Abstract
Tomosyn, a syntaxin-binding protein, is known to inhibit vesicle priming and synaptic transmission via interference with the formation of SNARE complexes. Using a lentiviral vector, we specifically overexpressed tomosyn1 in hippocampal dentate gyrus neurons in adult mice. Mice were then subjected to spatial learning and memory tasks and electrophysiological measurements from hippocampal slices. Tomosyn1-overexpression significantly impaired hippocampus-dependent spatial memory while tested in the Morris water maze. Further, tomosyn1-overexpressing mice utilize swimming strategies of lesser cognitive ability in the Morris water maze compared with control mice. Electrophysiological measurements at mossy fiber-CA3 synapses revealed impaired paired-pulse facilitation in the mossy fiber of tomosyn1-overexpressing mice. This study provides evidence for novel roles for tomosyn1 in hippocampus-dependent spatial learning and memory, potentially via decreased synaptic transmission in mossy fiber-CA3 synapses. Moreover, it provides new insight regarding the role of the hippocampal dentate gyrus and mossy fiber-CA3 synapses in swimming strategy preference, and in learning and memory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Our understanding of synaptic proteins involved in mechanisms of synaptic transmission and plasticity has progressed significantly in the last few decades (Rettig and Neher 2002; Richmond and Broadie 2002; Sudhof 2004; Morgan et al. 2005; Powell 2006). Of great relevance to these processes is the formation of the soluble N-ethylamine-sensitive factor attachment protein receptor (SNARE) complexes, a step that is highly regulated by several proteins including tomosyn, rabphilin, and complexin, and is thought to affect synaptic efficacy and plasticity (Lonart and Sudhof 2000; Rosenmund et al. 2002; Sorensen et al. 2002; Wojcik and Brose 2007;Mohrmann et al. 2010). In contrast to the myriad studies on synaptic plasticity and long-term potentiation (LTP) (Castillo et al. 1997; Soderling and Derkach 2000; Castillo et al. 2002; Richmond and Broadie 2002; Rosenmund et al. 2002; Weimer et al. 2003; Breustedt et al. 2010), only a few studies examined the interplay between presynaptic proteins and animal behavior (Migaud et al. 1998; Augustin et al. 2001; D’Adamo et al. 2004; Powell et al. 2004, 2006; Chen et al. 2011; Xu et al. 2012).
Tomosyn, also known as syntaxin-binding protein, is known to inhibit vesicle priming and synaptic transmission (Richmond and Broadie 2002; Gracheva et al. 2006; Sakisaka et al. 2008; Ashery et al. 2009; Chen et al. 2011). It is believed to function via interference with the formation of SNARE complexes (Yamamoto et al. 2010; Fujita et al. 1998; Sakisaka et al. 2008). Two mammalian tomosyn genes have been identified, encoding tomosyn1 and tomosyn2 with several distinct isoforms (Yokoyama et al. 1999; Groffen et al. 2005; Ashery et al. 2009). Tomosyn is highly enriched in mossy fiber (MF) terminals in the hilus and the CA3 stratum lucidum, but not in CA1 (Sakisaka et al. 2008; Barak et al. 2010). The unique enrichment of tomosyn1 in the MF and our interest to link between the activity of presynaptic proteins and animal behavior motivated us to explore the behavioral consequences of upregulation of tomosyn specifically in the MF of dentate gyrus (DG) granule neurons. An impairment of the MF pathway would be expected to result in loss of information flow in the hippocampus, potentially leading to impaired learning abilities. Interestingly, knocking down tomosyn levels affected odor learning in Drosophila by enhancing synaptic strength, resulting in substantially reduced late, but not short, aversive odor memory (Chen et al. 2011).
In the present study, we performed learning and memory behavioral tests in mice with DG neurons expressing elevated levels of tomosyn1 using targeted local stereotaxic injections of a lentivirus expressing tomosyn1. We provide evidence for a significant disruption in hippocampus-dependent spatial learning and memory by tomosyn1-overexpression. Moreover, electrophysiological measurements at MF-CA3 neuron synapses showed that tomosyn1 significantly reduces paired-pulse facilitation (PPF) at the MF terminals indicating an alteration in synaptic plasticity. These findings reveal a novel and important role for tomosyn1 in hippocampus-dependent learning and memory.
Results
DG- and MF-Specific Expression of YFP-Tomosyn1
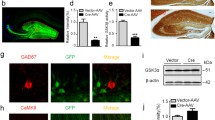
To investigate the roles of mTomosyn1 isoform (referred throughout as tomosyn1) in hippocampus-dependent learning and memory in vivo, we stereotaxically injected lentiviruses that overexpress tomosyn1 under the control of the neuron-specific promoter of human synapsin1(Kugler et al. 2003) in the DG of adult mice (see “Methods” for more details). Exogenous YFP-tomosyn1 (yellow fluorescent protein) had a similar expression to that of endogenous tomosyn in the hippocampus (Barak et al. 2010); both showed tomosyn1-expression in the granule cell bodies of the DG (GrDG), and tomosyn1 was sorted to their neurites in the molecular layer of the DG (MolDG), the hilus area, the stratum lucidum (SLu) of the MF, and the intra- and infrapyramidal (IIP) MF (Fig. 1a–c). This was further verified using immunofluorescence staining with anti-tomosyn and anti-synaptophysin antibodies; YFP-tomosyn1 colocalized with anti-tomosyn antibody in the GrDG cell bodies and MF terminals (Fig. 1d) and with anti-synaptophysin antibody in the MF terminals (Fig. 1e), suggesting that YFP-tomosyn1 was properly expressed and sorted to the MF terminals. In YFP-overexpressing mice, YFP colocalized with anti-tomosyn antibody in MF terminals (Fig. 1f), suggesting proper sorting of the transgene-derived tomosyn1 to the localization of endogenous tomosyn. There was no colocalization of YFP and the astrocytes-marker, glial fibrillary acidic protein (GFAP), in the hippocampal DG (Fig. 1g) or the MF area (Supplementary Fig. S1). Quantification of the expression levels of YFP (for YFP-tomosyn1- and YFP-overexpression) in the hippocampus demonstrated that lentiviral expression engulfed 85 % of the entire hippocampus along the rostro-caudal axis (Supplementary Fig. S2) and 95 % of the MF terminals located in the SLu (Supplementary Fig. S3) and lasted for more than 8 months (data not shown). Immunoblot analysis of hippocampal cell cultures overexpressing YFP-tomosyn1 verified similar endogenous tomosyn levels between transfected and non-transfected neurons with no breakdown products of the overexpressed protein observed and approximately fivefold overexpression of tomosyn1 in infected neurons as compared to non-transfected neurons (Supplementary Fig. S4A). No significant differences were found in the expression levels of presynaptic proteins (Synaptophysin, Munc13-1), SNARE-associated proteins (Syntaxin, SNAP25), or postsynaptic proteins (GluR2, NR2B, PSD95) while comparing their levels after YFP and YFP-tomosyn1 lentiviral infection (Supplementary Fig. S4B).
Lentiviral expression patterns after local injection into the dentate gyrus (DG) of the mouse hippocampus. (a) Fluorescence image of horizontal brain slice from a mouse expressing YFP in the hilus (Hi), mossy fibers (MFs), and dendritic tree of the granule cells (MolDG). Arrow and arrowhead: MF area enlarged in b and c, respectively. (b) YFP is highly expressed in MF terminals, in both SLu and IIP, and (c) in the CA3 area. (d) Immunofluorescence results show that YFP-tomosyn1 in GrDG cell bodies and neurites colocalizes with tomosyn antibody. (e) YFP-tomosyn1 in MF terminals colocalizes with synaptophysin antibody. (f) YFP-only in MF terminals colocalizes with tomosyn antibody. (g) The lentivirus is not expressed in astrocytes; YFP-tomosyn1 in GrDG cell bodies and neurites show no colocalization of YFP and the astrocyte-marker, glial fibrillary acidic protein (GFAP). All images were taken 4 months after injection of lentiviral vectors. Blue, nuclei marker, DAPI. MolDG molecular layer of the dentate gyrus, GrDG granular layer of the dentate gyrus, Hi hilus, IIP intra- and infrapyramidal, SLu stratum lucidum (Color figure online)
DG-Specific YFP-Tomosyn1-Overexpression Does not Affect Hippocampus-Dependent Spatial Learning and Memory in the Morris Water Maze Test while Using the Large Platform
To determine whether DG-specific tomosyn1-overexpression affects hippocampus-dependent spatial learning and memory, we utilized the well-established Morris water maze (MWM) using previously established methods (Morris et al. 1982; Okun et al. 2010) (see also Supplementary Methods). A visible platform test (see Supplementary Methods) revealed no differences in motivation, swimming, and visual abilities of YFP-tomosyn1 and YFP-injected mice (P = 0.907, Supplementary Fig. S5). When tested using a large squared platform (17 cm in length, surface area of 289 cm2), both groups displayed a similar decrease in latency to reach the hidden platform with increasing training days during the 6-day training phase (P = 0.329, Fig. 2a, Supplementary Fig. S6a, b). To test memory retention, the platform was removed, and mice were tested in probe trials at 24, 48, and 72 h after the last learning session. Both groups retained memory of the platform location 24 h after training (YFP-tomosyn1: P < 0.0001, Bonferroni P < 0.05; YFP: P = 0.0004, Bonferroni P < 0.05; Fig. 2b. Probe trials were analyzed using one-way ANOVA repeated measures, with a Bonferroni post hoc test to verify that the time mice spent in the targeted quadrant is significantly longer than in all 3 other non-targeted quadrants). After 48 h, both groups of mice retained the memory of the platform location, swimming more time in the target quadrant (YFP-tomosyn1: P = 0.2, Bonferroni P > 0.05; YFP: P = 0.0009, Bonferroni P > 0.05; Fig. 2c). At 72 h after the last training session, both groups exhibited extinction of the platform location (YFP-tomosyn1: P = 0.44, Bonferroni P > 0.05; YFP: P = 0.002, Bonferroni P > 0.05; Fig. 2d). In addition, no impairment was noted in short-term working memory (Saab et al. 2011) when measuring inter-trial extinction (P = 0.259, Supplementary Fig. S7). These results suggest that mice with overexpression of tomosyn1 display intact short-term working memory.
Selective overexpression of tomosyn1 in dentate gyrus (DG) neurons results in delayed spatial learning and memory in the MWM test using a medium- or small-sized platform but not while using a large platform. Mice with overexpressed tomosyn1 (Tomosyn, n = 14) in the DG and their control mice (YFP, n = 14) were tested in the MWM to assess their performance in hippocampus-dependent spatial learning and memory. Using a large platform (17 cm length), both groups of mice performed similarly as measured by (a) latency to reaching the platform, and (b) memory retention in a probe trial at 24 h after the last learning trial. (c) At 48 h after the last learning trial, both groups of mice spent more time in the target quadrant (UR) although not significantly, and (d) by 72 h, both groups lost retention of that location. To reveal differences in spatial navigation abilities, mice were tested using a medium-sized platform (14 cm length). (e) YFP-tomosyn1-overexpression in the DG resulted in significantly delayed spatial learning and memory as measured by longer latency to reach the platform during days 2 and 3. Following learning session of each of days 4–7 mice were tested for their memory retention in probe trials (referred here as P1-P4, see arrows in Fig. 2e). YFP-, but not YFP-tomosyn1-overexpressing mice, spent significantly more time in the target quadrant (UR) compared to all other quadrants in (f) probe trial P1 (g) probe trial P2, and in (h) probe trial P3, although a gradual improvement was shown by YFP-tomosyn1-overexpressing mice along probe trials. (i) Only in probe trial P4 YFP-tomosyn1-overexpressing mice exhibited proper memory retention. To further increase the difficulty of the task, mice were tested using a small-sized platform (12 cm length). (j) YFP-tomosyn1-overexpression in the DG resulted in significantly delayed spatial learning and memory as measured by longer latency to reaching the platform. (k) In probe trial run 24 h after the last learning trial, neither group remembered the platform location. UL upper left, UR upper right, LR lower right, LL lower left. Values are mean ± SE. # P < 0.05, ## P < 0.01, **P < 0.005, ***P < 0.0005
DG-Specific YFP-Tomosyn1-Overexpression Impairs Hippocampus-Dependent Spatial Learning and Memory in more Stringent Conditions of the Morris Water Maze Test
Because tomosyn plays a regulatory role in synaptic transmission (Sakisaka et al. 2008; Ashery et al. 2009), we hypothesized that manipulation of its levels in the hippocampus will result in subtle effects on spatial navigation in behavioral tests that require higher levels of spatial learning. To address this, we further tested mice under more stringent experimental conditions, using a medium-sized squared platform with 67 % of the large platform surface area (14 cm in length, surface area of 196 cm2). Under these conditions, mice overexpressing YFP-tomosyn1 exhibited significantly longer latency to reach the platform (F 1,26 = 10.21, P = 0.0036, Fig. 2e), significantly longer swimming distance (F 1,26 = 11.29, P = 0.0024, Supplementary Fig. S8a) and mean distance from the platform (F 1,26 = 5.832, P = 0.023, Supplementary Fig. S8b) during the second and third day of training compared to YFP-overexpressing mice. Only on day 4 did YFP-tomosyn1-overexpressing mice reach similar learning parameters as YFP-overexpressing mice. In accordance, in probe trials performed 24 h after the last learning session on day 4 (designated herein as P1; Fig 2e), mice in the YFP group spent significantly more time in the target quadrant than in all other quadrants, while mice expressing YFP-tomosyn1 showed no significant memory retention (YFP-tomosyn1: P = 0.312, Bonferroni P > 0.05; YFP: P < 0.0001, Bonferroni P < 0.05; Fig. 2f). To allow YFP-tomosyn1-overexpressing mice to better learn the location of the hidden platform, these mice and their control group were trained for an additional 4 trials after each probe trial. YFP-tomosyn1-overexpressing mice were gradually able to increase their searching time in the target quadrant during probe trials P2-P4 (Fig. 2g–i), and after 4 additional days, in probe trial P4, their performance equaled that of the YFP-overexpressing mice (YFP-tomosyn1: P < 0.0001, Bonferroni P < 0.05; YFP: P < 0.0001, Bonferroni P < 0.05; Fig. 2i).
Because increasing the difficulty of the task revealed impairments in tomosyn1-overexpressing mice, we increased the difficulty of the task by further decreasing the size of the platform to 49 % of the large platform surface area (12 cm in length, surface area of 144 cm2). YFP-tomosyn1-overexpressing mice exhibited significantly longer latency to reach the platform (F 1,26 = 11.85, P = 0.002; Fig. 2j), and significantly longer swimming distance (F 1,26 = 13.97, P = 0.0008; Supplementary Fig. S9a) and mean distance from the platform (F 1,26 = 5.204, P = 0.03; Supplementary Fig. S9b) throughout the learning period. In probe trials conducted 24 h after the last learning session on day 7, both groups displayed no significant memory retention (YFP-tomosyn1: P = 0.212, Bonferroni P > 0.05; YFP: P = 0.0002, Bonferroni P > 0.05; Fig. 2k). This might be a result of the hidden platform’s small surface area that added difficulty with properly navigate to and learn its location, as can be seen by relatively long latency even in last acquisition days (Fig. 2k) and only mild improvement in mean swimming distance from the platform (Supplementary Fig. S9b). Based on this set of experiments, we suggest that under stringent conditions, mice with elevated levels of tomosyn1 in their DG granule neurons exhibit a clear deficit in learning and memory compared to their control group. Interestingly, using a very small platform (see Supplementary methods), no group showed improvement in latency following the second day of the learning period (Supplementary Fig. S10).
DG-Specific Expression of YFP-Tomosyn1 Alters Swimming Strategy in the Morris Water Maze Test
Mice utilize a variety of swimming strategies which can be classified according to their cognitive level (Wolfer and Lipp 2000). In order to assess whether differences in swimming strategy are responsible for the delayed learning and memory of tomosyn1-overexpressing mice compared to YFP-overexpressing mice under stringent conditions, we analyzed the mice swimming strategies (Supplementary Fig. S11a, see Supplementary methods for the exact parameters used to classify strategies).
Similar swimming strategies were utilized by both groups of mice throughout the training days when the large platform (17 cm) was used (Supplementary Fig. S11b–g). Swimming strategies shifted from low to high levels of cognitive swimming strategies as training advanced, which is in accordance with reduced latency to reach the hidden platform (Fig. 2a). Interestingly, although groups performed similarly during the first day of acquisition when the medium platform (14 cm) was used (Fig. 3a, b), YFP-tomosyn1-overexpressing mice showed significantly higher levels of random swimming during days 2 and 3 (P < 0.01; Fig. 3c), and similar levels of circling strategy (P = 0.23; Fig. 3d) compared to YFP-overexpressing mice. Moreover, YFP-tomosyn1-overexpressing mice showed significantly lower levels of corrected (F 1,26 = 7.58, P = 0.01; Fig. 3e) and direct swim strategies (F 1,26 = 4.531, P = 0.043; Fig. 3f). These differences were in conjunction with the same days in which YFP-tomosyn1-overexpressing mice displayed a higher latency to reach the hidden platform (Fig. 2e).
YFP-tomosyn1-overexpressing mice use swimming strategies of a lower cognitive ability compared to YFP-overexpressing mice during learning days under more stringent conditions in the MWM test. When using the medium-sized platform (14 cm length), during learning days (a) YFP-tomosyn1-overexpressing mice used significantly higher levels of non-cognitive strategies compared to (b) YFP-overexpressing who used higher levels of high cognitive ability strategies. While YFP-tomosyn1-overexpressing mice used higher levels of (c) random swimming and (d) circling during days 2 and 3, YFP-overexpressing mice showed significantly higher levels of (e) corrected and (f) direct swim. More drastic differences were measured between (g) YFP-tomosyn1- and (h) YFP-overexpressing mice using the small-sized platform (12 cm length). YFP-tomosyn1-overexpressing mice used significantly higher levels of the low cognitive ability strategies (i) random swimming and (j) circling, while YFP-overexpressing mice used significantly higher levels of the high cognitive ability strategies (k) corrected and (l) direct swim. Graphs show percent of trials each swimming strategy was performed by mice per each day. Values are mean ± SE. # P < 0.05, ## P < 0.01, ### P < 0.001, *P < 0.05, **P < 0.005, ***P < 0.0001
Using the small platform (12 cm), the differences in swimming strategy were even more profound (Fig. 3g, h); YFP-tomosyn1-overexpressing mice showed significantly higher levels of random swimming (F 1,26 = 34.26, P < 0.0001; Fig. 3i), significantly higher levels of circling (F 1,26 = 9.32, P = 0.005; Fig. 3j), significantly lower levels of corrected swim (F 1,26 = 46.08, P < 0.0001; Fig. 3k), and significantly lower levels of direct swim (F 1,26 = 10.39, P = 0.0032; Fig. 3l). Overall, these differences suggest an explanation for the delayed learning in YFP-tomosyn1-overexpressing mice.
In order to explain the differences in memory retention between the two groups, we analyzed the swimming strategies during the different probe trials (Fig. 4a, see Supplementary methods for the exact parameters used to classify strategies). In probe trials that followed the use of the large platform (17 cm), no substantial differences in swimming strategies were observed between the groups (Supplementary Fig. S12a, b). Using the medium platform (14 cm), YFP-tomosyn1-overexpressing mice showed different swimming strategies compared with YFP-overexpressing mice. At P1 and P2, 57 % of YFP-tomosyn1-overexpressing mice used random swimming (Fig. 4b), whereas only 20 % of YFP-overexpressing mice used this strategy (Fig. 4c). The percentage of YFP-tomosyn1-overexpressing mice utilizing random swimming decreased at P3 and by P4 no mice used this strategy (Fig. 4b), consistent with a gradual improvement in this set of probe tests. YFP-overexpressing mice, however, showed higher levels of scanning and focused searching during all probe trials (Fig. 4c), consistent with their memory retention in all probe trials.
DG-restricted YFP-tomosyn1-overexpression alters swimming strategies performance during probe trials in the MWM test. YFP-tomosyn1-overexpressing mice use swimming strategies of a lower cognitive ability than YFP-overexpressing mice under more stringent conditions (using medium-sized platform) during probe trials in the MWM test. (a) Examples for swimming strategies employed by mice during probe trials in the MWM. From left to right, non-cognitive strategies (random swim, circling) are followed by strategies with cognitively higher spatial accuracy. (b) YFP-tomosyn1-overexpressing mice showed higher levels of random swim in P1 and P2 than (c) YFP-overexpressing mice, who displayed higher levels of scanning and focused searching
Using the small platform (12 cm), both groups of mice showed similar high levels of random swimming (Supplementary Fig. S12c, d). These differences could not be attributed to differences in motivation to swim, as a visible platform test showed similar performance by both experimental groups immediately following cessation of all MWM tasks (P = 0.89, Supplementary Fig. S13).
These results suggest that selective elevation of tomosyn1 levels in DG neurons delays spatial learning and memory in a hippocampus-dependent spatial task, which is associated with differences in swimming strategies.
Expression of YFP-Tomosyn1 in DG Cells Does not Affect Hippocampus-Dependent Contextual Memory and Amygdala-Dependent Learning and Memory
Both groups of mice learned normally and similarly to associate the conditioned stimulus (CS) with the unconditioned stimulus (US) and showed increase in freezing time during the association stage (P = 0.87, Supplementary Fig. S14a).
Twenty-four hours after, mice were placed in the same context in which they received the US and freezing time was measured in the absence of the CS. Both groups of mice exhibited similar freezing behavior, with high freezing percentages in the familiar context (P = 0.91, Supplementary Fig. S14b). Following a 3-h delay, mice were placed in a different context where they were analyzed for baseline freezing followed by CS, but not US, exposure, a task which is thought to be amygdala-dependent. No significant differences were observed between both groups (P = 0.34, Supplementary Fig. S14c).
Expression of YFP-Tomosyn1 in DG Cells Does not Affect Hippocampus-Dependent Recognition Memory
The novel object recognition paradigm, thought to rely on the dentate gyrus (Bevins and Besheer 2006), is a short-term (minutes–hours) non-spatial test that probes for the ability of mice to differentiate between familiar and novel objects. No difference in preference index (see details in Supplementary methods) toward the novel object was noted between both experimental groups, indicating no changes in hippocampus-dependent object recognition (P = 0.3, Supplementary Fig. S14d, e). In addition, the surgical procedures and viral transgene expression had no effects on gross motor function (Supplementary Fig. S15, S16a); body weight (Supplementary Fig. S16b); or anxiety levels, as measured using the elevated plus maze paradigm (Supplementary Fig. S16c) and the open field arena (Supplementary Fig. S16d–f).
Collectively, behavioral results suggest that YFP-tomosyn1-overexpression in DG cells specifically suppresses hippocampus-dependent spatial learning and reference memory under stringent conditions and has no effect on hippocampus-dependent contextual memory or object recognition.
Expression of YFP-Tomosyn1 in DG Cells Impairs Basal Synaptic Transmission and PPF at MF-CA3 Synapses
Next, we explored the cellular mechanism responsible for the impaired hippocampus-dependent learning and memory observed in mice with DG-targeted tomosyn1-overexpression. Tomosyn inhibits vesicle priming and synaptic transmission (Fujita et al. 1998; Yizhar et al. 2004, 2007; Gracheva et al. 2006; Sakisaka et al. 2008; Ashery et al. 2009), and we therefore hypothesized that tomosyn1-overexpression in DG reduces synaptic output of the MF synapse. Several weeks after the last behavioral test, we performed electrophysiological measurements on hippocampal slices from mice in the YFP-tomosyn1 and YFP groups (Fig. 5a) as well as on hippocampal neuronal cultures overexpressing YFP-tomosyn1. Measuring the slope of the field excitatory postsynaptic potential (fEPSP) across a range of stimulation intensities, mice expressing YFP-tomosyn1 in their DG cells exhibited significantly smaller EPSP amplitudes compared to mice expressing YFP alone (Fig. 5b, P < 0.0003). To examine short-term plasticity (STP), we measured PPF by stimulating granule cells in the GrDG and recording the ratio between two consecutive responses 40 ms apart at baseline frequency in the MF terminals. YFP-tomosyn1-overexpression resulted in significantly decreased PPF at MF-CA3 synapses (P < 0.001; 236.54 ± 4.2 % vs. 268.65 ± 4.6 %, respectively; Fig. 5c, g) compared to synapses in YFP-overexpressing mice. In accordance with the results from brain slices, autapses from primary hippocampal neurons infected with YFP-tomosyn1 also exhibited significantly decreased PPF (P = 0.02; 94.6 ± 4.2 % vs. 114 ± 6.4 %; Fig. 5d), while no effect on the amplitude of single excitatory postsynaptic currents (EPSCs) was measured compared to YFP-overexpressing autapses (P = 0.85; 575.94 ± 74.50 pA vs. 551.73 ± 115.23 pA, respectively; Supplementary Fig. S17a). We then applied high-frequency trains of action potentials to examine synaptic depression. YFP-tomosyn1-overexpression did not result in changes in either synaptic depression (Supplementary Fig. S17c) or asynchronous glutamate release (Supplementary Fig. S17d).
DG-restricted YFP-tomosyn1-overexpression impairs basal synaptic transmission and paired-pulse facilitation but not frequency facilitation or LTP at MF-CA3 synapses. Basal synaptic transmission and plasticity in the MF-CA3 synapses were measured in acute hippocampal slices derived from YFP-tomosyn1- and YFP-overexpressing mice. (a) Representative hippocampal slice expressing lentivirus with schematic representation of the stimulating and recording electrodes locations. (b) fEPSP slopes in an input–output curve versus the fiber volley of the MF-CA3 synapses in response to increasing stimulation intensities as was measured by extracellular field recordings. The curve reveals that basal synaptic transmission was significantly lower (P < 0.0003) in slices derived from YFP-tomosyn1-overexpressing (n = 6 slices from five different mice) versus YFP-overexpressing mice (n = 6 slices from five different mice). Paired-pulse facilitation (PPF) is impaired by YFP-tomosyn1-overexpression in (c) acute hippocampal slices derived from YFP-tomosyn1-overexpressing mice (n = 8 slices for YFP and n = 6 for YFP-tomosyn1, both from four different mice), and in (d) autapses from primary hippocampal neurons growing on microislands (YFP-tomosyn1, n = 11 cells; YFP, n = 9 cells). Bar graph shows the paired-pulse response ratio (2nd EPSP/1st EPSP) at 40 ms paired-pulse intervals. (e) No difference in frequency facilitation response was measured by extracellular field recordings between YFP-tomosyn1- and YFP-overexpressing mice (n = 8 slices for YFP and n = 6 for YFP-tomosyn1, both from four different mice). EPSP amplitude was plotted against stimulation frequencies. (f) No difference in long-term potentiation was measured by extracellular field recordings (n = 8 slices for YFP and n = 6 for YFP-tomosyn1, both from four different mice) between YFP-tomosyn1- and YFP-overexpressing mice. To confirm that the measured responses resulted from release from MF terminals, we blocked release from the MF terminals by application of DCG-IV (a selective agonist of presynaptic mGluR receptors on the MF terminals) at the end of each experiment. DCG-IV caused gradual and complete blockage of the responses. (g) Sample traces of PPF (left column) and LTP (right column) from YFP-overexpressing mice (upper row) and YFP-tomosyn1-overexpressing mice (lower row). PPF sample traces showing average of 15 traces; black, 0.05 Hz; blue, 0.2 Hz; red, 0.5 Hz. LTP; EPSP amplitude showing the time course of potentiation after tetanic stimulation; red, pre-LTP; blue, post-LTP, black, after DCG-IV application. GrDG granular cells of the dentate gyrus, SLu stratum lucidum. Values are mean ± SE. **P = 0.02, ***P < 0.0003 (Color figure online)
Next, we recorded postsynaptic responses of CA3 neurons following different stimulation frequencies of MF, to evaluate the presynaptic process termed “frequency-dependent facilitation.” Slices derived from YFP-tomosyn1- and YFP-overexpressing mice showed similar frequency facilitation at both 0.2 Hz (P = 0.94; 246.84 ± 30.2 % vs. 249.19 ± 16.9 %, respectively; Fig. 5e) and 0.5 Hz (P = 0.8; 419.06 ± 44 % vs. 404.72 ± 38.6 %, respectively; Fig. 5e). Similarly, LTP showed no significant differences between groups of mice 50 min after tetanus induction (P = 0.88; 131.47 ± 9.8 % and 131.28 ± 12 %; Fig. 5f, g). Input–output relationship, frequency-dependent facilitation, and LTP were evaluated using extracellular field recordings.
Overall, our electrophysiological and behavioral data suggest that high levels of tomosyn in MF terminals influence the degree of synaptic transmission and PPF in the MF-CA3 synapses and impair the performance in learning and memory tasks.
Discussion
Many studies have described the effects of hippocampal lesions and the role of the hippocampus in spatial navigation. However, few studies have employed local genetic manipulation of synaptic proteins to study their role in hippocampus-dependent behavior (Powell 2006; Xu et al. 2012). Our findings show that tomosyn1-overexpression in DG neurons of the mouse hippocampus results in a subtle but well-defined impairment in spatial learning and memory retention. Herein, we suggest that tomosyn1-overexpression causes mild impairment in hippocampus-dependent spatial navigation abilities. A decrease in the surface area of the hidden platform resulted in significantly delayed learning and memory in tomosyn1-overexpressing mice. The strategies employed by mice during spatial tasks are characterized by several indexes such as swim speed, path efficiency, duration, and body rotations and are often considered representative of the levels of cognitive resources being used for the task (Graziano et al. 2003). Our results indicate that swimming strategies employed by tomosyn1-overexpressing mice tended to be less cognitive in nature during acquisition days and probe trials, in correlation with the measured impairment in spatial navigation ability.
When first exposed to the MWM, animals typically swim in random patterns to explore their environment and to have a more accurate representation of the space they are navigating through (Day et al. 1999). As more training trials are completed, a more accurate representation of the platform’s position is encoded, allowing the mice to swim in a more direct path to the target platform while using surrounding cues. Random swimming was drastically lowered after the first day, and in parallel, direct swimming gradually increased in both groups. Nevertheless, testing with the smaller platforms, which required much higher spatial resolution navigation, determined that YFP-tomosyn1-overexpressing mice required additional trials to accurately map their surroundings in respect to the platform. The fact that both groups used all the swimming strategies to similar extents while using the large platform but not when using the smaller platforms may imply that this impairment is identifiable only while using platforms that require high spatial resolution navigation. The strong correlation between delayed learning and preferred strategy in tomosyn1-overexpressing mice prompted us to hypothesize that these fundamental differences are possibly causative. During probe trials, in which all mice swam for the same period of time, the two groups of mice used different swimming strategies indicating that swimming strategy and latency are independent variables and therefore supporting the use of such strategies as a comparable variable between the groups. Although the physiological basis for differences in swimming strategy selection is unknown, our results show an association between DG physiology and swimming strategy.
Our study adds to the body of evidence that suggests that the DG-CA3 has a prominent role in spatial reference learning and is less relevant in other hippocampus-dependent functions such as contextual fear memory and short-term object recognition memory. Object recognition memory is DG- but less CA3-dependent (Winters et al. 2010), and contextual fear memory is ventral CA1/CA3-dependent (McHugh and Tonegawa 2009); we found no evidence for a role for tomosyn1 in these hippocampus-dependent functions. Nevertheless, since exogenous tomosyn was highly expressed and active in the DG specifically, our results show that mild impairment of the DG–STP is not associated with altered function in behavioral tests which are known to be DG-dependent such as the novel object recognition test.
Physiological measurements revealed that in hippocampal slices derived from tomosyn1-overexpressing mice, impairment in the synaptic transmission process at MF-CA3 synapses is characterized by smaller EPSP amplitudes across a range of stimulation intensities. Additionally, the degree of PPF was significantly lower than in control counterparts, while no differences were observed in LTP and frequency facilitation in MF. Tomosyn inhibits vesicle priming (Yizhar et al. 2004; Baba et al. 2005; Gracheva et al. 2006) by attenuating vesicle immobilization at the plasma membrane (Yizhar and Ashery 2008; Ashery et al. 2009), a prerequisite step before fusion (Nofal et al. 2007). Therefore, high levels of tomosyn in the MF terminals possibly attenuated vesicle immobilization at the plasma membrane, resulting in vesicle priming inhibition and alterations in PPF. While reduction in P r usually leads to an increase in the paired-pulse ratio, we found that tomosyn1-overexpression decreased this ratio. It is possible that as a result of tomosyn1-overexpression, the high levels of tomosyn attenuate the priming of vesicles not only before the first stimulation, but also before the second one in the paired-pulse protocol. However, when the inter-stimulus interval between stimulations is longer, as in the case of the frequency facilitation protocol, vesicle priming overcomes tomosyn inhibition and synaptic transmission recovers to its baseline levels. This resembles a similar finding in chromaffin cells, in which inhibition imposed by tomosyn-overexpression was relieved in a late, sustained component of exocytosis (Yizhar et al. 2004, 2007), and suggests that tomosyn inhibitory function might be activity-dependent. Similarly to our electrophysiological measurements, in tomosyn1-knockout mice, impairment of the MF physiology was measured in STP only, but not in LTP (Sakisaka et al. 2008). Our data show that changes in STP, rather than LTP, might be associated with subtle behavioral alterations and are in accordance with the findings of Silva et al. (1996) who showed that STP has a significant role in information processing and learning; manipulation of a single synaptic protein (αCaMKII, SyII or SyI/II) decreases PPF and resulted in impaired learning (Silva et al. 1996). Hence, our results support the notion that small changes in STP in the mouse hippocampus have significant effects on specific hippocampus-dependent learning and memory.
One mechanism that could explain the delay in spatial learning in tomosyn1-overexpressing mice involves the impairment of DG function and CA3 place cells; the DG-CA3 MF pathway and CA3 sub-region are considered to be crucial for the encoding of novel spatial environments in the hippocampus (Lee and Kesner 2002, 2003; Hagena and Manahan-Vaughan 2011). The formation of place fields in CA3 depends on a synergy between the inputs derived from the GrDG and grid cells from the medial entorhinal cortex (MEC) (de Almeida et al. 2010). Under normal conditions, the number of place fields per CA3 place cell is ~1.1 (Leutgeb et al. 2007), in such manner that the place field expands with the ventral location of the place cell (Kjelstrup et al. 2008). Interestingly, as the dentate input becomes less significant, the number of place fields per CA3 place cell and the average place field area increases (de Almeida et al. 2010). Such alterations affect the resolution attributed to these place fields and can reduce the spatial scale as well as the resolution of the environment’s presentation.
We therefore speculate that impairment in DG-MF STP, resulting from tomosyn1-overexpression in the DG, may disrupt input to the CA3 through the MF synapse of the DG. Consequently, CA3 place cells may have additional place fields per cell with increased field area that will result in a lower spatial resolution, delaying these mice from efficiently navigating to a hidden platform with smaller surface area. Moreover, since learning was shown to sharpen and stabilize place fields (Si and Treves 2009; Savelli and Knierim 2010), it is possible that the CA3 place fields properties after few learning days in the MWM allowed proper presentation of the surrounding, enabling tomosyn1-overexpressing mice to reach the performance of their control group while using smaller hidden platforms.
In conclusion, this study presents novel evidence of the involvement of tomosyn in mouse behavior and provides new insight regarding the role of DG in swimming strategy preference and in learning and memory.
Methods
Animals
Eight-week-old C57BL/6 male mice (n = 15 per group, obtained from Jackson Laboratories, Bar Harbor, ME, USA) were housed in a controlled environment with a 12 h light/12 h dark cycle and provided with food and water ad libitum. All procedures followed National Institutes of Health guidelines and were approved by the National Institute on Aging Animal Care and Use Committee. All mice were of normal weight and size (Supplementary Fig. S16b).
Lentivirus Preparation
Rat tomosyn1 was subcloned into a third-generation lentivirus under the human synapsin promoter to ensure neuron-specific expression. Tomosyn1 is 98 % conserved between rat and mouse with amino acids transitions mainly between similar groups of amino acids. High-titer lentiviruses were produced for both viruses used in this work according to Naldini et al. (1996). Briefly, four vectors encoding pLentihSyn-YFP-tomosyn1 (for example), REV, MDL, and pvSVG were transfected by Lipofectamine LTX (Invitrogen) into HEK293T cells grown on plastic dishes coated with polyethylenimine (PEI; Sigma, St. Louis, MI, USA) (PEI was used to ensure tight adherence of the cells to the plastic). Forty-four hours after transfection, the medium containing the viruses was centrifuged at 1,100 rpm and filtered through a 0.45 μm filter to remove cell debris, then centrifuged at 42,152 rcf in an ultracentrafuge at 4 °C for 2 h. The supernatant was discarded and 100 μl of phosphate buffered saline (PBS) was used to gently resuspend the virus. Aliquots containing 5 μl PBS with viruses were quickly frozen and stored at −80 °C. To verify the degree and cellular localization of tomosyn1 gene expression and to facilitate identification of the brain areas expressing those for the electrophysiological measurements, we subcloned the fluorescent marker YFP fused to the N terminus of tomosyn1. The possible influence of the vector’s properties on the function of tomosyn1 was previously published by our group (Yizhar et al. 2004) and showed indistinguishable results and similar physiology of tomosyn and tomosyn fused to GFP.
Local Transcranial Injection of YFP-Tomosyn1 and YFP Lentiviruses
Concentrated viruses were injected during a single-short stereotaxic surgery taken before mice were subjected to behavioral tests and electrophysiological measurements. Briefly, mice were maintained on a heating pad to ensure constant body temperature, anesthetized with 4 % isoflurane (maintained on 1.5 % isoflurane anesthesia), and their head was fixed in a stereotaxic apparatus, where a longitudinal midsagittal cut with a sterile scalpel exposed their skull. To ensure maximal expression of the transgene in the hippocampal DG, lentiviruses were bilaterally injected into the dorsal and medial hippocampus. Injections were taken to the following coordinates, representing distances in mm from skull bregma: for dorsal hippocampus; anterior-posterior −2.1, mediolateral ± 1.35, and dorsoventral −2.1; for medial hippocampus; anterior-posterior −3.1, mediolateral ± 2.3, and dorsoventral −2.5. Injections were performed through a small hole in the skull drilled along the anterior-posterior and mediolateral coordinates using a fine drill. Concentrated virus (1 μl) was injected in a constant flow of 0.2 μl/min followed by 3 min to allow the virus to be absorbed by the tissue. Injections were performed with an Exmire microsyringe (Ito Corp., Fuji, Japan). Following the injections, the skin was sutured, and mice were placed in a separate cage for 3 h to recover.
Western Blot
Hippocampi dissected from newborn mice (P0-P1) were incubated for 15 min in a solution of papain (100 U) in Ca2+/Mg2+-free Hank’s balanced salt solution (HBSS) (Biological Industries, Beit HaEmek, Israel). Hippocampi were then mechanically dissociated, and cells were plated onto the coverslips and maintained at 37 °C in Neurobasal medium containing 2 mM B-27 supplement (Invitrogen), 1 % l-glutamine, 0.5 % penicillin/streptomycin for 10–14 days in vitro (DIV) and were then infected with the YFP-tomosyn1 lentiviral vector. Seven days after, infection lysates of infected and non-infected cultured cells were obtained by washing cells in ice-cold PBS and resuspending them in a modified RIPA lysis buffer (150 mM sodium chloride, 50 mM Tris–HCl pH 7.4, 1 mM EDTA, 1 % Triton-X100, 1 % sodium deoxicholic acid, 0.1 % sodium dodecylsulfate, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride). Protein concentration was determined using the Bradford reagent (BioRad, Hercules, Canada). Protein samples (50 μg) were prepared and electrophoresed on a 12 % Bis–Tris gel. Gels were then transferred to a nitrocellulose membrane. Membranes were blocked in 5 % non-fat milk for 1 h at room temperature (RT), followed by an overnight incubation at 4 °C with primary antibodies against: tomosyn [1:2,000, home-made affinity-purified rabbit anti-tomosyn polyclonal, as was described in (Hatsuzawa et al. 2003)], psd95 (1:1,000, rabbit anti-psd95, Millipore, Billerica, MA, USA), snap25 (1:2,000, mouse anti-snap25, Synaptic Systems, Goettingen, Germany), syntaxin (1:1,000, mouse anti-syntaxin, Synaptic Systems, Goettingen, Germany), GluR2 (1:1,000, mouse anti-Glur2, Neuro Mab, UC Davis/NIH NeuroMab Facility, Davis, CA, USA), NR2B (1:400, rabbit anti-NR2B, Millipore, Billerica, MA, USA), munc13-1 (1:2,000, rabbit anti-munc13-1, Synaptic Systems, Goettingen, Germany) synaptophysin (1:10,000, rabbit anti-synaptophysin, Santa Cruz Biotechnology, Santa Cruz, CA, USA), beta tubulin (1:1,000, rabbit anti-beta tubulin, abcam, Cambridge, MA, USA), and clathrin (1:1,000, mouse anti-clathrin, heavy chain, Thermo Fisher Scientific, Rockford, IL, USA), in 0.05 % sodium azide and 1 % bovine serum albumin (BSA) in TBS with 0.1 % Tween-20 at RT for 1 h. Protein bands were visualized using a chemiluminescence detection kit (Amersham Biosciences, Piscataway, NJ, USA).
Immunofluorescence Staining and Confocal Microscopy
Methods for immunofluorescence staining and confocal microscopy are detailed in Supplementary methods.
Behavioral Testing
Methods for MWM, rotarod, open field exploration, novel object recognition, elevated plus maze and fear conditioning are detailed in Supplementary methods.
Electrophysiological Measurements
Methods for neuronal cell cultures, slices preparation, electrophysiological measurements, microisland cultures and lentiviral infection, and autapse electrophysiological measurements are detailed in Supplementary methods.
Statistical Analysis
Analyses of the MWM (except probe trials), fear conditioning, rotarod, and input–output curves experiments were performed using two-way ANOVA with repeated measures. Probe trials in the MWM test were analyzed using one-way ANOVA repeated measures, with a Bonferroni post hoc test to verify that the time mice spent in the targeted quadrant is significantly longer than in all 3 other non-targeted quadrants. All other tests in this study were analyzed using unpaired two-tailed Student’s t test. Results are expressed as mean ± SEM.
References
Ashery, U., Bielopolski, N., et al. (2009). Friends and foes in synaptic transmission: The role of tomosyn in vesicle priming. Trends in Neuroscience, 32(5), 275–282.
Augustin, I., Korte, S., et al. (2001). The cerebellum-specific Munc13 isoform Munc13-3 regulates cerebellar synaptic transmission and motor learning in mice. The Journal of Neuroscience, 21(1), 10–17.
Baba, T., Sakisaka, T., et al. (2005). PKA-catalyzed phosphorylation of tomosyn and its implication in Ca2 + -dependent exocytosis of neurotransmitter. The Journal of cell biology, 170(7), 1113–1125.
Barak, B., Williams, A., et al. (2010). Tomosyn expression pattern in the mouse hippocampus suggests both presynaptic and postsynaptic functions. Frontiers in Neuroanatomy, 4, 149.
Bevins, R. A., & Besheer, J. (2006). Object recognition in rats and mice: A one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nature Protocols, 1(3), 1306–1311.
Breustedt, J., Gundlfinger, A., et al. (2010). Munc13-2 differentially affects hippocampal synaptic transmission and plasticity. Cerebral Cortex, 20(5), 1109–1120.
Castillo, P. E., Janz, R., et al. (1997). Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature, 388(6642), 590–593.
Castillo, P. E., Schoch, S., et al. (2002). RIM1alpha is required for presynaptic long-term potentiation. Nature, 415(6869), 327–330.
Chen, K., Richlitzki, A., et al. (2011). Tomosyn-dependent regulation of synaptic transmission is required for a late phase of associative odor memory. Proceedings of the National Academy of Sciences of the United States of America, 108(45), 18482–18487.
D’Adamo, P., Wolfer, D. P., et al. (2004). Mice deficient for the synaptic vesicle protein Rab3a show impaired spatial reversal learning and increased explorative activity but none of the behavioral changes shown by mice deficient for the Rab3a regulator Gdi1. The European Journal of Neuroscience, 19(7), 1895–1905.
Day, L. B., Weisand, M., et al. (1999). The hippocampus is not necessary for a place response but may be necessary for pliancy. Behavioral Neuroscience, 113(5), 914–924.
de Almeida, L., Idiart, M., et al. (2010). The single place fields of CA3 cells: A two-stage transformation from grid cells. Hippocampus, 22(2), 200–208.
Fujita, Y., Shirataki, H., et al. (1998). Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron, 20(5), 905–915.
Gracheva, E. O., Burdina, A. O., et al. (2006). Tomosyn inhibits synaptic vesicle priming in caenorhabditis elegans. PLoS Biology, 4(8), e261.
Graziano, A., Petrosini, L., et al. (2003). Automatic recognition of explorative strategies in the Morris water maze. Journal of Neuroscience Methods, 130(1), 33–44.
Groffen, A. J., Jacobsen, L., et al. (2005). Two distinct genes drive expression of seven tomosyn isoforms in the mammalian brain, sharing a conserved structure with a unique variable domain. Journal of Neurochemistry, 92(3), 554–568.
Hagena, H., & Manahan-Vaughan, D. (2011). Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cerebral Cortex, 21(11), 2442–2449.
Hatsuzawa, K., Lang, T., et al. (2003). The R-SNARE motif of tomosyn forms SNARE core complexes with syntaxin 1 and SNAP-25 and down-regulates exocytosis. The Journal of Biological Chemistry, 278(33), 31159–31166.
Kjelstrup, K. B., Solstad, T., et al. (2008). Finite scale of spatial representation in the hippocampus. Science, 321(5885), 140–143.
Kugler, S., Kilic, E., et al. (2003). Human synapsin 1 gene promoter confers highly neuron-specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Therapy, 10(4), 337–347.
Lee, I., & Kesner, R. P. (2002). Differential contribution of NMDA receptors in hippocampal subregions to spatial working memory. Nature Neuroscience, 5(2), 162–168.
Lee, I., & Kesner, R. P. (2003). Differential roles of dorsal hippocampal subregions in spatial working memory with short versus intermediate delay. Behavioral Neuroscience, 117(5), 1044–1053.
Leutgeb, J. K., Leutgeb, S., et al. (2007). Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science, 315(5814), 961–966.
Lonart, G., & Sudhof, T. C. (2000). Assembly of SNARE core complexes prior to neurotransmitter release sets the readily releasable pool of synaptic vesicles. The Journal of Biological Chemistry, 275(36), 27703–27707.
McHugh, T. J., & Tonegawa, S. (2009). CA3 NMDA receptors are required for the rapid formation of a salient contextual representation. Hippocampus, 19(12), 1153–1158.
Migaud, M., Charlesworth, P., et al. (1998). Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature, 396(6710), 433–439.
Mohrmann, R., de Wit, H., et al. (2010). Fast vesicle fusion in living cells requires at least three SNARE complexes. Science, 330(6003), 502–505.
Morgan, A., Burgoyne, R. D., et al. (2005). Regulation of exocytosis by protein kinase C. Biochemical Society Transactions, 33(Pt 6), 1341–1344.
Morris, R. G., Garrud, P., et al. (1982). Place navigation impaired in rats with hippocampal lesions. Nature, 297(5868), 681–683.
Naldini, L., Blomer, U., et al. (1996). Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proceedings of the National Academy of Sciences of the United States of America, 93(21), 11382–11388.
Nofal, S., Becherer, U., et al. (2007). Primed vesicles can be distinguished from docked vesicles by analyzing their mobility. The Journal of Neuroscience, 27(6), 1386–1395.
Okun, E., Griffioen, K., et al. (2010). Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. Proceedings of the National Academy of Sciences of the United States of America, 107(35), 15625–15630.
Powell, C. M. (2006). Gene targeting of presynaptic proteins in synaptic plasticity and memory: Across the great divide. Neurobiology of Learning and Memory, 85(1), 2–15.
Powell, C. M., Schoch, S., et al. (2004). The presynaptic active zone protein RIM1alpha is critical for normal learning and memory. Neuron, 42(1), 143–153.
Rettig, J., & Neher, E. (2002). Emerging roles of presynaptic proteins in Ca ++-triggered exocytosis. Science, 298(5594), 781–785.
Richmond, J. E., & Broadie, K. S. (2002). The synaptic vesicle cycle: Exocytosis and endocytosis in Drosophila and C. elegans. Current Opinion in Neurobiology, 12(5), 499–507.
Rosenmund, C., Sigler, A., et al. (2002). Differential control of vesicle priming and short-term plasticity by Munc13 isoforms. Neuron, 33(3), 411–424.
Saab, B. J., Saab, A. M., et al. (2011). Statistical and theoretical considerations for the platform re-location water maze. Journal of Neuroscience Methods, 198(1), 44–52.
Sakisaka, T., Yamamoto, Y., et al. (2008). Dual inhibition of SNARE complex formation by tomosyn ensures controlled neurotransmitter release. The Journal of Cell Biology, 183(2), 323–337.
Savelli, F., & Knierim, J. J. (2010). Hebbian analysis of the transformation of medial entorhinal grid-cell inputs to hippocampal place fields. Journal of Neurophysiology, 103(6), 3167–3183.
Si, B., & Treves, A. (2009). The role of competitive learning in the generation of DG fields from EC inputs. Cognitive Neurodynamics, 3(2), 177–187.
Silva, A. J., Rosahl, T. W., et al. (1996). Impaired learning in mice with abnormal short-lived plasticity. Current Biology, 6(11), 1509–1518.
Soderling, T. R., & Derkach, V. A. (2000). Postsynaptic protein phosphorylation and LTP. Trends in Neurosciences, 23(2), 75–80.
Sorensen, J. B., Matti, U., et al. (2002). The SNARE protein SNAP-25 is linked to fast calcium triggering of exocytosis. Proceedings of the National Academy of Sciences of the United States of America, 99(3), 1627–1632.
Sudhof, T. C. (2004). The synaptic vesicle cycle. Annual Review of Neuroscience, 27, 509–547.
Weimer, R. M., Richmond, J. E., et al. (2003). Defects in synaptic vesicle docking in unc-18 mutants. Nature Neuroscience, 6(10), 1023–1030.
Winters, B. D., Saksida, L. M., et al. (2010). Implications of animal object memory research for human amnesia. Neuropsychologia, 48(8), 2251–2261.
Wojcik, S. M., & Brose, N. (2007). Regulation of membrane fusion in synaptic excitation-secretion coupling: speed and accuracy matter. Neuron, 55(1), 11–24.
Wolfer, D. P., & Lipp, H. P. (2000). Dissecting the behaviour of transgenic mice: Is it the mutation, the genetic background, or the environment? Experimental Physiology, 85(6), 627–634.
Xu, W., Morishita, W., et al. (2012). Distinct neuronal coding schemes in memory revealed by selective erasure of fast synchronous synaptic transmission. Neuron, 73(5), 990–1001.
Yamamoto, Y., Fujikura, K., et al. (2010). The tail domain of tomosyn controls membrane fusion through tomosyn displacement by VAMP2. Biochemical and Biophysical Research Communications 399(1): 24–30.
Yizhar, O., & Ashery, U. (2008). Modulating vesicle priming reveals that vesicle immobilization is necessary but not sufficient for fusion-competence. PLoS ONE, 3(7), e2694.
Yizhar, O., Lipstein, N., et al. (2007). Multiple functional domains are involved in tomosyn regulation of exocytosis. Journal of Neurochemistry, 103(2), 604–616.
Yizhar, O., Matti, U., et al. (2004). Tomosyn inhibits priming of large dense-core vesicles in a calcium-dependent manner. Proceedings of the National Academy of Sciences of the United States of America, 101(8), 2578–2583.
Yokoyama, S., Shirataki, H., et al. (1999). Three splicing variants of tomosyn and identification of their syntaxin-binding region. Biochemical and Biophysical Research Communications, 256(1), 218–222.
Acknowledgments
We would like to thank Prof. Pablo Castillo for comments on the manuscript, Dr. Peisu Zhang for technical help, and Dr. Sarah Rothman for editing the manuscript. This research was supported, in part, by the Intramural Research Program of the National Institute on Aging, NIH, the Israel Science Foundation (Grant no. 1211/07 and 730/11; U.A.) and the BSF (Grant no. 2009279; U.A.), the National Institutes of Health (RO1 NS053978; E.S. and U.A.) and by travel grant from Boehringer Ingelheim Fonds (awarded to Boaz Barak).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Boaz Barak and Eitan Okun: contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barak, B., Okun, E., Ben-Simon, Y. et al. Neuron-Specific Expression of Tomosyn1 in the Mouse Hippocampal Dentate Gyrus Impairs Spatial Learning and Memory. Neuromol Med 15, 351–363 (2013). https://doi.org/10.1007/s12017-013-8223-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-013-8223-4