sánchez-Abarca, L. I., Tabernero, A. & Medina, J. M. (2001). Oligodendrocytes use lactate as a source of energy and as a precursor of lipids. Glia, 36(3), 321–329. https://doi.org/10.1002/glia.1119.
Scandella, V., & Knobloch, M. (2019). Sensing the environment: extracellular lactate levels control adult neurogenesis. Cell Stem Cell, 25(6), 729–731. https://doi.org/10.1016/j.stem.2019.11.008.
Sultan, S., Li, L., Moss, J., Petrelli, F., Cassé, F., Gebara, E., et al. (2015). Synaptic integration of adult-born hippocampal neurons is locally controlled by astrocytes. Neuron, 88(5), 957–972. https://doi.org/10.1016/j.neuron.2015.10.037.
Suzuki, A., Stern, S. A., Bozdagi, O., Huntley, G. W., Walker, R. H., Magistretti, P. J., et al. (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell, 144(5), 810–823. https://doi.org/10.1016/j.cell.2011.02.018.
Vezzoli, E., Calì, C., De Roo, M., Ponzoni, L., Sogne, E., Gagnon, N., et al. (2020). Ultrastructural evidence for a role of astrocytes and glycogen-derived lactate in learning-dependent synaptic stabilization. Cerebral Cortex, 30(4), 2114–2127. https://doi.org/10.1093/cercor/bhz226.
Wang, J., Cui, Y., Yu, Z., Wang, W., Cheng, X., Ji, W., et al. (2019). Brain endothelial cells maintain lactate homeostasis and control adult hippocampal neurogenesis. Cell Stem Cell, 25(6), 754-767.e759. https://doi.org/10.1016/j.stem.2019.09.009.
Yang, J., Ruchti, E., Petit, J. M., Jourdain, P., Grenningloh, G., Allaman, I., et al. (2014). Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci U S A, 111(33), 12228–12233. https://doi.org/10.1073/pnas.1322912111.
Zhang, H., Kim, Y., Ro, E. J., Ho, C., Lee, D., Trapp, B. D., et al. (2020). Hippocampal neurogenesis and neural circuit formation in a cuprizone-induced multiple sclerosis mouse model. Journal of Neuroscience, 40(2), 447–458. https://doi.org/10.1523/JNEUROSCI.0866-19.2019.
Zhou, J., Liu, T., Guo, H., Cui, H., Li, P., Feng, D., et al. (2018). Lactate potentiates angiogenesis and neurogenesis in experimental intracerebral hemorrhage. Experimental & Molecular Medicine, 50(7), 78. https://doi.org/10.1038/s12276-018-0113-2.
Acknowledgements
This work was funded by the Paul Feder fund for Alzheimer’s disease research, and the manuscript was edited by Yael Laure.
Funding
This work was funded by the Paul Feder fund for Alzheimer’s disease research.
Author information
Authors and Affiliations
Contributions
RN and EO wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nicola, R., Okun, E. Adult Hippocampal Neurogenesis: One Lactate to Rule Them All. Neuromol Med 23, 445–448 (2021). https://doi.org/10.1007/s12017-021-08658-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-021-08658-y