Abstract
Ventral midbrain (VM) dopaminergic (DA) neurons project to the dorsal striatum via the nigrostriatal pathway to regulate voluntary movements, and their loss leads to the motor dysfunction seen in Parkinson’s disease (PD). Despite recent progress in the understanding of VM DA neurogenesis, the factors regulating nigrostriatal pathway development remain largely unknown. The bone morphogenetic protein (BMP) family regulates neurite growth in the developing nervous system and may contribute to nigrostriatal pathway development. Two related members of this family, BMP2 and growth differentiation factor (GDF)5, have neurotrophic effects, including promotion of neurite growth, on cultured VM DA neurons. However, the molecular mechanisms regulating their effects on DA neurons are unknown. By characterising the temporal expression profiles of endogenous BMP receptors (BMPRs) in the developing and adult rat VM and striatum, this study identified BMP2 and GDF5 as potential regulators of nigrostriatal pathway development. Furthermore, through the use of noggin, dorsomorphin and BMPR/Smad plasmids, this study demonstrated that GDF5- and BMP2-induced neurite outgrowth from cultured VM DA neurons is dependent on BMP type I receptor activation of the Smad 1/5/8 signalling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the central nervous system (CNS), more than three-quarters of all DA neurons are found in the VM (Blum 1998; German et al. 1983; Pakkenberg et al. 1991). These are subdivided into three distinct clusters, termed the A8, A9 and A10 groups of VM DA neurons. The A9 group of VM DA neurons, located in the substantia nigra pars compacta (SNpc), projects to the dorsolateral striatum via the nigrostriatal pathway (Dahlstroem and Fuxe 1964; Bjorklund and Dunnett 2007). These A9 DA neurons, and their striatal targets, are part of the basal ganglia circuitry that regulates the control of voluntary movement. Their functional importance is highlighted by the neurodegenerative disorder PD, the primary neuropathological signature of which is the loss of these neurons and their striatal projections, which results in the motor deficits that are the characteristic of this disease (Toulouse and Sullivan 2008; Lees et al. 2009).
During embryonic development, A9 DA neurons are generated in the VM under the influence of two key signalling centres, the isthmus and the floor plate (Hynes et al. 1995; Crossley and Martin 1995; Liu and Joyner 2001). Much work in recent decades has focused on elucidating the molecular circuitry that is involved in the generation of A9 VM DA neurons (Hegarty et al. 2013c); however, the molecular mechanisms that regulate the growth and guidance of the axonal projections of these DA neurons to their appropriate target regions in the striatum are less well understood.
VM DA neurons extend their axons towards the telencephalon via the medial forebrain bundle in response to extrinsic directional cues (both chemo-attractive and chemo-repulsive) from the caudal brain stem, midbrain, diencephalon, striatum and cortex (Gates et al. 2004; Nakamura et al. 2000). Despite the paucity of studies identifying the regulatory molecules involved in the formation of DA projections, a number of molecules have been implicated. Cell surface ephrins and their Eph receptor tyrosine kinases, which are important in axonal guidance (Egea and Klein 2007), have been shown to play roles in target innervation by nigrostriatal axons (Sieber et al. 2004; Halladay et al. 2004; Van den Heuvel and Pasterkamp 2008; Calo et al. 2005; Yue et al. 1999; Cooper et al. 2009). Similarly, netrin signalling via the deleted colorectal cancer (DCC) receptor, which is known to actively regulate axonal growth (Round and Stein 2007), has been strongly implicated in the formation of the VM DA circuitry (Xu et al. 2010; Flores et al. 2005; Manitt et al. 2011; Lin et al. 2005; Sgado et al. 2012; Vitalis et al. 2000). Additionally, signalling between Slits and their Robo receptors (Bagri et al. 2002; Dugan et al. 2011; Lin et al. 2005; Lopez-Bendito et al. 2007), and by semaphorins (Hernandez-Montiel et al. 2008; Torre et al. 2010; Tamariz et al. 2010; Kolk et al. 2009), has been shown to regulate the formation of DA projections from the VM to the striatum. These identified molecules are well-established regulators of axonal growth and guidance in other regions of the nervous system. It is thus likely that further candidate molecules with similar functions in other areas of the NS may contribute to the regulation of DA axonal growth. One candidate group of molecules is the BMP family (Zou and Lyuksyutova 2007; Bovolenta 2005).
BMPs are regulators of axonal growth in a number of neuronal populations, with this role best characterised in the dorsal spinal cord (SC) (Parikh et al. 2011; Hazen et al. 2012; Lein et al. 1995; Hegarty et al. 2013a; Gratacos et al. 2002). The two members of the BMP family of proteins, BMP2 and a related molecule GDF5, have been shown to regulate neurite growth in the dorsal SC (Parikh et al. 2011; Hazen et al. 2011, 2012; Phan et al. 2010; Niere et al. 2006). GDF5 and BMP2 both activate a canonical signalling pathway involving two types of serine/threonine kinase receptors, type I and type II BMPRs (ten Dijke et al. 1994; Koenig et al. 1994; Yamashita et al. 1996; Shi and Massague 2003). Upon ligand binding, the constitutively active BMPRII transphosphorylates the cytoplasmic domain of the BMPRI (BMPRIa or BMPRIb), causing phosphorylation of the receptor-regulated Smads, Smads 1/5/8, by the activated BMPRI. The phosphorylated Smads 1/5/8 then form a heterocomplex with the co-Smad, Smad4, which mediates their nuclear translocation to allow modulation of target gene expression (Miyazono et al. 2010; Sieber et al. 2009).
BMP2 and GDF5 are expressed in the developing rat VM during the period of DA axogenesis, suggesting that they may play a role in this process (Krieglstein et al. 1995; O’Keeffe et al. 2004b; Storm et al. 1994; Chen et al. 2003; Jordan et al. 1997; Soderstrom and Ebendal 1999; Hegarty et al. 2014). In support of such a suggestion, both GDF5 and BMP2 have been shown to promote the survival of rat VM DA neurons (O’Keeffe et al. 2004a; Reiriz et al. 1999; Jordan et al. 1997; Sullivan et al. 1997) and induce neurite growth of rat VM DA neurons in vitro (O’Keeffe et al. 2004a; Reiriz et al. 1999). Despite these studies, the expression patterns of the BMP receptors (BMPRs) in the VM and the target striatum during nigrostriatal pathway development are unknown. Furthermore, the mechanisms by which GDF5 and BMP2 mediate their neurite growth-promoting effects on VM DA neurons remain to be determined. However, these effects have recently been proposed to occur via the canonical Smad signalling pathway in a cell line model of dopaminergic neurons (Hegarty et al. 2013b).
To address the gaps in our current knowledge of BMP-mediated DA neuronal growth, this study examined the expression of BMP receptors over the developmental period between embryonic day (E) 14 and post-natal day (P) 90 in rats, since the generation and maturation of nigrostriatal dopaminergic neurons, the invasion and arborisation of their striatal targets, and the refinement of these connections occur over this time period (Van den Heuvel and Pasterkamp 2008). Furthermore, the molecular mechanisms by which BMP2 and GDF5 regulate axonal growth of VM DA neurons were investigated.
Materials and Methods
Cell Culture
For the preparation of E14 rat VM cultures, E14 embryos were obtained by laparotomy from date-mated female Sprague–Dawley rats following decapitation under terminal anaesthesia induced by the inhalation of isoflurane (Isoflo®). Dissected VM tissue was centrifuged at 1,100 rpm for 5 min at 4 °C. The tissue pellet was incubated in a 0.1 % trypsin–Hank’s balanced salt solution for 5 min, at 37 °C with 5 % CO2. Foetal calf serum (FCS) was then added to the tissue followed by centrifugation at 1,100 rpm for 5 min at 4 °C. The resulting cell pellet was resuspended in 1 ml of differentiation media (Dulbecco’s modified Eagle’s medium/F12, 33 mM d-glucose, 1 % l-glutamine, 1 % FCS, supplemented with 2 % B27) using a P1000 Gilson pipette and carefully triturated using a sterile plugged flame-polished Pasteur pipette, followed by a 25-gauge needle and syringe, ensuring not to add air bubbles into the cell suspension. Cell density was estimated using a haemocytometer. Cells were plated on poly-d-lysine (Sigma)-coated 24-well tissue culture plates at a density of 5 × 104 cells per well in 500 μl of differentiation media at 37 °C with 5 % CO2. SH-SY5Y cells were used as a model of human DA neurons in this study, and their cell culture was performed as previously outlined (Hegarty et al. 2013b).
Cells were treated with 200 ng/ml of GDF5 (kindly provided by Biopharm GmbH) or recombinant human BMP2 (R&D Systems) and pre-treated (30 min prior to GDF5 or BMP2 application) with 1 μg/ml of Dorsomorphin (Sigma), 200 ng/ml of Noggin (R&D Systems), or 0.3 U/ml of Heparinase III (R&D Systems). For the neurite growth assay, cells were treated daily for 4 DIV. To test Smad pathway activation, cells were treated for 0, 30 or 120 min.
Electroporation of E14 Rat VM Cells
Electroporation of E14 VM cells was carried out using the Neon™ Transfection System (Invitrogen). E14 VM cell suspensions were prepared for counting (as outlined above), and the required volume of cells to give 200,000 cells per well was centrifuged at 4 °C at 1,100 rpm for 5 min. The cell pellet was washed twice with 10 mM phosphate-buffered saline (PBS) (without CaCl2 and MgCl2) (Sigma) and then resuspended in the required amount of the manufacturers resuspension buffer (12 μl per transfection/plasmid) (Invitrogen). About 0.5 μg of a GFP plasmid, 1 μg of plasmid DNA (caBMPRIb and/or Smad4 siRNA vector (Hegarty et al. 2013b)) and/or 1 μM of desired siRNA (Control or BMPRIb; Life Technologies) were added to the resuspended cells. About 10 μl of the cell/plasmid mixture was then electroporated according to the manufacturer’s protocol under specific parameters (1,100 V; 30 ms; 2 pulses).
Immunocytochemistry
Cultures were fixed for 10 min using 100 % ice-cold methanol. Following 3 washes in 10 mM PBS-T (0.02 % Triton X-100 in 10 mM PBS) for permeabilization, cultures were incubated in blocking solution (5 % bovine serum albumin) for 1 h at room temperature. Cultures were subsequently incubated in the following antibodies: BMPRII (1:200; R&D Systems), BMPRIb (1:200; R&D Systems), phopsho-Smad 1/5/8 (1:200; Cell Signalling), tyrosine hydroxylase (TH; 1:200; mouse monoclonal; Millipore, or 1:300; rabbit polyclonal; Millipore) and β-actin (1:200; Sigma) diluted in 1 % bovine serum albumin in 10 mM PBS at 4 °C overnight. Following 3 × 5 min washes in PBS-T, cells were incubated in Alexa Fluor 488- and/or 594-conjugated secondary antibodies (1:500; Invitrogen) reactive to the species of the primary antibodies and diluted in 1 % bovine serum albumin in 10 mM PBS, at room temperature for 2 h in the dark. Cultures were counterstained with bisbenzimide (1:1,000 in 10 mM PBS; Sigma). Negative controls in which the primary antibody was omitted were also prepared (not shown). Cells were imaged under an Olympus IX70 inverted microscope fitted with an Olympus DP70 camera and AnalysisD™ software. For densitometric analysis, the fluorescence intensity of individual cells stained for phospho-Smad 1/5/8 was measured using ImageJ analysis software (Rasband, WJ, http://rsb.info.nih.gov/ij/). The relative fluorescence intensity was calculated for each individual cell after subtraction of the background noise.
Immunohistochemistry
Four adult (8- to 12-week old) female Sprague–Dawley rats were killed by terminal anaesthesia (150 mg/kg sodium pentobarbitone, i.p.) and perfused intracardially with 100 ml of 10 mM PBS, pH 7.4, containing 500 Units of heparin sulphate, followed immediately by 200 ml of freshly prepared 4 % ice-cold paraformaldehyde in PBS. The brains were removed and placed in 4 % paraformaldehyde overnight, cryoprotected in 30 % sucrose in PBS, and then snap-frozen in isopentane on liquid nitrogen.
Three pairs of coronal cryosections (15 μm; Cryostat manufacturer: Leica—model CM1900) were collected at each of three levels through the midbrain (AP −4.8, −5.6, −6.4 relative to bregma; (Paxinos and Watson 1988)). The sections were mounted on gelatine-coated slides and then stained immunocytochemically for TH and/or BMPRIb or BMPRII. Firstly, endogenous peroxidase was inactivated by incubation in 20 % methanol, 0.2 % Triton X-100, 1.5 % hydrogen peroxide in 10 mM PBS for 10 min. Sections were incubated in blocking solution (3 % normal goat serum, 0.2 % Triton X-100 in 10 mM PBS) for 1 h at room temperature and then in a solution (1:1,000) of antiserum to TH (rabbit) and/or BMPRIb or BMPRII (mouse) overnight at 4 °C. After four washes in 0.02 % Triton X-100 in 10 mM PBS for 10 min each, sections were incubated in Alexa Fluor 488- and/or 594-conjugated secondary antibodies (1:500) reactive to the species of the primary antibodies diluted in 10 mM PBS, at room temperature for 2 h in the dark. Sections were then cover-slipped in PVA-DABCO before fluorescent imaging.
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
The VM and striatum from E14 to P90 rats were dissected, and following the extraction and purification of total RNA, semi-quantitative RT-PCR for a variety of genes involved in DA development and maintenance (TH, Nurr1, Lmx1b and Pitx3) (Hegarty et al. 2013c) was performed on the midbrain samples to confirm the accuracy of the dissection at each age. RT-PCR was also performed on E11–E14 rat VM tissue for BMPRII and BMPRIb, as well as on SH-SH5Y cells for BMPRIb.
Dissected embryonic and adult tissue in ice-cold Hank’s balanced salt solution was centrifuged at 500 rpm for 2 min, the supernatant discarded and the tissue pellet stored immediately at −80 °C until RNA extraction. Cultured SH-SH5Y cells (~1 × 106 cells) were centrifuged at 10,000 rpm for 10 min before storage/usage. RNA was isolated using an RNeasy mini extraction kit (Qiagen). An ImProm-II Reverse Transcription System (Promega) was used to synthesise cDNA using 1 μg of RNA in an 11.5 μl reaction volume for 90 min at 37 °C. Amplification was carried out using a GoTaq Flexi DNA Polymerase system (Promega) as per the manufacturer’s instructions. Each reaction mixture consisted of 2 μl cDNA, 2 μl forward and reverse primer mix, 5X PCR buffer, 1.5 mM MgCl2, 1.25 mM PCR dNTPs, 0.25 μl Taq polymerase and made up to a total volume of 25 μl with nuclease-free water. Forward and reverse primer pairs for TH (275 bp), Nurr1 (434 bp), Lmx1b (485 bp), Pitx3 (193 bp), BMPRIb (425 bp), BMPRII (349 bp) and GAPDH (388 bp) are listed in Supplementary Fig. 1.
Quantitative Real-Time PCR (RT-QPCR)
Midbrain and striatum samples were disrupted and homogenised in 1 ml of QIAzol Lysis Reagent (Qiagen). After the addition of 200 μl chloroform, homogenates were separated into aqueous and organic phases by centrifugation at 13,000 rpm for 15 min. The upper aqueous phase was mixed with an equal volume of 70 % ethanol, to precipitate the RNA, and then transferred to an RNeasy Mini spin column placed in a 2-ml collection tube. Total RNA was purified using the Qiagen RNeasy Lipid Tissue Mini extraction kit and RNase-free DNase set, according to the manufacturer’s instructions. Following purification, total RNA was reverse transcribed using Stratascript reverse transcriptase (Agilent Technologies), for 1 h at 45 °C, in a 30 μl reaction according to the manufacturer’s instructions.
In order to amplify cDNAs encoding the normalising reference genes, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), succinate dehydrogenase complex, subunit A (SDHA) and ubiquitin C (UBQC), 2.5 μl of cDNA was amplified in a 25 μl PCR containing 1X FastStart Universal SYBR Green Master Mix (Rox) (Roche) and 150 nM forward and reverse primers. In the case of amplifying cDNAs encoding TH, BMPRIb and BMPRII, 2 μl of cDNA was amplified in a 20 μl PCR containing 1X of Brilliant III Ultra-Fast QPCR Master Mix (Agilent Technologies), 150 nM each forward and reverse primers and 300 nM cDNA-specific FAM/BHQ1 dual-labelled hybridization probe (Eurofins), and 3 nM ROX reference dye.
Quantitative real-time PCR amplification was performed using the Stratagene MX3000P thermal cycler. GAPDH, SDHA and UBQC quantitative real-time PCR amplification products were verified as being correct by melting curve analysis (melting temperatures 83.5, 80 and 85 °C, respectively) of the completed PCR. The initial quantities of each cDNA in each PCR were determined by comparison to a standard curve incorporated into the PCR run and constructed from serial dilutions of cDNA reverse transcribed from RNA extracted from P11 striatum and midbrain samples. Values for each gene of interest were normalised to the geometric mean of the three reference genes.
Primer and probe sequences for the amplification of each cDNA are listed in Supplementary Fig. 1. Cycling parameters for GAPDH, SDHA and UBQC were 10 min at 95 °C followed by 40 cycles of 95 °C for 30 s; 55 °C for 1 min; 72 °C for 1 min. Cycling parameters for TH, BMPRIb and BMPRII were 3 min at 95 °C followed by 45 cycles of 95 °C for 13 s and 60 °C for 30 s.
Analysis of Neuronal Complexity
The total neurite length of individual E14 VM neurons was measured at 1 and 3 DIV using Sholl analysis as previously described (Gutierrez and Davies 2007; Collins et al. 2013). Traces of GFP+/TH+ neurons were carried out using the CorelDRAW ×4 software and analysed as previously described (O’Keeffe et al. 2004a). Briefly, neurite length (NL) was calculated using the following formula: NL = α × T × (π/2), where α is the number of times the neurite intersects the grid lines, and T is the distance between the gridlines on the magnified image (taking into account the magnification factor). VM neurons with intact processes were analysed from 20 random fields per condition, where any neuron with a process that was at least one and half times the length of the soma was determined as an intact process (which precludes the analysis of apoptotic neurons). For SH-SY5Y cells, cellular morphology was assessed as previously described (Hegarty et al. 2013b).
Western Blotting
Western blotting was carried out as previously described (Crampton et al. 2012). The cells were lysed in RIPA buffer, and insoluble debris was removed by centrifugation. Samples were run on an agarose gel and transferred to nitrocellulose membranes using a Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad, CA, USA). The membranes were incubated with primary antibodies against BMPRIb (1:1,000) or β-actin (1:1,000) overnight at 4 °C, washed, incubated with horseradish peroxidase-labelled anti-rabbit IgG (1:2,000; Promega), washed and developed with ECL-Plus (Amersham).
Statistical Analysis
Unpaired Student’s t test or one-way ANOVA with a post hoc Tukey’s test was performed, as appropriate, to determine significant differences between groups. Results were expressed as means with SEM and deemed significant when p < 0.05.
Results
BMPRs are Expressed in the Rat VM and Striatum During Embryonic and Post-natal Development
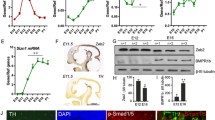
If BMP–Smad signalling promotes the neurite growth of VM DA neurons, then the BMP receptors, BMPRII and BMPRIb, should be expressed in the VM and possibly the striatum, during the period of DA axogenesis. To examine this, RT-QPCR was used to quantify the expression levels of TH, BMPRII and BMPRIb transcripts in the VM and striatum during embryonic and post-natal development, having confirmed the accuracy of the VM dissections by examining DA gene expression at each age (Fig. 1a–d). In the VM, TH mRNA levels are highest from E14 to P1 (Fig. 1b). A significant drop in TH transcript levels occurs between P1 and P11, after which the expression of TH mRNA remains stable through to adulthood (P90) (Fig. 1b). In the striatum, TH mRNA levels are significantly lower than those in the midbrain throughout the developmental period studied (Fig. 1b).
BMP receptors are expressed in the midbrain and striatum during embryonic and post-natal development. a RT-PCR of TH, Nurr1, Lmx1b, and Pitx3 in E14 and adult rat VM (SN = substantia nigra). b–d Quantitative RT-QPCR data showing the levels of (b) TH, (c) BMPRII and (c) BMPRIb mRNA in the developing midbrain and striatum, from E14 to P90, relative to the levels of the reference mRNAs GAPDH, SDHA and UBQC. Each data point represents pooled data from four samples from three separate litters/animals, and all data are presented as the mean ± SEM. e RT-PCR showing the expression of BMPRII and BMPRIb in the adult rat SN. f, g In situ hybridization images taken from the Allen Developing Brain Atlas (©(Allen) Developing Mouse Brain Atlas, 2012) showing BMPRII and BMPRIb expression (purple colour) in sagittal sections of the P56 adult mouse brain. h Atlas showing the major nuclei in the midbrain region, including the SNpc, substantia nigra pars reticulate (SNpr) and subthalamic nucleus (STN). Corresponding in situ hybridization images of this region showing strong expression of i BMPRII and j BMPRIb in the SNpc (identified by red arrows). Scale bar = 2103 μm. k Quantification of the percentage of DA neurons in the adult rat SN expressing BMPRII and BMPRIb. l Photomicrographs showing immunostaining for BMPRII and BMPRIb co-expressed with TH in the adult rat SNpc
BMPRII mRNA levels are relatively stable throughout development in the VM (Fig. 1c), while in the developing striatum BMPRII mRNA levels increase 1.5-fold between E14 and P1. Between P1 and P31, the level of BMPRII transcripts expressed in the striatum falls almost threefold and this lower expression level is maintained through to adulthood (Fig. 1c). BMPRII mRNA levels in P90 midbrain are similar to those in P90 striatum (Fig. 1c). In the midbrain, BMPRIb mRNA levels increase threefold between E14 and P1, and thereafter remain unchanged until adulthood (Fig. 1d). In the developing striatum, BMPRIb mRNA levels increase by twofold between E14 and P1, before increasing a further twofold between P1 and P60 (Fig. 1d). BMPRIb striatal mRNA levels remain relatively steady thereafter through to P90 and are comparable to that of the adult midbrain at this time point (Fig. 1d). The expression levels of BMPRII and BMPRIb transcripts in the adult midbrain (P31–P90) are very similar. Indeed, RT-PCR and in situ hybridization showed that BMPRII and BMPRIb are strongly expressed in the adult rodent SNpc (Fig. 1e–j). Furthermore, approximately 75 % of DA neurons in the adult rat midbrain expressed BMPRII and BMPRIb (Fig. 1k, l).
Since the initial phase of DA axogenesis begins at E11 in the rat (Gates et al. 2004; Nakamura et al. 2000), this study also showed that BMPRII and BMPRIb are expressed in the developing rat VM from E11 to E14 VM (Fig. 2a). Western blotting and immunocytochemistry were then used to confirm that the effector part of the BMP receptor complex, the BMPRIb protein, is expressed in the rat VM during this developmental period (Fig. 2b, c). To determine whether these receptors are expressed on DA neurons, immunocytochemical analysis was used to confirm protein expression of BMPRII and BMPRIb on TH-positive neurons in E14 rat VM cultures (Fig. 2d, e and data not shown). The co-localisation of BMPRII and BMPRIb immunostaining with TH immunostaining indicates that these receptors are expressed by DA neurons, although there is also expression of these BMPRs on TH-negative, non-DA cells (Fig. 2d, e).
BMPRs are expressed on DA neurons during the peak period of DA axogenesis. a RT-PCR of BMPRII, BMPRIb and GAPDH in E11 to E14 rat VM. b Western blotting showing BMPRIb protein expression in the developing rat VM. c Photomicrographs showing immunostaining for BMPRIb co-expressed with DAPI and the relevant negative controls ((−) control) in cultures of the E14 rat VM after 24 h in vitro. Photomicrographs showing immunostaining for d BMPRIb with e being the negative control, co-stained with DAPI and TH, in cultures of E14 rat VM after 24 h in vitro. Scale bar = 50 μm
BMP2 and GDF5 Promote Neurite Growth and Activate Canonical Smad Signalling in VM DA Neurons
Following the characterisation of BMPR expression in the VM and striatum during development, we next assessed the effects of BMP2 and GDF5 on the promotion of neurite growth from cultured E14 VM DA neurons. Treatment with either BMP2 or GDF5 for 4 DIV resulted in a significant increase in the neurite length of TH-positive neurons in E14 VM cultures, when compared to untreated controls (Fig. 3a, b).
BMP2 and GDF5 promote neurite growth and activate canonical Smad signalling in cultured DA neurons. a Total neurite length of BMP2- and GDF5-treated (10 ng/ml daily for 4 DIV) DA neurons in cultures of E14 rat VM. b Representative photomicrographs of control and BMP2-treated DA neurons in cultures of E14 rat VM at 4DIV, immunocytochemically stained for TH. Scale bar = 100 μm. c, d Densitometric analysis of phospho-Smad 1/5/8 in c BMP2- and d GDF5-treated DA neurons and non-DA neurons in E14 rat VM cultures at 0 (control), 30 and 60 min, as indicated. e Representative photomicrographs of phospho-Smad 1/5/8 immunostaining (yellow arrow heads), co-localised with TH immunostaining, in DA neurons treated BMP2 or GDF5 for 60 min in E14 rat VM cultures. Scale bar = 100 μm. f Smad-dependent transcriptional activity in BMP2- and GDF5-treated SH-SY5Y cells 48 h after transfection with a Smad-GFP reporter. g Photomicrographs showing increased Smad-GFP reporter fluorescence in SH-SH5Y cells treated with BMP2 or GDF5 for 2DIV. Scale bar = 10 μm. (*P < 0.05, **P < 0.01, ***P < 0.001 vs. control; ANOVA with post hoc Tukey’s test; 50 cells analysed per group per experiment; N = 3 experiments)
BMPs are well-known activators of a canonical signalling pathway involving activation of Smad 1/5/8 (Miyazono et al. 2010; Sieber et al. 2009). Densitometric analysis of the nuclear levels of phospho-Smad 1/5/8 showed that both BMP2 and GDF5 significantly increased the amount of phospho-Smad 1/5/8 in the nucleus of TH-positive DA neurons at 30 and 120 min, compared to the untreated controls (0 min) (Fig. 3c–e). To determine whether this effect of GDF5 and BMP2 on Smad phosphorylation was specific to DA neurons, nuclear phospho-Smad levels were also measured in TH-negative cells. BMP2 did not induce Smad phosphorylation in these cells at any time point examined (Fig. 3c). Although GDF5 did not activate Smad phosphorylation in TH-negative cells at 30 min, it did so at 120 min (Fig. 3d). Using SH-SH5Y cells as a model of human DA neurons (Hegarty et al. 2013b), BMP2 and GDF5 were both shown to significantly increase Smad-mediated transcriptional activity (as measured by the relative levels of GFP expression) at 2 DIV in SH-SH5Y cells transfected with a Smad reporter plasmid (GFP under the control of a Smad responsive element), compared to the control (Fig. 3f, g). Collectively, these data show that BMP2 and GDF5 promote neurite growth from DA neurons in E14 VM cultures and activate the canonical Smad signalling pathway in these neurons.
BMPR Inhibitors Prevent BMP2- and GDF5-Induced Neurite Outgrowth in VM DA Neurons
To explore the possibility that the effects of BMP2 and GDF5 on the neurite outgrowth from E14 VM DA neurons are mediated through BMPR-dependent activation of the canonical Smad 1/5/8 pathway, two approaches were employed to inhibit BMP–BMPR signalling. Firstly noggin, an extracellular inhibitor of BMPs, which blocks their binding epitopes for BMPRs (Groppe et al. 2002; Smith and Harland 1992), and secondly dorsomorphin, a small molecular inhibitor of BMPRI (Yu et al. 2008), were used. It has previously been shown that dorsomorphin is an effective inhibitor of BMP2 and GDF5 signalling in SH-SY5Y cells (Hegarty et al. 2013b). The ability of noggin to inhibit BMP2 and GDF5 in these cells was assessed first, and pre-treatment with either noggin or dorsomorphin prevented BMP2- and GDF5-induced neurite growth in SH-SY5Y cells (Supplementary Fig. 2). Similarly, the pre-treatment of E14 VM cultures with noggin or dorsomorphin completely prevented the BMP2- and GDF5-induced increases in the neurite length of TH-positive cells at 4 DIV (Fig. 4a, b). It has been suggested that the neurotrophic effects of GDF5 on DA neurons may be mediated indirectly through the action of glial cell line-derived neurotrophic factor (GDNF) (Sullivan and O’Keeffe 2005). To test this possibility, we adopted a similar approach to Orme et al. (2013) who prevented the DA neurotrophic effects of GDNF by blocking its heparan sulphate-dependent signalling (Barnett et al. 2002; Iwase et al. 2005; Orme et al. 2013). The pre-treatment of SH-SH5Y cells with Heparinase III did not affect BMP2- and GDF5-induced neurite growth in SH-SY5Y cells (Supplementary Fig. 3). Collectively, these data show that the neurite growth-promoting effects of BMP2 and GDF5 on VM DA neurons are directly mediated through a BMPR-dependent pathway.
Noggin and dorsomorphin prevent the promotion of DA neurite growth by BMP2 and GDF5. a Total neurite length of noggin- or dorsomorphin-pre-treated and/or BMP2- or GDF5-treated (daily for 4 DIV) DA neurons in E14 rat VM cultures, as indicated (***P < 0.001 vs. control; ANOVA with post hoc Tukey’s test; 50 TH-positive neurons analysed for each group per experiment; N = 3 experiments). b Representative photomicrographs of noggin- and dorsomorphin-pre-treated and/or BMP2- or GDF5-treated DA neurons in E14 rat VM cultures, immunocytochemically stained for TH. Scale bar = 100 μm. Data are expressed as mean ± SEM
Canonical BMPR–Smad Activation Promotes Neurite Outgrowth in VM DA Neurons
It is well established that BMP2 can signal through both BMPRIa and BMPRIb, whereas GDF5 predominantly signals through BMPRIb (Nishitoh et al. 1996), which suggests that BMP2 and GDF5 may signal through BMPRIb to exert their neurotrophic effects on VM DA neurons. To test this possibility, E14 VM neurons were transfected with a constitutively active BMPRIb (caBMPRIb) plasmid, and the neurite growth of the neurons was assessed and compared to that of neurons transfected with a control plasmid. Transfection of E14 VM neurons with the caBMPRIb plasmid induced a significant increase in their neurite length at 3 DIV, but not 1 DIV, when compared to cells transfected with the relevant control plasmid (Fig. 5a, c). Importantly, electroporation of E14 VM neurons with a wild-type BMPRIb plasmid did not result in significant increases in neurite length (data not shown), demonstrating the importance of the activation of the BMPR for this effect.
Overexpression of constitutively active BMPRIb promotes neurite outgrowth in cultured VM neurons. a Neurite length of control- or caBMPRIb-transfected neurons in E14 rat VM cultures at 1 and 3 DIV, as indicated (**P < 0.01; ##P < 0.01 vs. 1 DIV; ANOVA with post hoc Tukey’s; 40 cells for each group per experiment; N = 3 experiments). b Neurite length of control- or caBMPRIb-transfected neurons and/or co-transfected with a Smad4 siRNA expression vector in E14 rat VM cultures at 3 DIV, as indicated (***P < 0.001; ##P < 0.01, ##P < 0.001 vs. control; ANOVA with post hoc Tukey’s; 40 cells for each group per experiment; N = 3 experiments). c Representative line drawing of neurons from each of these groups at 3 DIV. All data are presented as mean ± SEM
To determine a functional link between BMPRIb-induced neurite growth and Smad 1/5/8 signalling, an siRNA that targets the co-Smad Smad4, which has been shown to be effective in inhibiting BMP2 and GDF5 signalling (Hegarty et al. 2013b), was used. The complex of phosphorylated Smad 1/5/8 with Smad4 following BMPRIb activation is required for the nuclear translocation of activated Smad 1/5/8 and thus their regulation of target gene expression (Miyazono et al. 2010; Sieber et al. 2009). To determine whether modulation of Smad4 expression affected the growth of E14 VM neurons, the neurite length of cells transfected with Smad4 siRNA or with Smad4 overexpression vectors was measured. Modulation of Smad4 expression did not affect the neurite length of transfected E14 VM neurons (data not shown). When E14 VM cells were co-transfected with the caBMPRIb and Smad4 siRNA, Smad4 siRNA significantly reduced the caBMPRIb promotion of E14 VM neuronal growth (Fig. 5b, c). These data show that the activation of the Smad signalling pathway by BMPRIb mimics the neurite growth-promoting effects of BMP2 and GDF5 in E14 VM neurons. To ensure that this effect was specific to DA neurons, we immunostained the electroporated neurons at 3 DIV for TH. This allowed the identification of TH-positive/GFP-positive DA neurons, indicating that they were transfected (Fig. 6a, b). Traces of the TH-positive/GFP-positive DA neurons were prepared for the analysis of neuronal growth (Fig. 6c), which showed that DA neurons expressing caBMPRIb had significantly longer neurites than their control counterparts (Fig. 6d). Finally, to further demonstrate the requirement of the BMPRIb for the neurite growth-promoting effects of the BMP ligands, an siRNA against BMPRIb was employed, which induced efficient BMPRIb knockdown (Fig. 6e). The ability of GDF5 to promote growth in cells transfected with either a control siRNA or the BMPRIb siRNA was then investigated. GDF5 promoted a significant increase in neurite length in cells expressing the control siRNA, whereas this effect was lost in cells expressing the BMPRIb siRNA (Fig. 6f, g). Taken together, these data show that the activation of canonical BMP–BMPRIb–Smad 1/5/8 signalling promotes neurite outgrowth in VM DA neurons.
Overexpression of constitutively active BMPRIb promotes neurite outgrowth in cultured DA neurons. a Photomicrograph of an E14 rat VM culture transfected with ca-BMPRIb (GFP-positive) at the time of plating, and immunocytochemically stained for TH at 3 DIV. b Higher magnification of the dashed area in (a), showing co-localisation of TH and GFP to identify transfected DA neurons. c Representative line drawing of control- or caBMPRIb-transfected DA neurons at 3DIV. Scale bar = 50 μm. d Neurite length of control- or caBMPRIb-transfected DA neurons in E14 rat VM cultures at 3 DIV, as indicated. e RT-PCR showing BMPRIb mRNA expression in SHSY5Y cells at 24 h following transfection with either a control or BMPRIb siRNA. f Neurite length and g representative photomicrographs of control siRNA and BMPRIb siRNA transfected SH-SY5Y cells with or without GDF5 treatment, as indicated. (***P < 0.001, vs. control; ANOVA with post hoc Tukey’s; 30 cells for each group per experiment; N = 3 experiments). All data are presented as mean ± SEM
Discussion
Understanding the molecular signals that regulate the development of DA neurons is crucial for advancing cell replacement therapy for PD (Toulouse and Sullivan 2008; Lees et al. 2009). While much progress has been made in understanding the signals that control DA neuron development, less is known about the molecules that promote the growth of DA neurites, which is crucial for the functional integration of transplanted cells into the host parenchyma. However, some molecules, such as Ephs and netrin1, have been identified as regulators of nigrostriatal pathway development in recent years (Hegarty et al. 2013a; Van den Heuvel and Pasterkamp 2008). In an attempt to identify new candidate molecules and signalling pathways that may be involved in nigrostriatal development, this study focused on two BMPs, GDF5 and BMP2, since both of these factors have been implicated in axonal growth in other NS populations (Parikh et al. 2011; Hazen et al. 2011, 2012; Phan et al. 2010; Niere et al. 2006; Lein et al. 1995; Hegarty et al. 2013a) and have been shown to have neurotrophic effects on VM DA neurons, specifically survival- and neurite growth-promoting effects (O’Keeffe et al. 2004a; Reiriz et al. 1999; Jordan et al. 1997; Sullivan et al. 1997; Hegarty et al. 2014). Despite these studies, the downstream molecular mechanisms that mediate the effects of GDF5 and BMP2 on VM DA neurons are unknown. The present study thus aimed to define these molecular mechanisms and to investigate the potential of BMP2 and GDF5 as regulators of nigrostriatal development.
To investigate this proposed role of BMP2 and GDF5 in the neurite growth of DA neurons, this study first characterised the temporal expression profiles of their receptors in the rat VM and striatum during embryonic and post-natal development. In the rat, the axons of the DA neurons in the VM extend towards the forebrain via the medial forebrain bundle from E13, and progressively innervate the striatum shortly thereafter, reaching the dorsal striatum around E20 (Gates et al. 2004; Nakamura et al. 2000; Specht et al. 1981a, b; Verney 1999; Voorn et al. 1988). In the first three post-natal weeks, striatal innervation becomes more extensive, while naturally occurring cell death refines these connections (Jackson-Lewis et al. 2000; Oo and Burke 1997; Burke 2003; Hegarty et al. 2013a; Van den Heuvel and Pasterkamp 2008). This study found that BMPRII and BMPRIb were expressed at steady levels in the VM throughout embryonic development (from E14) and into adulthood (until at least P90), with strong expression levels being detected on DA neurons in the P56 SNpc. Crucially, the expression of these BMPRs, both of which are required for canonical BMP–Smad signalling (Miyazono et al. 2010; Sieber et al. 2009), in the VM from E14 onwards correlates with the timing of the generation of nigrostriatal projections. These data suggest that BMPs, such as BMP2 and GDF5 that are expressed in the developing and adult VM and striatum (Krieglstein et al. 1995; O’Keeffe et al. 2004b; Storm et al. 1994; Chen et al. 2003; Jordan et al. 1997; Soderstrom and Ebendal 1999; Hegarty et al. 2014), may regulate the establishment of nigrostriatal projections from VM DA neurons. In support of this suggestion, the present study has demonstrated that both BMP2 and GDF5 promote neurite outgrowth from E14 VM neurons in culture. BMP2 and GDF5 may also act to orientate the axons of VM DA neurons away from the VM, since other BMPs, such as BMP7 and GDF7, have been shown to orient the commissural axons of dorsal SC interneurons via BMPRIb (Butler and Dodd 2003; Dent et al. 2011; Phan et al. 2010; Yamauchi et al. 2008; Wen et al. 2007). The sustained expression of BMPRs in the VM during adulthood suggests that they may function in the maintenance of DA neurons, with both BMP2 and GDF5 being shown to promote the survival of VM DA neurons in vitro (O’Keeffe et al. 2004a; Wood et al. 2005; Reiriz et al. 1999; Jordan et al. 1997) and in vivo (Sullivan et al. 1997, 1998, 1999; Hurley et al. 2004; O’Sullivan et al. 2010; Espejo et al. 1999). This study also demonstrated the expression of these BMPRs from E11 to E14 in the rat VM, further supporting their role in DA axogenesis, but also suggesting that BMPs may function in adoption of a DA phenotype during DA neurogenesis, which occurs during this period (Lumsden and Krumlauf 1996; Lauder and Bloom 1974; Gates et al. 2006; Hegarty et al. 2013c). In agreement with this proposal, BMP–BMPR–Smad-dependent transcriptional activity is found in the VM region during DA neurogenesis at E10.5 in mice (Monteiro et al. 2008), which also corresponds to the time of DA axon extension. BMP–Smad signalling may therefore concomitantly contribute to VM DA neuronal specification and their subsequent neurite outgrowth, which is the case for BMPs in the dorsal SC (Chizhikov and Millen 2005; Ulloa and Briscoe 2007).
In the striatum, there is a peak of BMPRII mRNA expression at P11, during the time period (P0–P20) when naturally occurring cell death is occurring due to limitations in the availability of target-derived neurotrophic factors (Jackson-Lewis et al. 2000; Oo and Burke 1997; Burke 2003; Van den Heuvel and Pasterkamp 2008). Similarly, BMPRIb is also expressed at relatively high levels in the early post-natal (P1 to P11) striatum. These data suggest that BMP2 and GDF5 may function as target-derived neurotrophic factors for VM DA neurons at this stage of development. Indeed, both factors have been shown to promote the survival of VM DA neurons (O’Keeffe et al. 2004a; Wood et al. 2005; Reiriz et al. 1999; Jordan et al. 1997; Sullivan et al. 1997, 1998, 1999; Hurley et al. 2004; O’Sullivan et al. 2010; Espejo et al. 1999). Furthermore, BMPRII null mice have reductions in nigrostriatal neurons, and striatal DA innervation, when examined in adulthood (Chou et al. 2008), which is likely due to deficient neurotrophic support during this post-natal developmental period. There is a peak of BMPRIb expression during adulthood in the striatum, which may point towards the aforementioned potential role of BMPs in the maintenance of VM DA neurons. Furthermore, it may suggest that BMPRIb functions in promoting the arborisation of DA axons that survive the period of naturally occurring cell death. The sustained expression of BMPRs in the adult rat brain (up to P90) demonstrated in this study suggests a role for BMP2 and GDF5 in the maintenance of the nigrostriatal system during adulthood. In support of this role, BMPs (including BMP2) and BMPRs have been shown to be expressed in the midbrain and striatum from 6–24 months in the adult rat (Chen et al. 2003). Furthermore, in animal models of PD, exogenous GDF5 delivery into the nigrostriatal pathway has potent survival-promoting effects on adult nigral DA neurons (Sullivan et al. 1997, 1999; Hurley et al. 2004; O’Sullivan et al. 2010; Sullivan and Toulouse 2011). Disruption to the normal expression of BMPRs may thus render nigrostriatal DA neurons more vulnerable to degeneration and increase the risk of the development of PD. The phenotype of the BMPRII null mouse supports this suggestion, while haploinsufficiency of other transforming growth factor (TGF)β superfamily members, such as GDNF and TGFβ2, causes an accelerated decline of midbrain DA neurons during normal ageing (Boger et al. 2006; Andrews et al. 2006). Interestingly, after a 6-hydroxydopamine (6-OHDA)-induced lesion of the adult rat nigrostriatal pathway, BMPRs were significantly downregulated in the nigra, but upregulated in the striatum (Chen et al. 2003). These findings likely reflect the loss of BMPR expression by nigral DA neurons, which are destroyed by 6-OHDA, and a potential compensatory mechanism by the striatum to restore BMP-mediated survival-promoting effects on innervating VM DA neurons through upregulation of BMPR expression. The BMPR expression in the developing striatum may also reflect autocrine or paracrine trophic influences on cells within the striatum, since the BMPs have been shown to play roles in striatal neuronal development (Gratacos et al. 2001, 2002).
The present study found that TH mRNA levels in the VM are maximal at E14, which is expected as this is the time point at which the greatest amount of post-mitotic DA neurons are present in the VM (Lumsden and Krumlauf 1996; Lauder and Bloom 1974; Gates et al. 2006). There was a subsequent significant decline in TH expression from birth onwards, reaching the lowest levels at P11, which correlates with the onset of programmed cell death for nigrostriatal DA neurons. TH mRNA expression was found to remain stable in the adult VM, reflecting the established population of A9 DA neurons.
Following the demonstration of the expression of BMPRs in the VM and striatal regions during embryonic and post-natal development, we next demonstrated that BMPRs are expressed on both DA and non-DA cells in E14 rat VM cultures, indicating that BMP2 and GDF5 may act in either an autocrine or paracrine manner to exert neurotrophic effects on DA neurons. Immunocytochemical staining for phospho-Smad 1/5/8 showed that both DA and non-DA cells express these transcription factors, and the nuclear location of phospho-Smad 1/5/8 indicated that these VM cells also express Smad4, which is required for the nuclear translocation of Smad 1/5/8 following their activation. These results demonstrate that VM DA neurons have the machinery to carry out canonical Smad 1/5/8 signalling in response to BMPs.
The current study has demonstrated that both BMP2 and GDF5 induce the neurite outgrowth of E14 VM DA neurons, which is consistent with previous studies on BMP2 (Reiriz et al. 1999) and GDF5 (O’Keeffe et al. 2004a) in rat VM cultures. The molecular mechanisms mediating this neurite growth-promoting effect were then assessed. BMP2 and GDF5 were both shown to activate canonical Smad 1/5/8 in VM DA neurons, as demonstrated by nuclear accumulation of phosphorylated Smad 1/5/8. Interestingly, GDF5, but not BMP2, activated Smad 1/5/8 signalling in non-DA cells. This finding is not surprising considering that the numbers of astrocytes are dramatically increased in GDF5-treated E14 rat VM cultures (Krieglstein et al. 1995; O’Keeffe et al. 2004a; Wood et al. 2005). It has thus been suggested that GDF5 may have an indirect neurotrophic action on VM DA neurons, possibly by stimulating glial-derived growth factor(s) production, such as GDNF, that might be involved in the neurotrophic response (Sullivan and O’Keeffe 2005). To test this possibility, this study investigated whether GDF5 and BMP2 were capable of promoting neurite growth in the absence of heparan sulphate-dependent GDNF signalling and showed that GDF5 and BMP2 did not require GDNF for this effect. Similarly, Wood et al. (2005) showed that the inhibition of the GDF5-induced increase in astrocytes does not prevent the neurotrophic effects of GDF5 on DA neurons in E14 rat VM cultures, suggesting that GDF5 has a direct neuronal action. Similarly, Reiriz et al. (1999) used the gliotoxin α-aminoadipic acid to demonstrate that the neurotrophic effects of BMP2 on E14 rat VM DA neurons were not mediated by astrocytes. These data, along with the present finding that BMP2 specifically activates Smad signalling in VM DA neurons, suggest that BMP2 and GDF5 act directly on DA neurons to induce axonal growth. The neurotrophic and gliogenic effects of GDF5 in VM cultures may thus be independent of one another. Similarly, BMP–Smad signalling has previously been shown to have such a dual-inductive role in enteric neural crest cells (Chalazonitis et al. 2004, 2011; Chalazonitis and Kessler 2012). Collectively, these data suggest that canonical Smad signalling mediates the neurotrophic effects of BMP2 and GDF5 on VM DA neurons.
To explore this premise further, the effects of BMP2 and GDF5 were assessed following the inhibition of their binding to BMPRs. BMPR activation by BMP2 and GDF5 was blocked by using noggin, an extracellular inhibitor of BMPs, which blocks their binding epitopes for BMPRs (Groppe et al. 2002; Smith and Harland 1992), or dorsomorphin, a small molecular inhibitor of BMPRI (Yu et al. 2008). Pre-treatment with either noggin or dorsomorphin inhibited the neurite growth-promoting effects of BMP2 and GDF5 on E14 VM DA neurons. Noggin and dorsomorphin have both previously been used to prevent BMP-induced neurite outgrowth in other neuronal populations (Parikh et al. 2011; Li and LoTurco 2000), and the current study also demonstrated their inhibition of BMP-induced neurite growth in SH-SH5Y cells. BMPR activation is therefore crucial to BMP-induced axonal growth from VM DA neurons. BMP2 can signal through both BMPRIa and BMPRIb, whereas GDF5 predominantly signals through BMPRIb (Nishitoh et al. 1996), suggesting that BMPRIb is responsible for mediating the neurotrophic effects of BMP2 and GDF5. To test this hypothesis, E14 VM cultures were electroporated with a constitutively active BMPRIb, which has been previously shown to activate the Smad 1/5/8 signalling pathway (Hegarty et al. 2013b). E14 VM neurons expressing the caBMPRIb were significantly larger than those transfected with the control plasmid, suggesting that BMP2 and GDF5 activate BMPRIb to induce neurite extension. These findings are in agreement with a previous study in SH-SH5Y cells, a model of human DA neurons (Hegarty et al. 2013b). Furthermore, the application of GDF5 at the time of plating, when BMPR1b is expressed, results in neurotrophic effects on VM DA neurons; however, application after six days in vitro, when the BMPRIb is no longer expressed, has no effect (O’Keeffe et al. 2004a). The present study next demonstrated that the transcriptional activity of Smad 1/5/8 is required for this BMP-induced neurite growth of VM neurons. The inhibition of the nuclear translocation of the Smad 1/5/8 transcription factors, using siRNA to target Smad4, significantly inhibited neurite outgrowth of E14 VM neurons induced by caBMPRIb. Finally, this study confirmed that the neurite growth-promoting effects of the caBMPRIb are specific for VM DA neurons. The caBMPRIb therefore mimics the effects of BMP2 and GDF5 on E14 VM DA neurons. Furthermore, siRNA knockdown of the BMPRIb also prevented GDF5-induced neurite growth in SH-SH5Y cells. Collectively, these data show that BMPRIb activation of Smad 1/5/8 is the mechanism by which these BMPs promote the neurite growth of VM DA neurons.
This study has identified BMP2 and GDF5 as bona fide candidates to be regulators of nigrostriatal pathway development. The expression profiles of their BMPRs in the VM and striatum, and their neurotrophic effects on cultured VM DA neurons, propose roles for BMP2 and GDF5 in the extension/projection of DA axons from the VM. They may act as target-derived neurotrophic factors for innervating nigrostriatal fibres, and/or as factors that maintain the integrity of nigrostriatal projections during adulthood. However, the analysis of mice with deficiencies in GDF5 and/or BMP2 is essential to further establish these factors as regulators of nigrostriatal pathway development. It is not unlikely that these morphogens may play multiple roles during nigrostriatal system development, since locally expressed factors are employed throughout NS development to regulate multiple steps of particular developmental processes, with temporally regulated functions. A relevant example of this is seen during chick dorsal SC development, in which BMP–Smad signalling promotes neuronal specification rather than astrocytic specification at E5, but at E6 has the opposite effect (Agius et al. 2010). The present study has thus contributed to the growing body of knowledge regarding the development of the A9 pathway. A detailed, well-characterised understanding of nigrostriatal pathway development is vital, to provide important information regarding developmental abnormalities or age-related defects that may lead to the progressive degeneration of this pathway in PD. Furthermore, cell replacement therapy is one of the most promising therapies for the treatment for PD (Orlacchio et al. 2010; Bonnamain et al. 2012; De Feo et al. 2012; Toulouse and Sullivan 2008; Hedlund and Perlmann 2009). Due to the importance of the establishment of functional connections by transplanted DA cells in the host striatum, factors that promote neurite outgrowth are being considered as adjuncts to transplantation therapy. GDF5 and BMP2 are thus ideal candidates to be used as growth-promoting factors, with their survival-promoting effects on VM DA neurons being beneficial also. The present study has, for the first time, demonstrated that the downstream molecular mechanisms mediating the neurite outgrowth-promoting effects of GDF5 in VM DA neurons are dependent, upon BMPRIb-mediated activation of canonical Smad 1/5/8 signalling.
Abbreviations
- 6-OHDA:
-
6-Hydroxydopamine
- BMP(s):
-
Bone morphogenetic protein(s)
- BMPR(s):
-
Bone morphogenetic protein receptor(s)
- caBMPRIb:
-
Constitutively active BMPRIb
- CNS:
-
Central nervous system
- DA:
-
Dopaminergic/dopamine
- DIV:
-
Day(s) in vitro
- E:
-
Embryonic day
- FCS:
-
Foetal calf serum
- GDF(s):
-
Growth differentiation factor(s)
- GDNF:
-
Glial cell line-derived neurotrophic factor
- N:
-
Number of repetitions
- P:
-
Post-natal day
- PBS:
-
Phosphate-buffered saline
- PD:
-
Parkinson’s disease
- RT-QPCR:
-
Quantitative real-time PCR
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- SC:
-
Spinal cord
- SNpc:
-
Substantia nigra pars compacta
- TGF:
-
Transforming growth factor
- TH:
-
Tyrosine hydroxlase
- VM:
-
Ventral midbrain/mesencephalon
References
Agius, E., Decker, Y., Soukkarieh, C., Soula, C., & Cochard, P. (2010). Role of BMPs in controlling the spatial and temporal origin of GFAP astrocytes in the embryonic spinal cord. Development Biology, 344(2), 611–620.
Allen © 2012 Allen Institute for Brain Science. Allen Mouse Brain Atlas [Internet]. Available from: http://mouse.brain-map.org/.
Andrews, Z. B., Zhao, H., Frugier, T., Meguro, R., Grattan, D. R., Koishi, K., et al. (2006). Transforming growth factor beta2 haploinsufficient mice develop age-related nigrostriatal dopamine deficits. Neurobiology of Diseases, 21(3), 568–575.
Bagri, A., Marin, O., Plump, A. S., Mak, J., Pleasure, S. J., Rubenstein, J. L., et al. (2002). Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron, 33(2), 233–248.
Barnett, M. W., Fisher, C. E., Perona-Wright, G., & Davies, J. A. (2002). Signalling by glial cell line-derived neurotrophic factor (GDNF) requires heparan sulphate glycosaminoglycan. Journal of Cell Science, 115(Pt 23), 4495–4503.
Bjorklund, A., & Dunnett, S. B. (2007). Dopamine neuron systems in the brain: An update. Trends in Neurosciences, 30(5), 194–202.
Blum, M. (1998). A null mutation in TGF-alpha leads to a reduction in midbrain dopaminergic neurons in the substantia nigra. Nature Neuroscience, 1(5), 374–377.
Boger, H. A., Middaugh, L. D., Huang, P., Zaman, V., Smith, A. C., Hoffer, B. J., et al. (2006). A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Experimental Neurology, 202(2), 336–347.
Bonnamain, V., Neveu, I., & Naveilhan, P. (2012). Neural stem/progenitor cells as a promising candidate for regenerative therapy of the central nervous system. Frontiers in Cellular Neuroscience, 6, 17.
Bovolenta, P. (2005). Morphogen signaling at the vertebrate growth cone: A few cases or a general strategy? Journal of Neurobiology, 64(4), 405–416.
Burke, R. E. (2003). Postnatal developmental programmed cell death in dopamine neurons. Annals of the New York Academy of Sciences, 991, 69–79.
Butler, S. J., & Dodd, J. (2003). A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron, 38(3), 389–401.
Calo, L., Spillantini, M., Nicoletti, F., & Allen, N. D. (2005). Nurr1 co-localizes with EphB1 receptors in the developing ventral midbrain, and its expression is enhanced by the EphB1 ligand, ephrinB2. Journal of Neurochemistry, 92(2), 235–245.
Chalazonitis, A., D’Autreaux, F., Guha, U., Pham, T. D., Faure, C., Chen, J. J., et al. (2004). Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. Journal of Neuroscience, 24(17), 4266–4282.
Chalazonitis, A., D’Autreaux, F., Pham, T. D., Kessler, J. A., & Gershon, M. D. (2011). Bone morphogenetic proteins regulate enteric gliogenesis by modulating ErbB3 signaling. Development Biology, 350(1), 64–79.
Chalazonitis, A., & Kessler, J. A. (2012). Pleiotropic effects of the bone morphogenetic proteins on development of the enteric nervous system. Developmental Neurobiology, 72(6), 843–856.
Chen, H. L., Lein, P. J., Wang, J. Y., Gash, D., Hoffer, B. J., & Chiang, Y. H. (2003). Expression of bone morphogenetic proteins in the brain during normal aging and in 6-hydroxydopamine-lesioned animals. Brain Research, 994(1), 81–90.
Chizhikov, V. V., & Millen, K. J. (2005). Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Development Biology, 277(2), 287–295.
Chou, J., Harvey, B. K., Ebendal, T., Hoffer, B., & Wang, Y. (2008). Nigrostriatal alterations in bone morphogenetic protein receptor II dominant negative mice. Acta Neurochirurgica. Supplementum, 101, 93–98.
Collins, L. M., O’Keeffe, G. W., Long-Smith, C. M., Wyatt, S. L., Sullivan, A. M., Toulouse, A., et al. (2013). Mitogen-activated protein kinase phosphatase (MKP)-1 as a neuroprotective agent: Promotion of the morphological development of midbrain dopaminergic neurons. NeuroMolecular Medicine, 15(2), 435–446.
Cooper, M. A., Kobayashi, K., & Zhou, R. (2009). Ephrin-A5 regulates the formation of the ascending midbrain dopaminergic pathways. Developmental Neurobiology, 69(1), 36–46.
Crampton, S. J., Collins, L. M., Toulouse, A., Nolan, Y. M., & O’Keeffe, G. W. (2012). Exposure of foetal neural progenitor cells to IL-1beta impairs their proliferation and alters their differentiation: A role for maternal inflammation? Journal of Neurochemistry, 120(6), 964–973.
Crossley, P. H., & Martin, G. R. (1995). The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development, 121(2), 439–451.
Dahlstroem, A., & Fuxe, K. (1964). Evidence for the existence of monoamine-containing neurons in the bentral nervous syste, I. Demonstrations of monoamines in the cell bodies of brain stem neurons. Acta Physiologica Scandinavica. Supplementum., 232, 231–255.
De Feo, D., Merlini, A., Laterza, C., & Martino, G. (2012). Neural stem cell transplantation in central nervous system disorders: From cell replacement to neuroprotection. Current Opinion in Neurology, 25(3), 322–333.
Dent, E. W., Gupton, S. L., & Gertler, F. B. (2011). The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol, 3(3).
Dugan, J. P., Stratton, A., Riley, H. P., Farmer, W. T., & Mastick, G. S. (2011). Midbrain dopaminergic axons are guided longitudinally through the diencephalon by Slit/Robo signals. Molecular and Cellular Neuroscience, 46(1), 347–356.
Egea, J., & Klein, R. (2007). Bidirectional Eph-ephrin signaling during axon guidance. Trends in Cell Biology, 17(5), 230–238.
Espejo, M., Cutillas, B., Ventura, F., & Ambrosio, S. (1999). Exposure of foetal mesencephalic cells to bone morphogenetic protein-2 enhances the survival of dopaminergic neurones in rat striatal grafts. Neuroscience Letters, 275(1), 13–16.
Flores, C., Manitt, C., Rodaros, D., Thompson, K. M., Rajabi, H., Luk, K. C., et al. (2005). Netrin receptor deficient mice exhibit functional reorganization of dopaminergic systems and do not sensitize to amphetamine. Molecular Psychiatry, 10(6), 606–612.
Gates, M. A., Coupe, V. M., Torres, E. M., Fricker-Gates, R. A., & Dunnett, S. B. (2004). Spatially and temporally restricted chemoattractive and chemorepulsive cues direct the formation of the nigro-striatal circuit. European Journal of Neuroscience, 19(4), 831–844.
Gates, M. A., Torres, E. M., White, A., Fricker-Gates, R. A., & Dunnett, S. B. (2006). Re-examining the ontogeny of substantia nigra dopamine neurons. European Journal of Neuroscience, 23(5), 1384–1390.
German, D. C., Schlusselberg, D. S., & Woodward, D. J. (1983). Three-dimensional computer reconstruction of midbrain dopaminergic neuronal populations: From mouse to man. Journal of Neural Transmission, 57(4), 243–254.
Gratacos, E., Checa, N., Perez-Navarro, E., & Alberch, J. (2001). Brain-derived neurotrophic factor (BDNF) mediates bone morphogenetic protein-2 (BMP-2) effects on cultured striatal neurones. Journal of Neurochemistry, 79(4), 747–755.
Gratacos, E., Gavalda, N., & Alberch, J. (2002). Bone morphogenetic protein-6 is a neurotrophic factor for calbindin-positive striatal neurons. Journal of Neuroscience Research, 70(5), 638–644.
Groppe, J., Greenwald, J., Wiater, E., Rodriguez-Leon, J., Economides, A. N., Kwiatkowski, W., et al. (2002). Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature, 420(6916), 636–642.
Gutierrez, H., & Davies, A. M. (2007). A fast and accurate procedure for deriving the Sholl profile in quantitative studies of neuronal morphology. Journal of Neuroscience Methods, 163(1), 24–30.
Halladay, A. K., Tessarollo, L., Zhou, R., & Wagner, G. C. (2004). Neurochemical and behavioral deficits consequent to expression of a dominant negative EphA5 receptor. Molecular Brain Research, 123(1–2), 104–111.
Hazen, V. M., Andrews, M. G., Umans, L., Crenshaw, E. B, 3rd, Zwijsen, A., & Butler, S. J. (2012). BMP receptor-activated Smads confer diverse functions during the development of the dorsal spinal cord. Development Biology, 367(2), 216–227.
Hazen, V. M., Phan, K. D., Hudiburgh, S., & Butler, S. J. (2011). Inhibitory Smads differentially regulate cell fate specification and axon dynamics in the dorsal spinal cord. Development Biology, 356(2), 566–575.
Hedlund, E., & Perlmann, T. (2009). Neuronal cell replacement in Parkinson’s disease. Journal of Internal Medicine, 266(4), 358–371.
Hegarty, S. V., O’Keeffe, G. W., & Sullivan, A. M. (2013a). BMP-Smad 1/5/8 signalling in the development of the nervous system. Progress in Neurobiology, 109C, 28–41.
Hegarty, S. V., Sullivan, A. M., & O’Keeffe, G. W. (2013b). BMP2 and GDF5 induce neuronal differentiation through a Smad dependant pathway in a model of human midbrain dopaminergic neurons. Molecular and Cellular Neuroscience, 56C, 263–271.
Hegarty, S. V., Sullivan, A. M., & O’Keeffe, G. W. (2013c). Midbrain dopaminergic neurons: A review of the molecular circuitry that regulates their development. Development Biology, 379(2), 123–138.
Hegarty, S. V., Sullivan, A. M., & O’Keeffe, G. W. (2014). Roles for the TGFβ superfamily in the development and survival of midbrain dopaminergic neurons. Molecular Neurobiology,. doi:10.1007/s12035-014-8639-3.
Hernandez-Montiel, H. L., Tamariz, E., Sandoval-Minero, M. T., & Varela-Echavarria, A. (2008). Semaphorins 3A, 3C, and 3F in mesencephalic dopaminergic axon pathfinding. Journal of Comparative Neurology, 506(3), 387–397.
Hurley, F. M., Costello, D. J., & Sullivan, A. M. (2004). Neuroprotective effects of delayed administration of growth/differentiation factor-5 in the partial lesion model of Parkinson’s disease. Experimental Neurology, 185(2), 281–289.
Hynes, M., Porter, J. A., Chiang, C., Chang, D., Tessier-Lavigne, M., Beachy, P. A., et al. (1995). Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron, 15(1), 35–44.
Iwase, T., Jung, C. G., Bae, H., Zhang, M., & Soliven, B. (2005). Glial cell line-derived neurotrophic factor-induced signaling in Schwann cells. Journal of Neurochemistry, 94(6), 1488–1499.
Jackson-Lewis, V., Vila, M., Djaldetti, R., Guegan, C., Liberatore, G., Liu, J., et al. (2000). Developmental cell death in dopaminergic neurons of the substantia nigra of mice. Journal of Comparative Neurology, 424(3), 476–488.
Jordan, J., Bottner, M., Schluesener, H. J., Unsicker, K., & Krieglstein, K. (1997). Bone morphogenetic proteins: Neurotrophic roles for midbrain dopaminergic neurons and implications of astroglial cells. European Journal of Neuroscience, 9(8), 1699–1709.
Koenig, B. B., Cook, J. S., Wolsing, D. H., Ting, J., Tiesman, J. P., Correa, P. E., et al. (1994). Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Molecular and Cellular Biology, 14(9), 5961–5974.
Kolk, S. M., Gunput, R. A., Tran, T. S., van den Heuvel, D. M., Prasad, A. A., Hellemons, A. J., et al. (2009). Semaphorin 3F is a bifunctional guidance cue for dopaminergic axons and controls their fasciculation, channeling, rostral growth, and intracortical targeting. Journal of Neuroscience, 29(40), 12542–12557.
Krieglstein, K., Suter-Crazzolara, C., Hotten, G., Pohl, J., & Unsicker, K. (1995). Trophic and protective effects of growth/differentiation factor 5, a member of the transforming growth factor-beta superfamily, on midbrain dopaminergic neurons. Journal of Neuroscience Research, 42(5), 724–732.
Lauder, J. M., & Bloom, F. E. (1974). Ontogeny of monoamine neurons in the locus coeruleus, Raphe nuclei and substantia nigra of the rat. I. Cell differentiation. Journal of Comparative Neurology, 155(4), 469–481.
Lees, A. J., Hardy, J., & Revesz, T. (2009). Parkinson’s disease. Lancet, 373(9680), 2055–2066.
Lein, P., Johnson, M., Guo, X., Rueger, D., & Higgins, D. (1995). Osteogenic protein-1 induces dendritic growth in rat sympathetic neurons. Neuron, 15(3), 597–605.
Li, W., & LoTurco, J. J. (2000). Noggin is a negative regulator of neuronal differentiation in developing neocortex. Developmental Neuroscience, 22(1–2), 68–73.
Lin, L., Rao, Y., & Isacson, O. (2005). Netrin-1 and slit-2 regulate and direct neurite growth of ventral midbrain dopaminergic neurons. Molecular and Cellular Neuroscience, 28(3), 547–555.
Liu, A., & Joyner, A. L. (2001). EN and GBX2 play essential roles downstream of FGF8 in patterning the mouse mid/hindbrain region. Development, 128(2), 181–191.
Lopez-Bendito, G., Flames, N., Ma, L., Fouquet, C., Di Meglio, T., Chedotal, A., et al. (2007). Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. Journal of Neuroscience, 27(13), 3395–3407.
Lumsden, A., & Krumlauf, R. (1996). Patterning the vertebrate neuraxis. Science, 274(5290), 1109–1115.
Manitt, C., Mimee, A., Eng, C., Pokinko, M., Stroh, T., Cooper, H. M., et al. (2011). The netrin receptor DCC is required in the pubertal organization of mesocortical dopamine circuitry. Journal of Neuroscience, 31(23), 8381–8394.
Miyazono, K., Kamiya, Y., & Morikawa, M. (2010). Bone morphogenetic protein receptors and signal transduction. Journal of Biochemistry, 147(1), 35–51.
Monteiro, R. M., de Sousa Lopes, S. M., Bialecka, M., de Boer, S., Zwijsen, A., & Mummery, C. L. (2008). Real time monitoring of BMP Smads transcriptional activity during mouse development. Genesis, 46(7), 335–346.
Nakamura, S., Ito, Y., Shirasaki, R., & Murakami, F. (2000). Local directional cues control growth polarity of dopaminergic axons along the rostrocaudal axis. Journal of Neuroscience, 20(11), 4112–4119.
Niere, M., Braun, B., Gass, R., Sturany, S., & Volkmer, H. (2006). Combination of engineered neural cell adhesion molecules and GDF-5 for improved neurite extension in nerve guide concepts. Biomaterials, 27(18), 3432–3440.
Nishitoh, H., Ichijo, H., Kimura, M., Matsumoto, T., Makishima, F., Yamaguchi, A., et al. (1996). Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. Journal of Biological Chemistry, 271(35), 21345–21352.
O’Keeffe, G. W., Dockery, P., & Sullivan, A. M. (2004a). Effects of growth/differentiation factor 5 on the survival and morphology of embryonic rat midbrain dopaminergic neurones in vitro. Journal of Neurocytology, 33(5), 479–488.
O’Keeffe, G. W., Hanke, M., Pohl, J., & Sullivan, A. M. (2004b). Expression of growth differentiation factor-5 in the developing and adult rat brain. Developmental Brain Research, 151(1–2), 199–202.
Oo, T. F., & Burke, R. E. (1997). The time course of developmental cell death in phenotypically defined dopaminergic neurons of the substantia nigra. Developmental Brain Research, 98(2), 191–196.
Orlacchio, A., Bernardi, G., & Martino, S. (2010). Stem cells and neurological diseases. Discovery Medicine, 9(49), 546–553.
Orme, R. P., Bhangal, M. S., & Fricker, R. A. (2013). Calcitriol imparts neuroprotection in vitro to midbrain dopaminergic neurons by upregulating GDNF expression. PLoS ONE, 8(4), e62040.
O’Sullivan, D. B., Harrison, P. T., & Sullivan, A. M. (2010). Effects of GDF5 overexpression on embryonic rat dopaminergic neurones in vitro and in vivo. Journal of Neural Transmission, 117(5), 559–572.
Pakkenberg, B., Moller, A., Gundersen, H. J., Mouritzen Dam, A., & Pakkenberg, H. (1991). The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson’s disease estimated with an unbiased stereological method. Journal of Neurology, Neurosurgery and Psychiatry, 54(1), 30–33.
Parikh, P., Hao, Y., Hosseinkhani, M., Patil, S. B., Huntley, G. W., Tessier-Lavigne, M., et al. (2011). Regeneration of axons in injured spinal cord by activation of bone morphogenetic protein/Smad1 signaling pathway in adult neurons. Proceedings of the National Academy of Sciences of the United States of America, 108(19), E99–E107.
Paxinos, G., & Watson, C. (1988). The rat brain in stereotaxic coordinates. San Diego: Academic Press.
Phan, K. D., Hazen, V. M., Frendo, M., Jia, Z., & Butler, S. J. (2010). The bone morphogenetic protein roof plate chemorepellent regulates the rate of commissural axonal growth. Journal of Neuroscience, 30(46), 15430–15440.
Reiriz, J., Espejo, M., Ventura, F., Ambrosio, S., & Alberch, J. (1999). Bone morphogenetic protein-2 promotes dissociated effects on the number and differentiation of cultured ventral mesencephalic dopaminergic neurons. Journal of Neurobiology, 38(2), 161–170.
Round, J., & Stein, E. (2007). Netrin signaling leading to directed growth cone steering. Current Opinion in Neurobiology, 17(1), 15–21.
Sgado, P., Ferretti, E., Grbec, D., Bozzi, Y., & Simon, H. H. (2012). The atypical homeoprotein Pbx1a participates in the axonal pathfinding of mesencephalic dopaminergic neurons. Neural Development, 7, 24.
Shi, Y., & Massague, J. (2003). Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell, 113(6), 685–700.
Sieber, C., Kopf, J., Hiepen, C., & Knaus, P. (2009). Recent advances in BMP receptor signaling. Cytokine & Growth Factor Reviews, 20(5–6), 343–355.
Sieber, B. A., Kuzmin, A., Canals, J. M., Danielsson, A., Paratcha, G., Arenas, E., et al. (2004). Disruption of EphA/ephrin-a signaling in the nigrostriatal system reduces dopaminergic innervation and dissociates behavioral responses to amphetamine and cocaine. Molecular and Cellular Neuroscience, 26(3), 418–428.
Smith, W. C., & Harland, R. M. (1992). Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell, 70(5), 829–840.
Soderstrom, S., & Ebendal, T. (1999). Localized expression of BMP and GDF mRNA in the rodent brain. Journal of Neuroscience Research, 56(5), 482–492.
Specht, L. A., Pickel, V. M., Joh, T. H., & Reis, D. J. (1981a). Light-microscopic immunocytochemical localization of tyrosine hydroxylase in prenatal rat brain. I. Early ontogeny. Journal of Comparative Neurology, 199(2), 233–253.
Specht, L. A., Pickel, V. M., Joh, T. H., & Reis, D. J. (1981b). Light-microscopic immunocytochemical localization of tyrosine hydroxylase in prenatal rat brain. II. Late ontogeny. Journal of Comparative Neurology, 199(2), 255–276.
Storm, E. E., Huynh, T. V., Copeland, N. G., Jenkins, N. A., Kingsley, D. M., & Lee, S. J. (1994). Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature, 368(6472), 639–643.
Sullivan, A. M., & O’Keeffe, G. W. (2005). The role of growth/differentiation factor 5 (GDF5) in the induction and survival of midbrain dopaminergic neurones: Relevance to Parkinson’s disease treatment. Journal of Anatomy, 207(3), 219–226.
Sullivan, A. M., Opacka-Juffry, J., Hotten, G., Pohl, J., & Blunt, S. B. (1997). Growth/differentiation factor 5 protects nigrostriatal dopaminergic neurones in a rat model of Parkinson’s disease. Neuroscience Letters, 233(2–3), 73–76.
Sullivan, A. M., Opacka-Juffry, J., Pohl, J., & Blunt, S. B. (1999). Neuroprotective effects of growth/differentiation factor 5 depend on the site of administration. Brain Research, 818(1), 176–179.
Sullivan, A. M., Pohl, J., & Blunt, S. B. (1998). Growth/differentiation factor 5 and glial cell line-derived neurotrophic factor enhance survival and function of dopaminergic grafts in a rat model of Parkinson’s disease. European Journal of Neuroscience, 10(12), 3681–3688.
Sullivan, A. M., & Toulouse, A. (2011). Neurotrophic factors for the treatment of Parkinson’s disease. Cytokine & Growth Factor Reviews, 22(3), 157–165.
Tamariz, E., Diaz-Martinez, N. E., Diaz, N. F., Garcia-Pena, C. M., Velasco, I., & Varela-Echavarria, A. (2010). Axon responses of embryonic stem cell-derived dopaminergic neurons to semaphorins 3A and 3C. Journal of Neuroscience Research, 88(5), 971–980.
ten Dijke, P., Yamashita, H., Sampath, T. K., Reddi, A. H., Estevez, M., Riddle, D. L., et al. (1994). Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. Journal of Biological Chemistry, 269(25), 16985–16988.
Torre, E. R., Gutekunst, C. A., & Gross, R. E. (2010). Expression by midbrain dopamine neurons of Sema3A and 3F receptors is associated with chemorepulsion in vitro but a mild in vivo phenotype. Molecular and Cellular Neuroscience, 44(2), 135–153.
Toulouse, A., & Sullivan, A. M. (2008). Progress in Parkinson’s disease-where do we stand? Progress in Neurobiology, 85(4), 376–392.
Ulloa, F., & Briscoe, J. (2007). Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle, 6(21), 2640–2649.
Van den Heuvel, D. M., & Pasterkamp, R. J. (2008). Getting connected in the dopamine system. Progress in Neurobiology, 85(1), 75–93.
Verney, C. (1999). Distribution of the catecholaminergic neurons in the central nervous system of human embryos and fetuses. Microscopy Research and Technique, 46(1), 24–47.
Vitalis, T., Cases, O., Engelkamp, D., Verney, C., & Price, D. J. (2000). Defect of tyrosine hydroxylase-immunoreactive neurons in the brains of mice lacking the transcription factor Pax6. Journal of Neuroscience, 20(17), 6501–6516.
Voorn, P., Kalsbeek, A., Jorritsma-Byham, B., & Groenewegen, H. J. (1988). The pre- and postnatal development of the dopaminergic cell groups in the ventral mesencephalon and the dopaminergic innervation of the striatum of the rat. Neuroscience, 25(3), 857–887.
Wen, Z., Han, L., Bamburg, J. R., Shim, S., Ming, G. L., & Zheng, J. Q. (2007). BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF/cofilin. Journal of Cell Biology, 178(1), 107–119.
Wood, T. K., McDermott, K. W., & Sullivan, A. M. (2005). Differential effects of growth/differentiation factor 5 and glial cell line-derived neurotrophic factor on dopaminergic neurons and astroglia in cultures of embryonic rat midbrain. Journal of Neuroscience Research, 80(6), 759–766.
Xu, B., Goldman, J. S., Rymar, V. V., Forget, C., Lo, P. S., Bull, S. J., et al. (2010). Critical roles for the netrin receptor deleted in colorectal cancer in dopaminergic neuronal precursor migration, axon guidance, and axon arborization. Neuroscience, 169(2), 932–949.
Yamashita, H., Ten Dijke, P., Heldin, C. H., & Miyazono, K. (1996). Bone morphogenetic protein receptors. Bone, 19(6), 569–574.
Yamauchi, K., Phan, K. D., & Butler, S. J. (2008). BMP type I receptor complexes have distinct activities mediating cell fate and axon guidance decisions. Development, 135(6), 1119–1128.
Yu, P. B., Hong, C. C., Sachidanandan, C., Babitt, J. L., Deng, D. Y., Hoyng, S. A., et al. (2008). Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nature Chemical Biology, 4(1), 33–41.
Yue, Y., Widmer, D. A., Halladay, A. K., Cerretti, D. P., Wagner, G. C., Dreyer, J. L., et al. (1999). Specification of distinct dopaminergic neural pathways: Roles of the Eph family receptor EphB1 and ligand ephrin-B2. Journal of Neuroscience, 19(6), 2090–2101.
Zou, Y., & Lyuksyutova, A. I. (2007). Morphogens as conserved axon guidance cues. Current Opinion in Neurobiology, 17(1), 22–28.
Acknowledgments
The authors declare no conflict of interest. This work was supported by grant support from the Irish Research Council (SVH/AS/G’OK), the Health Research Board of Ireland (HRA/2009/127) (GO’K/AS) and Science Foundation Ireland (10/RFP/NES2786) (GO’K).
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Aideen M. Sullivan and Gerard W. O’Keeffe have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12017_2014_8299_MOESM2_ESM.tif
Noggin and dorsomorphin prevent the promotion of SH-SH5Y neurite growth by BMP2 and GDF5. (a) Total neurite length of noggin- or dorsomorphin-pre-treated and/or BMP2- or GDF5-treated (daily for 4 DIV) SH-SY5Y cells, as indicated (*** P < 0.001 vs. control; ANOVA with post hoc Tukey’s test; 20 images analysed for each group per experiment; N = 3 experiments). Data are expressed as mean ± SEM. (b) Representative photomicrographs of noggin pre-treated and/or BMP2- or GDF5-treated SH-SY5Y cells, as indicated, immunocytochemically stained for β-actin. Scale bar = 100 μm (TIFF 4240 kb)
12017_2014_8299_MOESM3_ESM.tif
Heparinase III does not affect the promotion of SH-SH5Y neurite growth by BMP2 and GDF5. (a) Total neurite length of Heparinase III-pre-treated and/or BMP2- or GDF5-treated (daily for 4 DIV) SH-SY5Y cells, as indicated (*** P < 0.001 vs. control; ANOVA with post hoc Tukey’s test; 20 images analysed for each group per experiment; N = 3 experiments). Data are expressed as mean ± SEM. (b) Representative photomicrographs of Heparinase III-pre-treated and/or GDF5-treated SH-SY5Y cells, as indicated, immunocytochemically stained for β-actin. Scale bar = 100 μm (TIFF 1711 kb)
Rights and permissions
About this article
Cite this article
Hegarty, S.V., Collins, L.M., Gavin, A.M. et al. Canonical BMP–Smad Signalling Promotes Neurite Growth in Rat Midbrain Dopaminergic Neurons. Neuromol Med 16, 473–489 (2014). https://doi.org/10.1007/s12017-014-8299-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-014-8299-5