Abstract
Inhalational anesthetic preconditioning can induce neuroprotective effects, and the notch signaling pathway plays an important role in neural progenitor cell differentiation and the inflammatory response after central nervous system injury. This study evaluated whether the neuroprotective effect of isoflurane preconditioning is mediated by the activation of the notch signaling pathway. Mice were divided into two groups consisting of those that did or did not receive preconditioning with isoflurane. The expression levels of notch-1, notch intracellular domain (NICD), and hairy and enhancer of split (HES-1) were measured in mice subjected to transient global cerebral ischemia–reperfusion injury. The notch signaling inhibitor DAPT and conditional notch-RBP-J knockout mice were used to investigate the mechanisms of isoflurane preconditioning-induced neuroprotection. Immunohistochemical staining, real-time polymerase chain reaction assays, and Western blotting were performed. Isoflurane preconditioning induced neuroprotection against global cerebral ischemia. Preconditioning up-regulated the expression of notch-1, HES-1, and NICD after ischemic–reperfusion. However, these molecules were down-regulated at 72 h after ischemic–reperfusion. The inhibition of notch signaling activity by DAPT significantly attenuated the isoflurane preconditioning-induced neuroprotection, and similar results were obtained using notch knockout mice. Our results demonstrate that the neuroprotective effects of isoflurane preconditioning are mediated by the pre-activation of the notch signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The notch signaling pathway is highly conserved and widely expressed across the animal kingdom. Functionally, notch plays an important role in cell self-renewal, individual growth, and development as well as a range of physiologic and pathologic processes (Artavanis-Tsakonas et al. 1999). In the central nervous system, notch is associated with the differentiation, maturation, regeneration, and functional maturation of neural progenitor cells and has been implicated in neurodegenerative diseases (Yoon and Gaiano 2005; Gaiano and Fishell 2002). Notch signaling, in combination with ciliary neurotrophic factor (CNTF), causes neural stem cells to generate astrocytes in the embryonic period (Nagao et al. 2007). However, the notch protein and its ligands are not only present in the embryonic stages but are also continuously present in the adult nervous system (Presente et al. 2001). Hence, the notch signaling pathway is actively involved in dynamic changes in the cellular architecture and function of the nervous system during the entire life span. The binding of notch ligands to notch receptors leads to the cleavage of notch receptors and the nuclear translocation of notch intracellular domain (NICD), which induces the transcriptional activation of notch-targeted genes. The hairy and enhancer of split-1 (HES-1) directly affects cell fate decisions as a primary target gene of notch signaling (Presente et al. 2001). The activation of γ-secretase results in transient notch-1 cleavage and increased levels of NICD after focal cerebral ischemic–reperfusion in the rat, although notch-1 antisense transgenic mice and normal mice treated with γ-secretase inhibitors exhibit less severe stroke-induced brain damage and improved functional outcome (Arumugam et al. 2006). Recently, notch signaling was discovered to play an important role in the regulation of neurogenesis and the functional recovery of rodents with brain damage from stroke (Androutsellis-Theotokis et al. 2006; Yilmaz and Granger 2010).
As a classical inhalational anesthetic, isoflurane is generally considered to induce ischemic tolerance in the myocardium, cerebrum, and spinal cord (Redel et al. 2009; Xiong et al. 2003; Kitano et al. 2007; Sang et al. 2006). Previously, our laboratory, along with others, showed that isoflurane preconditioning can induce neuroprotective effects on focal cerebral ischemia. This neuroprotection may be mediated by many factors, including antioxidants, the mitochondrial adenosine triphosphate-sensitive potassium channel, nitric oxide synthase, and excitatory amino acids (Wang et al. 2008; Matchett et al. 2009). However, it remains unknown which of these factors are relevant to acute isoflurane preconditioning-mediated neuroprotection in the global cerebral ischemia–reperfusion model.
In this study, we investigated the relationship between changes in the notch signaling pathway and neuroprotection induced by isoflurane preconditioning. Our hypothesis was that the neuroprotection of isoflurane preconditioning is mediated by the pre-activation of the notch signaling pathway. Considering the pleiotropic functions of the notch signaling pathway, the effects of cerebral ischemia–reperfusion injury on the notch cascade is potentially of great interest with respect to neuroprotection and neuroregeneration strategies.
Materials and Methods
Experimental Protocols
All animal-related procedures were approved by the Ethics Committee for Animal Experimentation of the Fourth Military Medical University (Xi’an, China) and proceeded in accordance with the guidelines for Animal Experimentation of the University. Exclude for died or other reasons such as surgical failure or failure to meet rCBF criteria, 168 male C57BL/6 mice, aged 12 weeks and weighing 20–24 g, were used in our experiments and were provided by the Experimental Animal Center of the Fourth Military Medical University. Mice were kept in cages under a controlled-light environment (lights on from 8:00 a.m. to 8:00 p.m.) and allowed free access to a rodent diet (standardized rat food is obtained from the Experimental Animal Center of Fourth Military Medical University.) and tap water.
The male wild-type (WT) C57BL/6 mice (aged 10–12 weeks, weighing 20–25 g) were provided by the Experimental Animal Center of the Fourth Military Medical University. Neuron-specific RBP-J knockout mice were generated by the Cre/loxP system. Conditional RBP-J allele (RBP-J floxed) mice were a generous gift from Professor Hua Han, M.D., Ph.D. (Department of Medical Genetics and Developmental Biology, Fourth Military Medical University). The CamKIIα-Cre mouse, in which Cre expression is controlled by the forebrain-specific calcium- or calmodulin-dependent kinase IIα(CamKIIα) promoter in neuronal cells, was presented by Gunther Schutz, M.D. (Professor, German Cancer Research Center, Heidelberg, Germany). By mating RBP-J-floxed mice with CamKIIα-Cre transgenic mice, we obtained bitransgenic mice CamKIIα-Cre-RBP-Jflox/flox, which were then mated with each other to generate RBP-J knockout mice (CamKIIα-Cre-RBP-Jflox/flox). Male mice were used for all experiments. Wild-type (WT) and heterozygous (CamKIIα-Cre-RBP-Jflox/−) littermates were used in the studies as controls. All mice were maintained in a pathogen-free environment with a 12-h light/dark cycle, a temperature of 21 ± 2 °C, and a humidity of 60–70 %. (Yang et al. 2012). The genotypes of the generated mice were verified by polymerase chain reaction (PCR) analysis (Yang et al. 2012).
Experiment I
The purpose of this experiment was to investigate the neuroprotective effects induced by isoflurane preconditioning in a transient global cerebral ischemia mouse model. Animals were randomly divided into three groups: Sham, isoflurane preconditioning ischemia–reperfusion (Pre-IR), and Control (Con). The mice in the Pre-IR group were placed in a chamber for isoflurane preconditioning (98 % O2 and 1.2 % isoflurane) at a rate of 1 h/day for 5 days. The mice in the Con group were placed in the chamber and inhaled oxygen (98 % O2 and 2 % N2) for 1 h/day for 5 days. At 24 h after the last day of preconditioning and inhalation, mice in the Pre-IR and Con groups were subjected to bilateral common carotid artery occlusion (BCCAO) for 20 min. The mice in the Sham group did not receive preconditioning or artery occlusion during surgery.
Experiment II
The purpose of this experiment was to observe changes in the expression of the notch signaling pathway during the isoflurane preconditioning and ischemia–reperfusion processes and to establish the relationship between changes in notch signaling and the neuroprotective effects induced by preconditioning. Mice were randomly divided into the following four groups: Sham, Pre-IR, Con, and isoflurane preconditioning only (Pre). In this experiment, the Sham group mice did not receive preconditioning or artery occlusion during surgery; the mice in the Pre-group only received successive preconditioning without artery occlusion during surgery.
Experiment III
To evaluate the effects of isoflurane preconditioning-induced neuroprotection after the notch signaling pathway was inhibited, mice were injected intraperitoneally with 100 mg/kg DAPT or an equal volume of vehicle (DMSO) 3 h prior to each preconditioning event for 5 days. DAPT (ALX-270-416-M025; Axxora Bioscience, NY, USA), a gamma-secretase inhibitor that can inhibit the notch signaling pathway, was dissolved in dimethyl sulfoxide and then diluted with saline. The DAPT dose and administration method in this experiment were selected based on the results of a DAPT inhibitor efficacy validation test conducted in our laboratory as well as the results of previously published studies (Dovey et al. 2001; Lanz et al. 2003). Mice were randomly divided into five groups: DAPT + Pre-IR, Vehicle + Pre-IR, DAPT + Con, Vehicle + Con, and Sham. The mice in the Sham group were not administered any drug and did not receive preconditioning or artery occlusion during surgery.
Experiment IV
A conditional RBP-J-knockout approach was used to further reveal the role of the notch signaling pathway in ischemic brain injury. WT, RBP-J knockouts (RBP-J−/−), and heterozygous littermates (RBP-J+/−) were used. Mice were divided into five groups: two received 98 % O2 and 2 % N2 inhalation (RBP-J+/− and RBP-J−/−), two received 1.2 % isoflurane and 98 % O2 preconditioning (Pre-IR + RBP-J+/− and Pre-IR + RBP-J−/−), and the WT group did not receive preconditioning or artery occlusion during surgery. All mice except the WT group were subjected to ischemia–reperfusion injury at 24 h after the last preconditioning or inhalation.
A detailed description of the experimental grouping and protocols is presented in Fig. 1.
Global Cerebral Ischemia and Regional Cerebral Blood Flow Monitoring
Bilateral common carotid artery occlusion (BCCAO) was used as a model of global cerebral ischemia (Kitagawa et al. 1998a; Ohtaki et al. 2008). Surgical operation was performing by a person who was blinded to the animal groups. Mice were anesthetized with 3 % isoflurane. After induction, isoflurane was maintained at 1.5 %. Isoflurane was delivered via a face mask that was constructed to fit over the animals’ frontal area. A midline incision was made to the region between the neck and sternum to expose the trachea. Both the right and left common carotid arteries were located lateral to the sternocleidomastoid and were carefully separated from the surrounding tissues and vagus nerve. Cerebral ischemia was induced by clamping both of the arteries with two miniature artery clips. A laser Doppler flowmeter (PeriFlux system 5000, Perimed, Sweden) was used to measure the regional cerebral blood flow (rCBF; 2–3 mm lateral to bregma) from the time of anesthetic induction to 5 min after reperfusion (Kitagawa et al. 1998b; Wacker et al. 2009). In our experimental model, only mice whose mean cortical rCBF was reduced to < 10 % of the pre-ischemic value were used for the data analysis. Totally, 22 animals were excluded for failure to meet rCBF inclusion criteria and 17 mice were excluded for surgical failure. After 20 min of cerebral ischemia, the clips were removed from both arteries to allow for the reperfusion of blood through the carotid arteries. Sham-operated mice underwent the same surgical procedure without artery occlusion. The incision was sutured using 4–0 Mersilk (Ethicon, Johnson & Johnson Company, CA, USA). During the surgical procedure, the pericranial temperature was monitored using a temperature probe and maintained at 37.0–37.5 °C using a heating pad. After surgery, animals were placed in a warm environment (30–33 °C) to avoid biased results due to hypothermia. According to our observation, except the animals that died during surgery and failing in making the model, all of the animals in Pre-IR and Con group survived after ischemia–reperfusion in Experiment I/IV. In addition, two animals died in the Con group, one on the second day and the other on the third day in Experiment II. One animal died in the DAPT Con group on the third day after ischemia–reperfusion in Experiment III.
Isoflurane Preconditioning and Measurement of Physiologic Parameters:
Mice from the Pre-IR group in Experiments I, II, III, and IV were placed in a chamber (containing 1.2 % isoflurane and 98 % O2) for 1 h/day for 5 days as previously described (Zhang et al. 2010). Mice from the Con group in Experiments I, II, III, and IV were placed in the same chamber without isoflurane preconditioning (98 % O2 and 2 % N2) for 1 h/day for 5 days. At 24 h after the last day of preconditioning, mice in the Pre-IR and Con/Vehicle groups were subjected to BCCAO for 20 min. To avoid the hypoxemia, 12 additional animals were used in a separate experiment to measure blood gas levels during isoflurane preconditioning (n = 6) or oxygen inhalation (n = 6). Blood gas measurements were taken in each mouse at three time points (before/during and post-preconditioning), that each animal had a femoral artery catheter placed, that sampling volume was 0.2 mL, and that a comparable volume of saline to replace withdrawn blood volume was injected so as to control for the effects of hypovolemia on blood pressure. Samples were then measured using a Bayer Rapidlab 1260 system (Bayer, Leverkusen, Germany).
Neurologic Tests
The treated mice were allowed to recover for 24 h before subsequent tests. Mice (n = 8) in Experiments I, II, and III were subjected to a modified neurologic examination designed to detect motor deficits. Briefly, mice were placed on a 10- to 20-cm screen (grid size 0.2 × 0.2 cm) that could be rotated from 0° (horizontal) to 90° (vertical). Mice were placed on this screen, which was in a horizontal position, and the screen was then rotated into the vertical plane. The duration for which each mouse was able to hold on to the vertical screen was recorded up to a maximum of 15 s (corresponding to a maximum of three points). Next, the mouse was placed at the center of a horizontal wooden rod (1.5 cm in diameter), and the duration that the mouse was able to remain balanced on the rod was recorded up to a maximum of 30 s (corresponding to a maximum of three points). Finally, a prehensile traction test was administered. The time that the mouse was able to cling to a horizontal rope was recorded up to a maximum of 5 s (corresponding to a maximum of three points). From these tests, a total motor score (9 possible points) was calculated. The neurologic tests were performed at 24-, 48-, and 72-h post-reperfusion by an observer who was unaware of the grouping. The total motor score (TMS), as applied in the current study, has been shown to be an accurate method for evaluating global cerebral ischemia injury in mice (Homi et al. 2003; Sheng et al. 1999).
Histologic and Apoptotic Assessments
To evaluate histologic damage, the mice (n = 8) from Experiments I, III, and IV were killed under deep anesthesia at 72-h post-reperfusion by perfusion with physiologic saline solution followed by perfusion with freshly prepared 4 % (v/v) paraformaldehyde in 0.01 M phosphate-buffered saline (PBS) buffer (pH = 7.4). Brains were removed and post-fixed in 4 % (v/v) paraformaldehyde for 6–8 h and then cut on a freezing microtome (Leica CM 1900, Nussloch, Germany). Backward from the optic chiasm consecutive six sections (12 μm) that included the dorsal hippocampus were stained with hematoxylin–eosin, and the pyramidal neurons of the CA1 region were examined, as these are the neurons that are most vulnerable to global ischemia–reperfusion injury (Kitagawa et al. 1998a; Fujii et al. 1997). Viable neurons were defined as neurons in which a clear nucleus could be seen, and ischemic damaged neurons were defined as neurons that exhibited features including pyknosis and shrunken cell bodies. For the detection of in situ DNA fragmentation, terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end-labeling (TUNEL) staining was performed using an In Situ Cell Death Detection Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. TUNEL staining was performed on 12-μm-thick frozen coronal consecutive sections backward from the optic chiasm. The sections were washed in 0.01 M PBS, pH 7.4, for 5 min, treated with 0.3 % (v/v) H2O2 for 20 min, rinsed in 0.01 M PBS for 5 min, and then incubated in the TUNEL reaction mixture for 1 h at 37 °C. The sections were then rinsed in 0.1 M PBS three times for 5 min and incubated in converter-peroxidase for 30 min at 37 °C. Following three washes in 0.01 M PBS for 5 min, sections were developed with 3,3′-diaminobenzidine for 5 min at room temperature. Six sections per animal were used for histology and apoptotic assessment at 72 h after ischemia–reperfusion. Viable neurons were defined as neurons where a clear nucleus could be seen, and ischemic damaged neurons exhibited features including pyknosis and shrunken cell bodies. A neuron with a nucleus stained dark brown was considered a TUNEL-positive cell. By counterstaining with hematoxylin (a blue dye), viable neurons were also observed. Viable neurons and apoptotic-positive cells were counting and statistics by an observer who was blind to the animal groups, respectively. To minimize variability of neuron after ischemia-reperfusion, we observed the same hippocampus CA1 area in 3 different fields for each section under 400 × for the analysis. The software we used was Image-Pro Plus 6. Images of the viable neurons and TUNEL-positive neurons in the hippocampal CA1 area in each section were captured (BX51 microscope; Olympus, Tokyo, Japan). The data are represented as the number of cells per mm2.
Dual Immunohistochemical Staining
In Experiment II, we also examined the dorsal hippocampus tissue because of its sensitivity to ischemia–reperfusion injury and because new neurons can be found in the hippocampal CA1 region post-ischemically (Nakatomi et al. 2002; Oya et al. 2009). The mice (n = 4) from the Sham, Pre-IR, and Con groups at 2, 24, and 72 h after reperfusion and from the Pre-group at 2 and 24 h after the last preconditioning step were deeply anesthetized and then transcardially perfused with physiologic saline followed by 4 % (v/v) ice-cold paraformaldehyde in PBS (pH = 7.4). Brains were post-fixed for 6–8 h in the same fixative at 4 °C and cryoprotected in 15 % (w/v) and 30 % (w/v) sucrose solutions. Coronal sections (12 μm) were cut on a freezing microtome (Leica CM 1900, Nussloch, Germany). After blocking with PBS containing 0.3 % (v/v) Triton X-100 and 10 % (v/v) normal goat serum, sections were incubated overnight at 4 °C with primary antibodies for both antineuronal nuclei mouse monoclonal antibody (1:2,000, Chemicon, MA, USA) and anti-NICD rabbit polyclonal antibody (1:500, Abcam, CA, UK). The sections were washed with PBS and incubated with the secondary antibody solution TRITC-labeled goat anti-rabbit antibody (IgG, 1:800, Vector, USA) and FITC-labeled goat anti-mouse antibody (IgG, 1:800, Vector, USA) at room temperature for 1 h. Three sections per animal were used for immunohistochemical staining, and four animals were selected in corresponding time in each group. Finally, sections were observed, and images were captured using a fluorescence microscope (BX51; Olympus, Tokyo, Japan).
Western Blotting
In Experiment II, mice (n = 6) from the Sham, Con, Pre-IR, and Pre-groups were killed at the same time points that were utilized in the immunohistochemical staining experiments. Animals were anesthetized and perfused with ice-cold PBS. Tissue was instantly frozen in liquid nitrogen and stored at −80 °C. Dorsal hippocampal tissue was homogenized on ice in lysis buffer containing protease inhibitors. After centrifugation (Allegra 64R Centrifuge, Beckman, CA, USA, 12,000 rpm/s, 10 min), the supernatant was collected and the protein concentration of each sample was estimated by the Lowry method using a protein assay kit. Aliquots of lysates containing 20 μg of protein were separated by electrophoresis on 8 % gradient sodium dodecyl sulfate–polyacrylamide gels (SDS–PAGE) and transferred to nitrocellulose membranes (Bio-Rad Laboratories, Richmond, CA, USA). After blocking for 1 h at room temperature with 5 % (w/v) skim milk powder, blots were incubated overnight at 4 °C with the primary rabbit anti-NICD polyclonal antibody (1:800, Abcam, CA, UK). A β-actin antibody (1:500; Biosynthesis, Beijing, China, bs-0061R) was used as an internal control. Anti-rabbit antibody was used as the secondary antibody, and antigens were detected using a standard chemiluminescence method (ECL; Millipore Biotech, Billerica, MA, USA). Image analyses were performed using computerized analysis software (Bio-Rad Laboratories, CA, USA).
Real-Time PCR
In Experiment II, mice (n = 6) from the Sham, Con, Pre-IR, and Pre-groups were killed at the same time points that were utilized for immunohistochemical staining. Dorsal hippocampal tissue that had been subjected to ischemia–reperfusion was harvested, and total RNA was extracted using RNAiso Plus (Takara Biotech Corporation) according to a standard protocol (Livak and Schmittgen 2001). Quantitative PCR was performed using the SYBR Green real-time PCR method on an Bio-Rad IQ5 real-time PCR instrument (Applied Biosystems). The following 3-stage program parameters provided by the manufacturer were used as follows: 2 min at 50 °C, 10 min at 95 °C, and then 40 cycles of 15 s at 95 °C and 60 s at 59 °C. Each sample was tested in triplicate, and the analyses of the relative gene protocol expression data using the 2−▵▵t method were performed. The following primers were used for real-time PCR: Notch1 forward, TGC CAG GAC CGT GAC AAC TC; Notch1 reverse, CAC AGG CAC ATT CGT AGC CAT C; HES-1 forward, AAA GAC GGC CTC TGA GCA C; HES-1 reverse, GGT GCT TCA CAG TCA TTT CCA; GAPDH forward, AGA ACA TCA TCC CTG CAT CC; and GAPDH reverse, CAC ATT GGG GGT AGG AAC AC.
DAPT γ-Secretase Inhibitor Efficacy Validation
In Experiment III, the animals in the inhibitor validation group (a separate group, n = 3) were intraperitoneally injected with the inhibitor DAPT (100 mg/kg) and then killed after 1, 2, 3, 6, 12, or 24 h observe changes in NICD expression via Western blot analysis.
Statistical Analysis
The statistical analyses were conducted using SPSS 11.0 for Windows software (SPSS Inc., Chicago, IL). All values, except for total motor scores, are presented as the means ± the SDs and were analyzed using a one-way analysis of variance. Between-group differences were detected based on post hoc Student–Newman–Keuls tests. The total motor scores are expressed as the medians and were analyzed using the Kruskal–Wallis test followed by the Mann–Whitney U test with Bonferroni corrections. Values of p < 0.05 were considered statistically significant.
Results
Physiologic Parameters
Changes in blood gas concentrations during isoflurane preconditioning and oxygen inhalation are observed. There were no significant differences between the isoflurane inhalation and oxygen inhalation groups at any time points.
rCBF Measurements
The rCBF was immediately reduced to < 10 % of the pre-ischemic baseline after BCCAO and remained constant during the ischemic period in all animals. After the clips were removed, and the rCBF returned to pre-ischemic values within 5 min. There were no significant differences in the rCBF between the Pre-IR and Con groups (Experiments I/II/III/IV) at any time points.
Isoflurane Preconditioning-Induced Improvements in Neurologic Function are Compromised by DAPT and Weakened in RBP-J Knockout Mice
At 24, 48, and 72 h after reperfusion, isoflurane preconditioning significantly improved the TMSs relative to those of the Con group at the same time points (Fig. 2a, p < 0.05). The notch signaling pathway inhibitor DAPT compromised the beneficial effect of isoflurane preconditioning (p < 0.05, Vehicle Pre-IR versus DAPT Pre-IR). Furthermore, the TMSs of the DAPT Con group at 48 and 72 h after reperfusion were higher than those of the Vehicle Con groups at the same time points (Fig. 2b–d, p < 0.05). At 24, 48, and 72 h after reperfusion, isoflurane preconditioning significantly improved the TMSs compared with those of the Vehicle groups at the same time points (p < 0.05). The RBP-J knockout RBP-J−/− nullified the beneficial effect of isoflurane preconditioning (p < 0.05, RBP-J+/− Pre-IR versus RBP-J−/− Pre-IR). Furthermore, the TMSs of the RBP-J−/− Vehicle group at 48 and 72 h after reperfusion were higher than those of the RBP-J+/− Vehicle groups at the same time points (Fig. 2e–g, p < 0.05).
a Neurologic scores recorded in each animal of seven groups 24, 48, and 72 h after reperfusion in Experiment I. Significantly, the total motor scores (TMS) in pre-IR group were significantly better than those in the Con groups at 24, 48, and 72 h after reperfusion (n = 8, *p < 0.05). b–d Neurologic scores recorded in each animal of five groups 24, 48, and 72 h after reperfusion in Experiment III. Obviously, the total motor scores (TMS) in the Vehicle Pre-IR group at 24 h/48 h/72 h after reperfusion were better than the DAPT Pre-IR group in the corresponding time points (n = 8, *p < 0.05), but the scores of DAPT Pre-IR group at 24 h/48 h/72 h after reperfusion were significantly better than the DAPT Con and Vehicle Con groups in the corresponding time points (n = 8, # p < 0.05); the scores of DAPT Con group at 48 h/72 h after reperfusion were better than the Vehicle Con groups in the corresponding time points (n = 8, § p < 0.05). e–g Neurologic scores recorded in each animal of five groups 24, 48, and 72 h after reperfusion in Experiment IV. The total motor scores (TMS) in the RBP-J+/− Pre-IR group at 24 h/48 h/72 h after reperfusion were better than the RBP-J−/− Pre-IR group in the corresponding time points (n = 6, *p < 0.05), but the scores of RBP-J−/− Pre-IR group at 24 h/48 h/72 h after reperfusion were significantly better than the RBP-J−/− Vehicle and RBP-J+/− Vehicle groups in the corresponding time points (n = 6, # p < 0.05); the scores of RBP-J−/− Vehicle group at 48 h/72 h after reperfusion were better than the RBP-J+/− Vehicle groups in the corresponding time points (n = 6, § p < 0.05)
Isoflurane Preconditioning-Induced Reduction in Neuronal Degeneration is Attenuated by DAPT and Absent in Notch Knockout Mice
Three days after reperfusion, isoflurane preconditioning significantly reduced the neuronal degeneration in the CA1 region compared with that observed in the Con group (Fig. 3a, p < 0.05). At the same observation time points, the number of viable neurons of the DAPT Pre-IR group was significantly reduced compared to the Vehicle Pre-IR group (p < 0.05), and the abundance of viable neurons in the DAPT Con group was greater than that of the Vehicle Con group (Fig. 3b, p < 0.05). Three days after reperfusion, isoflurane preconditioning significantly reduced the extent of neuronal degeneration in the CA1 region compared to the Vehicle groups. The number of viable neurons of the RBP-J−/− Pre-IR group was significantly reduced compared to the RBP-J+/− Pre-IR group (p < 0.05), and the number of viable neurons of the RBP-J−/− Vehicle group was higher than that of the RBP-J+/− Vehicle group (Fig. 3c, p < 0.05).
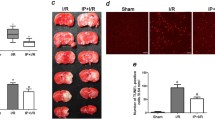
A Isoflurane preconditioning protects hippocampal CA1 neurons against ischemic injury in Experiment I. a Representative microphotographs of hematoxylin–eosin-stained hippocampal CA1 regions at 72 h after global ischemia in mice. A Sham, B Con 72 h, C Pre-IR 72 h. b Viable CA1 neurons were counted and analyzed at 72 h after global cerebral ischemia. Viable neuronal cells were significantly increased in the CA1 area in the Pre-IR group compared with those in the Con and Sham groups at the corresponding time points (n = 8, *p < 0.05). B Isoflurane preconditioning-induced reduction in neuronal degeneration is attenuated by DAPT in Experiment III. a Representative microphotographs of hematoxylin–eosin-stained hippocampal CA1 regions at 72 h after global ischemia in mice of administration. A Sham, B Vehicle Pre-IR 72 h, C DAPT Pre-IR 72 h, D Vehicle Con 72 h, E DAPT Con 72 h. b Viable CA1 neurons were counted and analyzed at 72 h after global cerebral ischemia. The numbers of viable neurons in the CA1 region were significantly increased in the Vehicle Pre-IR group at 72 h after reperfusion compared with those in the DAPT Pre-IR and Sham groups (n = 8, *p < 0.05), but the numbers of viable neurons in the DAPT Pre-IR group at 72 h after reperfusion were higher than the DAPT Con and Vehicle Con groups in the corresponding time points (n = 8, # p < 0.05), and DAPT Con group viable neurons at 72 h after reperfusion were higher than Vehicle Con in the corresponding time points (n = 8, § p < 0.05). C Isoflurane preconditioning-induced reduction in neuronal degeneration is absent prevented by notch knockout in Experiment IV. a Representative microphotographs of hematoxylin–eosin-stained hippocampal CA1 regions at 72 h after global ischemia in mice. A Wild-type, B RBP-J+/− Pre-IR 72 h, C RBP-J−/− Pre-IR 72 h, D RBP-J+/− Vehicle 72 h, E RBP-J−/− Vehicle 72 h. b Viable CA1 neurons were counted and analyzed at 72 h after global cerebral ischemia. The numbers of viable neurons in the CA1 region were significantly increased in the RBP-J+/− Pre-IR group at 72 h after reperfusion compared with those in the RBP-J−/− Pre-IR and wild-type groups (n = 6, *p < 0.05), but the numbers of viable neurons in the RBP-J−/− Pre-IR group at 72 h after reperfusion were higher than the RBP-J+/− Vehicle and RBP-J−/− Vehicle groups in the corresponding time points (n = 6, # p < 0.05), and RBP-J−/− Vehicle group viable neurons at 72 h after reperfusion were higher than RBP-J+/− Vehicle in the corresponding time points (n = 6, § p < 0.05)
Isoflurane Preconditioning-Induced Reductions in Apoptosis are Attenuated by DAPT and Absent in Notch Knockout Mice
The number of TUNEL-positive neurons in the CA1 region of the Con group was significantly increased at 72 h after reperfusion compared to the Pre-IR group (Fig. 4a, p < 0.05). At the same time point, the number of TUNEL-positive neurons in the CA1 region of the Vehicle Pre-IR group was significantly lower than that of the DAPT Pre-IR group (p < 0.05), and the number of TUNEL-positive neurons in the DAPT Con group was lower than that of the Vehicle Con group (Fig. 4b, p < 0.05). At the same observation time point, the number of TUNEL-positive neurons in the CA1 regions of the RBP-J+/− Pre-IR group was significantly reduced compared to the RBP-J−/− Pre-IR group (p < 0.05), and the number of TUNEL-positive neurons in the RBP-J−/− Vehicle group was reduced compared to the RBP-J+/− Vehicle group (Fig. 4c, p < 0.05).
A Isoflurane preconditioning decreases the number of hippocampal CA1 TUNEL-positive neurons following ischemia in Experiment I. a Representative microphotographs of TUNEL-stained hippocampal CA1 regions at 72 h after global ischemia in mice. A Sham, B Con 72 h, C Pre-IR 72 h. b TUNEL-positive neurons were observed and analyzed at 72 h after global cerebral ischemia. TUNEL-positive neurons in the CA1 area were significantly decreased in the Pre-IR group compared with those in the Con and Sham groups at the corresponding time points (n = 8, *p < 0.05). B Isoflurane preconditioning-induced reduction in apoptosis is attenuated by DAPT in Experiment III. a Representative microphotographs of TUNEL-stained hippocampal CA1 regions at 72 h after global ischemia in mice of administration. A Sham, B Vehicle Pre-IR 72 h, C DAPT Pre-IR 72 h, D Vehicle Con 72 h, E DAPT Con 72 h. b TUNEL-positive neurons were observed and analyzed at 72 h after global cerebral ischemia. The numbers of TUNEL staining-positive neurons in the CA1 region were significantly decreased in the Vehicle Pre-IR group at 72 h after reperfusion compared with the DAPT Pre-IR and Sham groups (n = 8, *p < 0.05); the numbers of TUNEL-positive neurons in the DAPT Pre-IR group at 72 h after reperfusion were lower than the DAPT Con and Vehicle Con groups in the corresponding time points (n = 8, # p < 0.05); and DAPT Con group positive neurons at 72 h after reperfusion were lower than Vehicle Con in the corresponding time points (n = 8, § p < 0.05). C Isoflurane preconditioning-induced reduction in apoptosis is absent by notch knockout in Experiment IV. a Representative microphotographs of TUNEL-stained hippocampal CA1 regions at 72 h after global ischemia in mice. A Wild-type, B RBP-J+/− Pre-IR 72 h, C RBP-J−/− Pre-IR 72 h, D RBP-J+/− Vehicle 72 h, E RBP-J−/− Vehicle 72 h. b TUNEL-positive neurons were observed and analyzed at 72 h after global cerebral ischemia. The numbers of TUNEL staining-positive neurons in the CA1 region were significantly decreased in the RBP-J+/− Pre-IR group at 72 h after reperfusion compared with the RBP-J−/− Pre-IR and wild-type groups (n = 6, *p < 0.05); the numbers of TUNEL-positive neurons in the RBP-J−/− Pre-IR group at 72 h after reperfusion were lower than the RBP-J+/− Vehicle and RBP-J−/− Vehicle groups in the corresponding time points (n = 6, # p < 0.05); and RBP-J−/− Vehicle group positive neurons at 72 h after reperfusion were lower than RBP-J+/− Vehicle in the corresponding time points (n = 6, § p < 0.05)
Isoflurane Preconditioning Pre-activates Notch Signaling
To identify the molecular mechanisms underlying the isoflurane preconditioning-mediated notch signaling pathway, the expression levels of Notch-1, NICD, and HES-1 were measured after ischemia–reperfusion in Experiment II (Zacharek et al. 2009; Chen et al. 2008). First, the expression and localization of NICD was examined by dual immunofluorescence staining of the neuronal nuclei and NICD (neuronal marker in green and NICD in red). As shown in Fig. 5a, NICD was mainly localized in the neurons of the hippocampus. In the Sham group, a low-level expression of NICD was observed in the hippocampus. In contrast, NICD expression gradually increased in the Con group between 2 and 24 h after reperfusion, although the fluorescence intensities were weaker than those of the Pre-IR group at the same time points. However, 72 h after reperfusion, NICD expression in the remaining neurons in the Con group was stronger than in the Pre-IR group.
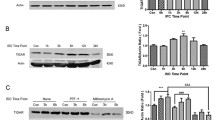
a Representative microphotographs showing immunofluorescent staining for NICD (red) and NeuN (neuronal marker, green). Note that NICD is expressed in neurons (Merge). In the Sham group, little expression of NICD was observed in the hippocampus, while Con group gradually increased NICD expression at 2 and 24 h after reperfusion, but the fluorescence intensity was weaker than that in the Pre-IR group at the same time points. However, 72 h after reperfusion, the NICD expression of Con group in remaining neurons was stronger than that in the Pre-IR group. Scale bar = 50 μm. b Isoflurane preconditioning makes notch signaling pre-activated as revealed by the presence of protein. c Representative Western blot and quantitative evaluation of NICD expression after reperfusion or preconditioning in the Con, Pre-IR, and Pre-groups. NICD expression was normalized to the expression of β-actin. The NICD expression in the Con group at 2, 24, and 72 h after reperfusion gradually increased was stronger than the Sham group (n = 6, # p < 0.05); the NICD expression in Pre-IR group at 2 h and 24 h after reperfusion was stronger than the Con group in the same time (n = 6, *p < 0.05), but at 72 h after reperfusion, the NICD expression in Pre-IR group was weaker than the Con group (n = 6, *p < 0.05). d The NICD expression in the Pre-group at 2 h was stronger than the Sham group (n = 6, *p < 0.05), but there was no significant difference between the Pre-group at 24 h and Sham. e-h Isoflurane preconditioning makes notch signaling pre-activated as revealed by the presence of mRNA. e mRNA expression of the receptor notch-1 in the Con group increased at 2 and 72 h after reperfusion, and higher than the Sham group (n = 6, # p < 0.05); notch-1 expression of the Pre-IR group at 2 h/24 h after reperfusion was higher than the Con group at the same time point (n = 6, *p < 0.05), but lower than the Con group at the time point of 72 h after reperfusion (n = 6, *p < 0.05). f The mRNA expression levels of target gene HES-1 in the Con group at 2 h/24 h after reperfusion increased gradually were higher than Sham group (n = 6, # p < 0.05). HES-1 expression of the Pre-IR group at 2 h/24 h after reperfusion was higher than the Con group at the same time point (n = 6, *p < 0.05), but at 72 h after reperfusion, the HES-1 mRNA expression of the these two groups between Pre-IR and Con groups was no significant difference compared with the Sham group. g, h The notch-1, HES-1, and NICD expression levels of Pre-group at 2 h were higher than the Sham group (n = 6, *p < 0.05), but there were no significant difference between the Pre-group at 24 h and Sham (Color figure online)
Isoflurane preconditioning induced an apparent activation of notch signaling in the hippocampus, as revealed by the protein (Fig. 5b–d) and mRNA (Fig. 5e–h) assays. Western blot analyses showed that isoflurane preconditioning significantly increased the expression of NICD protein compared with that in the Sham and Con groups at 2 and 24 h after reperfusion (p < 0.05). However, at 72 h after reperfusion, the expression of NICD protein in the Pre-IR group was weaker than that in the Con group (Fig. 5c, p < 0.05). Moreover, the NICD expression at 2 h in the Pre-group was stronger than that in the Sham group (Fig. 5d, p < 0.05), although there was no significant difference between the Pre- and Sham groups 24 h after reperfusion.
The expression levels of receptor Notch-1 and target gene HES-1 mRNA were significantly increased at 2 and 24 h after reperfusion in the Pre-IR groups compared to the Sham and Con groups (p < 0.05). However, at 72 h after reperfusion, Notch-1 mRNA expression in the Con group was higher than in the Pre-IR group (Fig. 5e, p < 0.05). At the same time point, there was no significant difference in HES-1 mRNA expression between the Pre-IR and Con groups (Fig. 5f, p < 0.05). Additionally, at 2 h, Notch-1 and HES-1 mRNA expression levels were higher in the Pre-group than in the Sham group (Fig. 5g–h, p < 0.05), whereas there was no significant difference between the Pre-and Sham groups at 24 h after reperfusion.
DAPT γ-Secretase Inhibitor Efficacy Validation
Verification of the effects of this inhibitor showed that intraperitoneal injection of DAPT (100 mg/kg) reached a peak efficacy at 3 h after injection and continued its inhibition for 24 h (Fig. 6a, b, *p < 0.05 vs. Admin. 1 and 24 h, # p < 0.05 vs. Admin. 1 h).
γ-Secretase inhibitor DAPT efficacy validation test. a Representative Western blot and quantitative evaluation of NICD expression at after injected DAPT (100 mg/kg) intraperitoneally. NICD expression was normalized to the expression of β-actin. b The NICD expression in this group at 1, 2, and 3 h after injected the inhibitor DAPT by intraperitoneal gradually decreased. Three hours of administration reached the inhibitor peak efficacy, and NICD expression was the lowest compared with the other time points (n = 3, *p < 0.05 vs. Admin. 1 and 24 h). NICD expression of 24 h after administration was the lower compared with the Admin. 1 h indicated injected DAPT (100 mg/kg) maintain the inhibitor effect to 24 h (n = 3, # p < 0.05 vs. Admin. 1 h)
Discussion
The results of this study demonstrate that isoflurane preconditioning has a neuroprotective effect in cerebral ischemia–reperfusion in mice. Isoflurane preconditioning significantly improved the TMS and reduced neuronal degeneration after cerebral ischemia–reperfusion. We found that isoflurane preconditioning induced a pre-activation of the notch signaling pathway and increased NICD expression. The neuroprotection induced by isoflurane preconditioning was reduced by both conditional RBP-J-knockout and the administration of the notch signaling inhibitor DAPT prior to cerebral ischemia–reperfusion.
According to the protocols of our previous studies and those of other groups, C57BL/6 mice were pre-treated with isoflurane for five consecutive days in the current study (Homi et al. 2003; Bekker et al. 2006; Zhang et al. 2010). The neuroprotective effects of isoflurane preconditioning were evidenced by significant improvements in neurologic function scores in the 3 days subsequent to ischemia–reperfusion injury. Furthermore, histopathologic studies showed that preconditioning remarkably decreased the necrosis and apoptosis of hippocampal neurons that was caused by ischemia–reperfusion injury.
Since volatile anesthetics are commonly used in surgical procedures, volatile anesthetic preconditioning has been speculated to be an applicable and promising method of inducing cardiac and cerebral ischemic tolerance during perioperative periods (Kitano et al. 2007; Bein 2011; Schifilliti et al. 2010). However, the mechanisms involved in the neuroprotective effects of volatile anesthetic preconditioning remain unclear and are the subject of ongoing study. As a highly conserved signaling pathway, notch plays a very important role in cell self-renewal, individual growth, and development as well as a range of physiologic and pathologic processes (Artavanis-Tsakonas et al. 1999). Notch signaling regulates the cell differentiation, maturation, and regeneration of neural progenitors (Yu et al. 2007). Previous studies have also shown that notch signaling is altered after focal cerebral ischemia and may be involved in the neuroprotective effects of ischemia preconditioning (Yang et al. 2012; Yang et al. 2011). To determine the role of the notch signaling pathway in the mediation of the neuroprotective effects of isoflurane preconditioning, we assessed changes in the expression of molecules related to the notch signaling pathway at different time points after ischemia–reperfusion. Using immunofluorescence staining, we found that NICD was mainly expressed in neurons after ischemia–reperfusion injury. NICD expression increased significantly after reperfusion in both the Pre-IR and Con groups. The expression levels of NICD were higher in the Pre-IR group than in the Con group at 2 and 24 h after reperfusion. However, the expression level of NICD in the Pre-IR group began to decrease at 72 h after reperfusion, whereas the control group maintained increased expression levels at this time point. Therefore, these results indicate that isoflurane preconditioning advanced the peak of NICD expression, which may be related to the mechanisms of ischemic tolerance induced by isoflurane preconditioning. Similar trends in the expression were found for other molecules related to notch signals, such as the notch signaling pathway ligands notch-1 and the target gene HES-1.
The function of notch signaling in cerebral ischemia–reperfusion injury remains unclear. Previous research has demonstrated that the activation of notch signaling reduces the tolerance of neurons to ischemic injury and induces an inflammatory response, which exacerbates injury (Arumugam et al. 2006). In contrast, Androutsellis-Theotokis et al. (2006) found that the activation of notch signaling promotes the growth of nerves and plays a key role in the self-repair process after nerve injury. The changes in notch signals observed in our experiment indicate that notch signaling may play different roles at different time points after ischemia–reperfusion injury. By observing changes in NICD, notch-1, and HES-1 expression, we found that isoflurane inhalation pre-activated the notch signaling pathway and advanced the peak of notch signaling to 24 h after reperfusion. To demonstrate that the pre-activation of notch signaling plays a crucial role in isoflurane preconditioning, a notch signal inhibitor was administered prior to isoflurane preconditioning in the current study. Neurologic behavioral scoring and morphologic neuron quantification showed that the neuroprotective effects of isoflurane preconditioning were partially reversed by DAPT administration prior to isoflurane inhalation. DAPT also inhibited the preconditioning-induced pre-activation of the notch signaling pathway, which reduced the tolerance to the detrimental stimulation of notch signaling. These results are consistent with those described (Wang et al. 2009), who reported that the activation of the notch pathway after ischemia–reperfusion injury inhibits the differentiation of progenitor cells and that the notch pathway ultimately participates in neuronal injury.
Our results support a neuroprotective effect of notch signaling, as isoflurane preconditioning up-regulated the notch signaling pathway before ischemia–reperfusion. However, we also found that the neuroprotective effect in the DAPT + Pre-IR group, although less robust, was greater than that in the Con group without inhibitor pre-treatment, suggesting that DAPT did not completely inhibit the protective effects of preconditioning. In addition, we observed that trends in neurologic function and morphology in neuronal notch-RBP-J knockout mice were similar to those observed after the administration of the inhibitor DAPT, suggesting that the notch signaling pathway is not the only pathway involved in the neuroprotective effects of isoflurane preconditioning. Notch signal activation contributes to post-ischemic inflammation by directly modulating the microglial innate response and establishes a role for notch signaling in modulating the microglia innate response based on these experimental results of activation of notch worsens ischemic brain damage (Wei et al. 2011). It indicates that the relationship between inflammation and notch signaling may be an important factor in neuroprotection and need to be further elucidated.
Another interesting finding of our study is that the inhibition of the notch signaling pathway had subtle protective effects on ischemia–reperfusion injury. These results are consistent with the previous findings (Arumugam et al. 2006). DAPT has a half-life of approximately 18 h (Dovey et al. 2001) and may accumulate within the body after five consecutive days of administration. This accumulation may have caused the subtle protective effects observed in the DAPT + Con group.
The present study has several limitations. First, this study did not reveal whether other molecules in the notch signal pathway are involved in this neuroprotective effect. Second, the mechanism of notch pathway was only studied in male and young mice. However, the patients at risk for perioperative stroke tend to be both genders and older. Thus, it would be interesting to determine whether the protection of isoflurane preconditioning occurs in female and aged animals when subjected to global brain ischemia and whether this neuroprotective effect is related to the activation of notch signals in future studies. Moreover, we did not measure or control for blood pressure, so changes in blood pressure may also have an impact on the neuroprotective effect, such as hypotension, which may produce a similar effect as ischemic preconditioning.
In conclusion, isoflurane preconditioning can activate and advance the peak expression of notch signaling after global cerebral ischemia–reperfusion. The neuroprotective effect of isoflurane preconditioning against cerebral ischemia is diminished by blocking the notch signal. These findings indicate that notch signals are crucial pathway for inducing ischemic tolerance by isoflurane preconditioning.
Summary
In this study, we established the relationship between preconditioning-induced neuroprotection and the notch signaling pathway. This relationship is based on the fact that isoflurane preconditioning induces neuroprotective effects after global cerebral ischemia in mice. This work establishes a foundation for future research to investigate new mechanisms and therapeutic targets.
References
Androutsellis-Theotokis, A., Leker, R. R., Soldner, F., Hoeppner, D. J., Ravin, R., Poser, S. W., et al. (2006). Notch signalling regulates stem cell numbers in vitro and in vivo. Nature, 442(7104), 823–826.
Artavanis-Tsakonas, S., Rand, M. D., & Lake, R. J. (1999). Notch signaling: Cell fate control and signal integration in development. [Research support, non-U.S. gov’t research support, U.S. gov’t, P.H.S. review]. Science, 284(5415), 770–776.
Arumugam, T. V., Chan, S. L., Jo, D. G., Yilmaz, G., Tang, S. C., Cheng, A., et al. (2006). Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. [Research support, N.I.H., intramural]. Nature Medicine, 12(6), 621–623.
Bein, B. (2011). Clinical application of the cardioprotective effects of volatile anaesthetics: PRO–get an extra benefit from a proven anaesthetic free of charge. [Research support, non-U.S. gov’t review]. European Journal of Anaesthesiology, 28(9), 620–622.
Bekker, A., Shah, R., Quartermain, D., Li, Y. S., & Blanck, T. (2006). Isoflurane preserves spatial working memory in adult mice after moderate hypoxia. Anesthesia and Analgesia, 102(4), 1134–1138.
Chen, J., Zacharek, A., Li, A., Cui, X., Roberts, C., Lu, M., et al. (2008). Atorvastatin promotes presenilin-1 expression and Notch1 activity and increases neural progenitor cell proliferation after stroke. Stroke, 39(1), 220–226.
Dovey, H. F., John, V., Anderson, J. P., Chen, L. Z., de Saint Andrieu, P., Fang, L. Y., et al. (2001). Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. Journal of Neurochemistry, 76(1), 173–181.
Fujii, M., Hara, H., Meng, W., Vonsattel, J. P., Huang, Z., & Moskowitz, M. A. (1997). Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57black/6 mice. Stroke, 28(9), 1805–1810. (discussion 1811).
Gaiano, N., & Fishell, G. (2002). The role of notch in promoting glial and neural stem cell fates. Annual Review of Neuroscience, 25, 471–490.
Homi, H. M., Mixco, J. M., Sheng, H., Grocott, H. P., Pearlstein, R. D., & Warner, D. S. (2003). Severe hypotension is not essential for isoflurane neuroprotection against forebrain ischemia in mice. Anesthesiology, 99(5), 1145–1151.
Kitagawa, K., Matsumoto, M., Tsujimoto, Y., Ohtsuki, T., Kuwabara, K., Matsushita, K., et al. (1998a). Amelioration of hippocampal neuronal damage after global ischemia by neuronal overexpression of BCL-2 in transgenic mice. Stroke, 29(12), 2616–2621.
Kitagawa, K., Matsumoto, M., Yang, G., Mabuchi, T., Yagita, Y., Hori, M., et al. (1998b). Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: Evaluation of the patency of the posterior communicating artery. Journal of Cerebral Blood Flow and Metabolism, 18(5), 570–579.
Kitano, H., Kirsch, J. R., Hurn, P. D., & Murphy, S. J. (2007). Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. [Research support, N.I.H., extramural research support, non-U.S. gov’t review]. Journal of Cerebral Blood Flow and Metabolism, 27(6), 1108–1128.
Lanz, T. A., Himes, C. S., Pallante, G., Adams, L., Yamazaki, S., Amore, B., et al. (2003). The gamma-secretase inhibitor N-[N-(3, 5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester reduces: A beta levels in vivo in plasma and cerebrospinal fluid in young (plaque-free) and aged (plaque-bearing) Tg2576 mice. Journal of Pharmacology and Experimental Therapeutics, 305(3), 864–871.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods, 25(4), 402–408.
Matchett, G. A., Allard, M. W., Martin, R. D., & Zhang, J. H. (2009). Neuroprotective effect of volatile anesthetic agents: Molecular mechanisms. Neurological Research, 31(2), 128–134.
Nagao, M., Sugimori, M., & Nakafuku, M. (2007). Cross talk between notch and growth factor/cytokine signaling pathways in neural stem cells. [Research support, non-U.S. gov’t]. Molecular and Cellular Biology, 27(11), 3982–3994.
Nakatomi, H., Kuriu, T., Okabe, S., Yamamoto, S., Hatano, O., Kawahara, N., et al. (2002). Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell, 110(4), 429–441.
Ohtaki, H., Ylostalo, J. H., Foraker, J. E., Robinson, A. P., Reger, R. L., Shioda, S., et al. (2008). Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proceedings of the National Academy of Science USA, 105(38), 14638–14643.
Oya, S., Yoshikawa, G., Takai, K., Tanaka, J. I., Higashiyama, S., Saito, N., et al. (2009). Attenuation of Notch signaling promotes the differentiation of neural progenitors into neurons in the hippocampal CA1 region after ischemic injury. [Research support, non-U.S. gov’t]. Neuroscience, 158(2), 683–692.
Presente, A., Andres, A., & Nye, J. S. (2001). Requirement of Notch in adulthood for neurological function and longevity. NeuroReport, 12(15), 3321–3325.
Redel, A., Stumpner, J., Tischer-Zeitz, T., Lange, M., Smul, T. M., Lotz, C., et al. (2009). Comparison of isoflurane-, sevoflurane-, and desflurane-induced pre- and postconditioning against myocardial infarction in mice in vivo. Experimental Biology and Medicine (Maywood), 234(10), 1186–1191.
Sang, H., Cao, L., Qiu, P., Xiong, L., Wang, R., & Yan, G. (2006). Isoflurane produces delayed preconditioning against spinal cord ischemic injury via release of free radicals in rabbits. Anesthesiology, 105(5), 953–960.
Schifilliti, D., Grasso, G., Conti, A., & Fodale, V. (2010). Anaesthetic-related neuroprotection: Intravenous or inhalational agents? [Research support, non-U.S. gov’t review]. CNS Drugs, 24(11), 893–907.
Sheng, H., Laskowitz, D. T., Mackensen, G. B., Kudo, M., Pearlstein, R. D., & Warner, D. S. (1999). Apolipoprotein E deficiency worsens outcome from global cerebral ischemia in the mouse. Stroke, 30(5), 1118–1124.
Wacker, B. K., Park, T. S., & Gidday, J. M. (2009). Hypoxic preconditioning-induced cerebral ischemic tolerance: Role of microvascular sphingosine kinase 2. Stroke, 40(10), 3342–3348.
Wang, L., Chopp, M., Zhang, R. L., Zhang, L., Letourneau, Y., Feng, Y. F., et al. (2009). The Notch pathway mediates expansion of a progenitor pool and neuronal differentiation in adult neural progenitor cells after stroke. Neuroscience, 158(4), 1356–1363.
Wang, L., Traystman, R. J., & Murphy, S. J. (2008). Inhalational anesthetics as preconditioning agents in ischemic brain. Current Opinion in Pharmacology, 8(1), 104–110.
Wei, Z., Chigurupati, S., Arumugam, T. V., Jo, D. G., Li, H., & Chan, S. L. (2011). Notch activation enhances the microglia-mediated inflammatory response associated with focal cerebral ischemia. [Research support, non-U.S. gov’t]. Stroke, 42(9), 2589–2594.
Xiong, L., Zheng, Y., Wu, M., Hou, L., Zhu, Z., Zhang, X., et al. (2003). Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesthesia and Analgesia, 96(1), 233–237.
Yang, Y., Duan, W., Jin, Z., Bi, S., Yan, J., Jin, Y., et al. (2011). New role of Notch-mediated signaling pathway in myocardial ischemic preconditioning. [Research support, non-U.S. gov’t]. Medical Hypotheses, 76(3), 427–428.
Yang, Q., Yan, W., Li, X., Hou, L., Dong, H., Wang, Q., et al. (2012). Activation of canonical Notch signaling pathway is involved in the ischemic tolerance induced by sevoflurane preconditioning in mice. Anesthesiology. doi:10.1097/ALN.0b013e31826cb469.
Yilmaz, G., & Granger, D. N. (2010). Leukocyte recruitment and ischemic brain injury. [Research support, N.I.H., extramural research support, non-U.S. gov’t]. NeuroMolecular Medicine, 12(2), 193–204.
Yoon, K., & Gaiano, N. (2005). Notch signaling in the mammalian central nervous system: Insights from mouse mutants. Nature Neuroscience, 8(6), 709–715.
Yu, Z. C., Liu, W. C., Liu, D. H., & Fan, L. (2007). Effect of Notch ligand Delta-1 on the differentiation and maturation of erythroid progenitors in humans. Zhonghua Xue Ye Xue Za Zhi, 28(6), 401–403.
Zacharek, A., Chen, J., Cui, X., Yang, Y., & Chopp, M. (2009). Simvastatin increases notch signaling activity and promotes arteriogenesis after stroke. Stroke, 40(1), 254–260.
Zhang, H. P., Yuan, L. B., Zhao, R. N., Tong, L., Ma, R., Dong, H. L., et al. (2010). Isoflurane preconditioning induces neuroprotection by attenuating ubiquitin-conjugated protein aggregation in a mouse model of transient global cerebral ischemia. [Research support, non-U.S. gov’t]. Anesthesia and Analgesia, 111(2), 506–514.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Beijing, China; Grants 30772059/30972853 and 81371510 to Hailong Dong; Grant 81171051 to Yanyan Sun; Grant 81100901 to Binxiao Su) and the National Science Foundation for Distinguished Young Scholars (Beijing, China; Grant 30725039 to Lize Xiong).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hao-peng Zhang, Yan-yan Sun, and Xiao-mei Chen have contributed equally to this work.
This work was conducted in the Department of Anesthesiology of Xijing Hospital, Fourth Military Medical University, Xi’an, Shaanxi, China.
Rights and permissions
About this article
Cite this article
Zhang, Hp., Sun, Yy., Chen, Xm. et al. The Neuroprotective Effects of Isoflurane Preconditioning in a Murine Transient Global Cerebral Ischemia–Reperfusion Model: The Role of the Notch Signaling Pathway. Neuromol Med 16, 191–204 (2014). https://doi.org/10.1007/s12017-013-8273-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-013-8273-7