Abstract
Anti-Jo-1 is the most frequently detectable antibody in the antisynthetase syndrome (ASSD), an autoimmune disease characterized by the occurrence of arthritis, myositis, and interstitial lung disease (ILD). Recently, we organized an international collaborative group called American and European NEtwork of Antisynthetase Syndrome (AENEAS) for the study of this rare and fascinating disease. The group collected and published one of the largest series of ASSD patients ever described and with one of the longer follow-up ever reported. The number of participating centers is steadily increasing, as well as the available cohort. In the first paper, we showed that arthritis, myositis, and ILD may be frequently the only feature at disease onset, raising problems to reach a correct diagnosis of this syndrome. Nevertheless, we first observed that the ex novo appearance of further manifestations is common during the follow-up, strengthening the importance of a correct diagnosis. In our cohort, the 24 % of the 243 patients up to now collected had isolated arthritis as a presenting feature. These patients represent the most intriguing group in terms of differential diagnosis and clinical time course. Furthermore, data on this aspect are scanty, the reason that lead us to evaluate these aspects in our cohort of patients, reviewing also available literature. In fact, the most relevant aspect is that ASSD is rarely suspected in this setting of patients, in particular in case of poliarticular involvement, positive rheumatoid factor (RF), or anti-cyclic citrullinated peptide antibodies (ACPA) or evidence of joint erosions at plain radiographs. These findings were not rare in our cohort, and they have been also described in other series. Furthermore, manifestations such as Raynaud’s phenomenon, mechanic’s hands, and fever that may lead to the suspect of ASSD are observed only in a third of cases. If we consider the high rate of clinical picture progression in these patients, we feel that ASSD should be carefully considered in all patients presenting with isolated arthritis, even in those with erosive, RF, and ACPA-positive arthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antisynthetase syndrome (ASSD) is an autoimmune disease characterized by the occurrence of antibodies directed against different aminoacyl-tRNA synthetase [1] within the spectrum of different forms of myositis [2]. Indeed, the association of inflammatory myositis with serum autoantibodies fulfills the criteria of autoimmunity with numerous modulating factors acting on B cells [3, 4]. Among associated antibodies [5, 6], the most frequent is the anti-Jo-1, that is a rare antibody [7] directed against the histidyl-tRNA synthetase, whereas other antisynthetase specificities (e.g., anti-PL-7, PL-12, EJ, KS, OJ, YRS, and Zo) are less frequently identified. From the clinical point of view, manifestations such as arthritis, myositis, and interstitial lung disease (ILD) are observed in up to 90 % of cases, whereas features such as Raynaud’s phenomenon (RP), fever, and mechanic’s hands (MH) are less frequently reported [8–11]. Although the typical disease onset is characterized by the concomitant occurrence of arthritis, myositis, and ILD [1], with or without RP, fever, or MH, patients presenting with only arthritis, or myositis, or ILD, have been reported [10, 12, 13]. On the other hand, the appearance of lacking findings during the follow-up is said to be possible [12]. Recently, we have shown that an isolated arthritis, isolated myositis, or ILD may occur in up to 50 % of cases of ASSD and that the ex novo appearance of further manifestations during the follow-up is really common in these patients [11]. Our data clearly indicate that an underlying ASSD should be considered in all patients presenting with arthritis, myositis, and ILD, even when they are isolated manifestations. Nowadays, the possibility of having an ASSD is well established in individuals presenting with myositis and ILD [14]. However, this is not well established in patients presenting with isolated arthritis. Despite the fact that positivity for IgM-rheumatoid factor (RF), anti-cyclic citrullinated peptide antibodies (ACPA), and joint erosions have been reported in ASSD patients [15–17], the presence of these features more likely raise the suspicion of rheumatoid arthritis (RA) than that of ASSD. In the present paper, we analyzed the experience of rheumatology centers included in the American and European NEtwork of Antisynthease Syndrome (AENEAS) collaborative group and reviewed available literature data.
Previous Reports
Arthritis, myositis, and ILD represent the typical clinical triad of ASSD, reported in up to 90 % of cases [1, 18, 19]. Despite the high frequency of the manifestation, for several years, no studies have been specifically aimed to arthritis pattern evaluation in ASSD. In one of the first review on the syndrome, Imbert-Masseau et al. [1] stated that joint involvement could range from simple polyarthralgias to destructive polyarthritis involving hands, wrists, elbows, and knees, even if the occurrence of joint erosions at plain X-rays has been considered a rare disease’s finding for several years [1, 20]. On the other hand, some early reports suggested the possibility that RF test could be occasionally positive in some ASSD patients, with subsequent troubles in differential diagnosis with RA [21, 22]. In 2009, Labrador-Horrillo et al. [23] first analyzed the meaning of ACPA in idiopathic inflammatory myopathies. In the included cohort of 90 patients, ACPA were positive in 12 cases (13.3 %) accounting for all patients, whereas the prevalence rate of ACPA was slightly reduced (8 %) when considering the 25 patients positive for antisynthease antibodies. Interestingly, none of the 90 included patients met the ACR classification criteria for RA [24], at disease onset or during the follow-up. On this basis, authors stated that in myositis ACPA may be considered as false-positive results and without clinical significance. Nagashima et al. [25] in 2009 reported two anti-Jo-1 and ACPA-positive patients without myositis but with destructive arthropathy, suggesting the occurrence of a new myositis subset, overlapping with RA and lacking of muscle involvement. In contrast with Labrador-Horrillo [23] and Nagashima [25], in 2010 [13], in a single center study, we described eight anti-Jo-1-positive patients with concomitant arthritis and biopsy-proven myositis. It is important to observe that some patients were positive for RF and/or ACPA or presented an erosive arthritis, in all cases fulfilling the ACR classification criteria for RA [24]. Following our paper, Nagashima et al. [25] suggested that the association between ASSD and RA is not so rare and that the small percentage of patients who have polymiositis or dermatomyositis overlapping with RA is also anti-Jo-1 antibody positive. In order to support this hypothesis, authors analyzed several case reports from literature [19, 20, 26, 27] and resumed their personal experience on the topic [15, 25, 28]. On this basis, it is clear how in few years, the way to consider arthritis and arthritis-related antibodies in ASSD patients, in particular in those anti-Jo-1 positive, has gradually changed overtime. According to this change of perspective, recently, Lefèvre et al. [29] in a retrospective study involving several French centers referring to Club Rhumatismes et Inflammation evidenced that ASSD may be revealed by a seronegative polyarthritis. Authors first performed a single center analysis, identifying 12 ASSD patients first presenting with an isolated polyarthritis, without muscular or respiratory symptoms. Subsequently, with the support of other centers, the final number of included patients was 40. If the prevalence of this peculiar subset of ASSD was 27 % in the single center, the prevalence in all participating centers was not specified, because the total number of ASSD patients screened was lacking. Positivity for RF or ACPA and the overlap with other connective tissue diseases were between the exclusion criteria, because considered possible confounding factors, suggesting the occurrence of other diseases accounting for articular manifestations. Manifestations such as RP, MH, and other cutaneous findings of dermatomyositis were not the exclusion criteria, as well as the occurrence of anti-Ro 52 kDA antibodies, observed in up to 40 % of cases. The pattern of joint involvement was mainly characterized by the occurrence of distal symmetrical polyarthralgia with at least one synovitis or distal polyarthritis involving interphalangeal, metacarpophalangeal joints and wrists. Only few patients (6 %) had joint erosions at plain X-rays of hands and feet. Regarding extra-articular features of ASSD, RP was present in 13 patients (32.5 %) at disease diagnosis and in seven cases (17.5 %) preceded polyarthritis onset. For authors, RP could be considered as a red flag for the occurrence of ASSD in patients with seronegative and apparently isolated polyarthritis.
Soon after this study, the French group published another paper [30] suggesting that in ASSD, the positivity for ACPA is associated with the occurrence of a severe and erosive arthritis. From the clinical point of view, the occurrence of ILD, muscle, dermatological, or articular involvements was mandatory for patients’ inclusion. The occurrence of an isolated arthritis was one of the exclusion criteria identified by authors. From the starting cohort of 284 ASSD patients, 17 (6 %) were ACPA positive. These patients were compared with 34 unselected ASSD patients matched for age, sex, and follow-up length. Joint involvement was statistically associated with ACPA positivity: in fact, the 100 % of 17 ACPA-positive patients had arthritis, with respect to 14 (41 %) ACPA-negative ones. Furthermore, ACPA-positive ASSD had not only more swollen, tender, and damaged joints at plain X-rays than ACPA-negative ASSD but also they meet the 2010 ACR classification criteria for RA [31] in all cases, with respect to 56 % of ACPA-negative patients. According to these results, authors suggested that ACPA positivity in ASSD patients may be considered as a marker of overlap with RA.
The AENEAS Collaborative Group
The AENEAS collaborative group involves 25 rheumatology centers from Italy (15), Spain (6), Germany (3), and the USA (1). In order to better understand the project and evaluate the differences with other casuistries up to now published, it is important to highlight the asked criteria for cohort entry. Patients were eligible if they had anti-Jo-1 testing positive in at least two determinations along with an isolated arthritis (joint swelling required) as the presenting manifestation of ASSD. At least one anti-Jo-1 positivity should be obtained in the leading reference/tertiary center. Patients included in the study should have not had any clinical symptoms or instrumental and laboratory signs of pulmonary and muscle involvement before arthritis appearance and for at least 3 months following the arthritis onset. The occurrence of other ASSD manifestations such as RP, MH, or fever was not an exclusion criterion. Disease duration was calculated from arthritis onset. Other autoantibodies such as anti-Ro, IgM RF, or ACPA were considered to be positive if they were confirmed in at least two different determinations, also in this case with one confirmation in the leading reference/tertiary center. Patients signed the informed consent that was approved by each local institutional ethics committee according to local and national rules. Type and characteristics of clinical features at onset and during the follow-up were retrospectively collected. Arthritis occurrence and its presentation pattern (e.g., symmetrical polyarthritis, oligoarticular, or asymmetrical arthritis) were assessed clinically, as well as the occurrence of fever, MH, and RP and other potentially relevant clinical findings. Patients were assessed and then followed up for joint erosions, ILD, and myositis onset. For the assessment of joint erosions, patients underwent plain radiographs of the joints. ILD occurrence was defined instrumentally by the occurrence of a restrictive pulmonary function test pattern (FVC ≤ 80 %, FEV1/FVC ≥ 70 %, decreased or normal FEV1, and/or <20 % reduction in DLCO) and/or by signs of alveolitis/fibrosis on chest high-resolution computed tomography (HRCT). The presentation of ILD was defined as acute/subacute when dyspnoea began acutely or progressed rapidly (within 4–6 weeks of symptom onset), chronic when dyspnoea began insidiously and progressed slowly, and asymptomatic when lung involvement was only instrumental without clinical correlates. Screening for myositis consisted of the regular monitoring of creatine phosphokinase (CPK) and/or aldolase and/or lactate dehydrogenase (LDH). Patients with muscle enzyme elevation and the presence of typical electromyography alterations and/or compatible muscle biopsy findings were considered as having muscle involvement. Myositis onset was defined as classic (muscle strength deficit) or hypomyopathic (instrumental/laboratory evidence of muscle impairment without strength deficit) patterns. For practical purposes, patients developing arthritis, ILD, and myositis were defined as having complete ASSD, while the remaining patients as having incomplete ASSD. Anti-Jo-1 positivity and other additional anti-extractable nuclear antigen specificities were tested and confirmed at least once by well-validated methods, as well as IgM-RF and ACPA (Appendix 1), in the leading reference/tertiary center. In our casuistry, descriptive data were reported or considered as absolute and relative frequencies, mean and standard deviation, median and interquartile range (IQR) based on the type of the variable distribution. Comparison between groups was firstly tested by chi-squared test, t test or Mann-Whitney test, based on the variable type and distribution. Given the retrospective design, the association between clinical variables at disease onset and evolution toward myositis, ILD, or complete forms was evaluated by univariable and multivariable logistic models. Analyses were performed using STATA software package (2009, release 11; StataCorp, TX, USA).
From the initial cohort of 243 anti-Jo-1-positive ASSD, we identified 58 patients (24 % of cases, 45 females, 13 males) first presenting with isolated arthritis. Baseline characteristics, subsequent evolution, and statistical results according to arthritis presentation pattern are summarized in Table 1. Arthritis was poly-articular in 41 cases (71 %) and oligo-articular/asymmetrical in 17 (29 %). IgM-RF was positive in 22 out of the 57 (39 %), ACPA in 13 out of 47 (28 %) patients assessed. Anti-Ro positivity was observed in 27 patients (47 %). At the onset, 5 patients (9 %) had fever, 15 RP (26 %), and 8 MH (14 %). The onset of these manifestations was concomitant to arthritis in all cases. The majority of patients (38, 65.5 %) had arthritis as the only presenting clinical ASSD-related manifestation, and 22 (38 %) were also anti-Ro negative. Forty-one patients (71 %) met the 1987 revised ACR classification criteria for RA [19], in particular all patients presenting with symmetrical polyarthritis (p < 0.001).
Median age at disease onset was 54 years (IQR, 43–62). We did not observe any statistically significant differences in the onset age according to the arthritis presentation pattern (p = 0.513). Median diagnostic delay was 12.5 months (IQR, 6–37). Nor did we observe any statistically significant differences in the diagnostic delay according to the arthritis presentation pattern (p = 0.864).
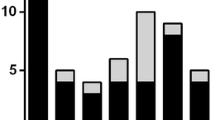
At the end of a median follow-up of 84 months (interquartile range 58–151 months), 20 of the 57 patients had plain radiographs of the hands and feet (35 %) which demonstrated the presence of an erosive disease. No clinical, laboratory, and clinical/laboratory combined variables were statistically associated with the occurrence of joint erosions (Tables 1 and 2). During the follow-up, only five patients (9 %) did not present myositis or ILD (median follow-up 71 months, IQR 36–102, with one patient followed for 5 months). All these patients had ACPA-negative symmetrical polyarthritis, in one case IgM-RF and anti-Ro positive, in one anti-Ro positive, and RP in two cases. Conversely, 53 patients (91 %) developed additional manifestations, even when RF (21 cases, 40 % of subset), ACPA (13 cases, 30 %) or both (10 cases, 23 %) were positive. In particular, 38 patients (65.5 %) developed an ex novo myositis, with a presentation pattern that was mainly classic (24 cases, 63 % of myositis) and less commonly hypomyopathic (14 cases, 37 %). Myositis occurred in median 17 months after arthritis (IQR 8.5–37). An ex novo ILD appeared in 48 cases (83 %). The pattern of presentation was acute/sub-acute in 13 cases (27 % of cases with ILD), chronic in 21 (55 %), and asymptomatic in 13 (34 %). Asymptomatic ILD onset was observed more commonly in oligoarticular/asymmetrical arthritis and acute/sub-acute or chronic onset more commonly in polyarticular and symmetrical arthritis (p = 0.045). ILD onset type was not registered in one patient. ILD occurred in median 14 months after arthritis onset (IQR 7.5–53). The delay between the appearance of myositis and ILD was not statistically significant (p = 0.650). We observed the appearance of both ILD and myositis in 33 patients (57 %). In 12 cases (21 %), the progression to a complete form of ASSD occurred in two different stages. In median, the overall first progression (40 ILD, 34 myositis, in 20 cases both manifestations) was observed 14 months after arthritis onset (IQR 8–42 months), the second progression (8 ILD and 4 myositis) 26 months (IQR 12–65 months) after the appearance of the second manifestation. From the statistical point of view, no variables were associated with the occurrence of myositis and ILD (Table 3). In Fig. 1, we reported the prevalence over time of myositis, ILD, and both myositis and ILD according to the number of patients followed. The follow-up is ongoing for 36 patients (median 83 months, IQR 41.5–139), whereas 9 patients were lost to follow-up (median 89 months, IQR 61–127, p = 0.860 with respect to patients on follow-up), and 13 died (median 120, IQR 69–172.5, p = 0.275 with respect to patients on follow-up and p = 0.254 with respect to patients lost to follow-up). The nine patients lost to follow-up developed both ILD and myositis in five cases, only myositis in one, only ILD in one, whereas no progression was registered in two cases, with a follow-up of 5 and 204 months, respectively. Disease progression rate in patients lost to follow-up was not different with respect to that of other groups (p = 0.350). Death was disease-related in three cases (23 % of subset, after 60, 120 and 144 months from disease onset), not disease-related in five (38.5 %, after 60, 72, 72, 168, 168 months) and not specified in five (38.5 %, after 60, 84, 186, 216, and 276 months).
Data Comparison
In our cohort of anti-Jo-1-positive ASSD, isolated arthritis was the presenting finding of the disease in 24 % of cases. Arthritis was mainly polyarticular, frequently RF and/or ACPA-positive, as well as erosive, with subsequent difficulty in the differential diagnosis with RA. Findings such as MH, RP, and fever were observed in one third of cases and in all cases never before arthritis onset, whereas anti-Ro positivity was found in up to 50 % of patients. The disease time course had greatly changed: the majority of patients developed ILD or myositis. The ex novo appearance of ILD (82 %) was more common than that of myositis (65.5 %), but interestingly, 55 % of patients developed both manifestations, thus configuring a complete ASSD. The timing of progression was very wide, ranging from a few months to several years. Our data suggested the relevance of early identification of ASSD in patients presenting with isolated arthritis, even when RA diagnosis is possible and also in the absence of other potential suspect findings of ASSD. Furthermore, we highlighted the need for a long and continuous multidisciplinary follow-up because of the high risk of the appearance of subsequent muscle and respiratory involvement.
Even if the possibility is recognized that arthritis could be the first clinical finding of ASSD [32, 33], up to now, only one retrospective study has addressed the analysis of this clinical aspect [29]. Although the prevalence of isolated arthritis as onset finding was similar (27 vs. 24 % of cases), some differences should be considered: in our cohort, positivity for RF or ACPA was not an exclusion criterion, because the occurrence of these antibodies in ASSD has been evidenced in several previous papers [13, 15–17]. Furthermore, Lefevre et al. [29] described patients from several French Rheumatology Departments/Units, but the prevalence reported represented only a single center result, whereas our study involved all ASSD referring to AENEAS participating centers, thus with a multicenter prevalence. Finally, we described a more homogeneous population, including only anti-Jo-1-positive patients. In fact, the clinical phenotype of ASSD is generally associated with the underlying specificity of antisynthetase antibodies detected [9], thus with a possible subsequent selection bias if also analyzing patients with other antisynthetase specificities. Interestingly and differently to our study, for Lefevre et al [29], the occurrence of connective tissue diseases such as systemic lupus erythematosus, Sjogren syndrome, systemic sclerosis, and mixed connective tissue disease, but not of RA, was an exclusion criteria, because these conditions could explain the occurrence of a joint involvement or pain not linked to ASSD, being potential confounding factors [34–38]. According to this point, we think that the purpose of authors was not to state that ASSD could not overlap with other connective tissue diseases but to obtain a more homogeneous population to study. However, from the theoretical point of view, in line with this choice, also patients diagnosed with RA should had been excluded from the study. The main conclusion of authors was that the occurrence of RP may lead to the suspect of an underlying ASSD in patients presenting with seronegative polyarthritis. We agree with this key message, but we suggested that also anti-Ro positivity may be considered as a suspect finding, because observed in a relevant percentage of patients presenting with isolated arthritis and having positive antisynthetase antibodies. Although these clinical and laboratory features may increase the number of patients diagnosed with ASSD, they cannot help clinicians in the identification of all ASSD patients, that, as we showed, may present only with arthritis without any other finding of suspect [39, 40]. Another point of discussion is that the prevalence of respiratory of muscle symptoms in this subset was reduced with respect to other ASSD patients, despite a similar frequency of ILD at HRCT scan and of muscle involvement diagnosis. According to these data, we cannot exclude that some of these patients were not purely presenting with an isolated arthritis but that they could have had an asymptomatic or a chronic ILD, as well as a hypomyopathic myositis not identified at arthritis onset, as the delay between arthritis occurrence and ASSD diagnosis seems to indicate.
With respect to other studies, our choice to also include RF and ACPA-positive patients highlights a relevant point of discussion: what do we need in order to define ASSD? In fact, although some criteria for ASSD have been proposed [41], approved classification criteria are lacking, thus with a potentially shared selection bias in all published studies. The prototypical example of this problem is the diagnostic definition of an anti-Jo-1-positive patient with symmetrical polyarthritis, in particular when seropositive for RF and/or for ACPA and/or with an erosive arthritis and without any other clinical and laboratory finding for suspect connective tissue disease. In a recent paper, Meyer et al. [30] did not diagnose with ASSD patients presenting with an ACPA-positive isolated arthritis and concomitant antisynthetase antibodies positivity. For ASSD diagnosis, the authors asked also the occurrence of concomitant pulmonary, dermatological, or muscle involvement. However, if we consider that in our cohort, all these patients developed either myositis or ILD, or both, it seems reasonable that the diagnosis of ASSD should be considered correct since arthritis onset. It is interesting that disease pattern progression was observed also in patients without any other laboratory (e.g., anti-Ro positivity) or clinical finding (mechanic’s hands, Raynaud’s phenomenon, fever in particular) that may lead to suspect ASSD. With respect to all anti-Jo-1 patients followed in participating centers [11, 42], in this subset, we did not confirm the association between RF or ACPA positivity and the occurrence of joint erosions at plain radiographs of hands and feet, but we observed a trend toward the statistical significance in symmetrical polyarthritis. The possible explanation for this difference is the different sample size of included patients. No baseline variables were clearly associated with the ex novo appearance of ILD or myositis. This data is another point strengthening the importance of the early identification of antisynthetase antibodies in patients presenting with symmetrical polyarthritis.
We are aware that our retrospective cohort analysis has potential limitations. First, retrospective studies are associated with an increased risk of incompleteness, in particular in the case of a very long follow-up [43, 44]. It is important to remember that up to now, no prospective studies addressed to ASSD are available. Regarding our casuistry, we included only patients diagnosed in rheumatology centers, with a subsequent risk of selection bias and overestimation of arthritis as a presenting finding. Another potential limitation is that anti-Jo-1 antibodies were assessed using different commercially available ELISA kits. The different kits used did not allow to evaluate anti-Jo-1 antibody levels, previously correlated with disease activity of ASSD in a large and relevant study [45]. Furthermore, we cannot exclude a possible delay in the diagnosis of asymptomatic ILD, and the temporal timing of 3 months to define contemporary the onset of different manifestations was arbitrary. It is important to remember that these pitfalls are common to the majority of multicenter studies up to now published. Finally, it was possible to check patients only for the 1987 revised classification criteria for RA [24], without considering the new available classification criteria [31] because of the unavailability of all necessary data in some patients.
To reduce the risk of false-positive tests and the subsequent selection bias, we required double test positivity and at least one positivity in a reference/tertiary center, not only for anti-Jo-1 but also for RF, ACPA, and anti-Ro. In fact, first time positivity is frequently obtained from primary level structures, which may have limited experience in autoantibody testing. Furthermore, to the best of our knowledge, the starting cohort of 243 anti-Jo-1-positive ASSD is one of the largest collected up to now.
According to our findings, disease course of anti-Jo-1 positive ASSD presenting with isolated polyarthritis is very variable. Up to 90 % of patients developed either myositis or ILD, or both, in a wide time frame, ranging from a few months to several years. Our data confirm that differential diagnosis may be challenging [46] because several patients had no other symptoms or signs that may lead to the suspicion not only of ASSD but also of connective tissue disease. Furthermore, in these patients, the pattern of presentation is often RA-like or polyarticular, with the possible positivity of IgM-RF and ACPA and the occurrence of joint erosions, as the high prevalence of patients satisfying the 1987 revised classification criteria for RA [24] clearly indicate.
On this basis, we could speculate that some cases of anti-TNF-alpha-induced anti-Jo-1-positive polymyositis reported in the setting of RA may be more related to the natural history of the disease rather than to the treatment with anti-TNF-alpha agents. This statement is strengthened by the presence of anti-Jo-1 positivity ab initio described in some of these cases [47–49].
In conclusion, according to our results and literature review, we think that the presence of anti-Jo-1 should be investigated not only in all patients with myositis and ILD [14] but also in subjects with peripheral arthritis, even though a diagnosis of RA is more likely [50, 51]. Furthermore, in these patients, periodic screening for the occurrence of myositis and in particular of ILD is mandatory. the overall survival rate was good, thus indicating a substantially good prognosis, independently of the subsequent potential occurrence of well-established negative risk factors such as ILD [9, 52, 53].
References
Imbert-Masseau A, Hamidou M, Agard C, Grolleau JY, Cherin P (2003) Antisynthetase syndrome. Joint Bone Spine 70:161–168
Milisenda JC, Selva-O’Callaghan A, Grau JM (2014) The diagnosis and classification of polymyositis. J Autoimmun 48-49:118–121
Jeganathan V, Peeva E, Diamond B (2014) Hormonal milieu at time of B cell activation controls duration of autoantibody response. J Autoimmun 53:46–54
Tang X, Zhang B, Jarrell JA, Price JV, Dai H, Utz PJ et al (2014) Ly108 expression distinguishes subsets of invariant NKT cells that help autoantibody production and secrete IL-21 from those that secrete IL-17 in lupus prone NZB/W mice. J Autoimmun 50:87–98
Betteridge Z, McHugh N (2015) Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Intern Med (in press)
Garcia-De La Torre I (2015) Clinical usefulness of autoantibodies in idiopathic inflammatory myositis. Front Immunol 6:331
Selmi C, Ceribelli A, Generali E, Scire CA, Alborghetti F, Colloredo G et al (2015) Serum antinuclear and extractable nuclear antigen antibody prevalence and associated morbidity and mortality in the general population over 15years. Autoimmun Rev 15:162–166
Dugar M, Cox S, Limaye V, Blumbergs P, Roberts-Thomson PJ (2011) Clinical heterogeneity and prognostic features of South Australian patients with anti-synthetase autoantibodies. Intern Med J 41:674–679
Hervier B, Devilliers H, Stanciu R, Meyer A, Uzunhan Y, Masseau A et al (2012) Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun Rev 12:210–217
Chatterjee S, Prayson R, Farver C (2013) Antisynthetase syndrome: not just an inflammatory myopathy. Cleve Clin J Med 80:655–666
Cavagna L, Nuno L, Scire CA, Govoni M, Longo FJ, Franceschini F et al (2015) Clinical spectrum time course in anti Jo-1 positive antisynthetase syndrome: results from an international retrospective multicenter study. Medicine (Baltimore) 94:e1144
Hervier B, Benveniste O (2013) Clinical heterogeneity and outcomes of antisynthetase syndrome. Curr Rheumatol Rep 15:349
Cavagna L, Caporali R, Abdi-Ali L, Dore R, Meloni F, Montecucco C (2013) Cyclosporine in anti-Jo1-positive patients with corticosteroid-refractory interstitial lung disease. J Rheumatol 40:484–492
Ghirardello A, Zampieri S, Tarricone E, Iaccarino L, Bendo R, Briani C et al (2006) Clinical implications of autoantibody screening in patients with autoimmune myositis. Autoimmunity 39:217–221
Nagashima T, Iwamoto M, Minota S (2011) Antisynthetase syndrome associated with long-standing rheumatoid arthritis. Rheumatol Int 31:705–706
Cavagna L, Fusetti C, Montecucco C, Caporali R (2010) Anticyclic citrullinated peptide antibodies as markers of erosive arthritis in antisynthetase syndrome. J Rheumatol 37:1967, author reply 8
Kaneko Y, Hanaoka H, Hirakata M, Takeuchi T, Kuwana M (2014) Distinct arthropathies of the hands in patients with anti-aminoacyl tRNA synthetase antibodies: usefulness of autoantibody profiles in classifying patients. Rheumatology (Oxford) 53:1120–1124
Chan MT, Owen P, Dunphy J, Cox B, Carmichael C, Korendowych E et al (2008) Associations of erosive arthritis with anti-cyclic citrullinated peptide antibodies and MHC Class II alleles in systemic lupus erythematosus. J Rheumatol 35:77–83
Gomard-Mennesson E, Fabien N, Cordier JF, Ninet J, Tebib J, Rousset H (2007) Clinical significance of anti-histidyl-tRNA synthetase (Jo1) autoantibodies. Ann N Y Acad Sci 1109:414–420
Park CK, Kim TJ, Cho YN, Kim IS, Lee HJ, Lee KE et al (2011) Development of antisynthetase syndrome in a patient with rheumatoid arthritis. Rheumatol Int 31:529–532
Bunch TW, O’Duffy JD, McLeod RA (1976) Deforming arthritis of the hands in polymyositis. Arthritis Rheum 19:243–248
Greenway G, Weisman MH, Resnick D, Zvaifler NJ, Guerra J Jr (1981) Deforming arthritis of the hands: an unusual manifestation of polymyositis. AJR Am J Roentgenol 136:611–612
Labrador-Horrillo M, Martinez MA, Selva-O’Callaghan A, Delgado JF, Martinez-Gomez X, Trallero-Araguas E et al (2009) Anti-cyclic citrullinated peptide and anti-keratin antibodies in patients with idiopathic inflammatory myopathy. Rheumatology (Oxford) 48:676–679
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Nagashima T, Sato H, Minota S (2009) Destructive arthropathy associated with dermatomyositis sine myositis positive for anti-Jo-1 and anti-cyclic citrullinated peptide antibodies. J Rheumatol 36:2133–2134
Schmidt WA, Wetzel W, Friedlander R, Lange R, Sorensen HF, Lichey HJ et al (2000) Clinical and serological aspects of patients with anti-Jo-1 antibodies—an evolving spectrum of disease manifestations. Clin Rheumatol 19:371–377
Meyer O, Charlanne H, Cherin P, Allanore Y, Coquerelle P, Grardel B et al (2009) Subluxing arthropathy: an unusual manifestation of the antisynthetase syndrome. Ann Rheum Dis 68:152–153
Nagashima T, Onishi S, Kamata Y, Minota S (2009) Dermatomyositis associated with thyroid cancer: a paraneoplastic syndrome? Rheumatol Int 29:1261–1262
Lefevre G, Meyer A, Launay D, Machelart I, DeBandt M, Michaud J et al (2015) Seronegative polyarthritis revealing antisynthetase syndrome: a multicentre study of 40 patients. Rheumatology (Oxford) 54:927–932
Meyer A, Lefevre G, Bierry G, Duval A, Ottaviani S, Meyer O et al (2015) In antisynthetase syndrome, ACPA are associated with severe and erosive arthritis: an overlapping rheumatoid arthritis and antisynthetase syndrome. Medicine (Baltimore) 94:e523
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd et al (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69:1580–1588
Mumm GE, McKown KM, Bell CL (2010) Antisynthetase syndrome presenting as rheumatoid-like polyarthritis. J Clin Rheumatol 16:307–312
Aggarwal R, Cassidy E, Fertig N, Koontz DC, Lucas M, Ascherman DP et al (2014) Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis 73:227–232
D’Amico F, Skarmoutsou E, Mazzarino MC (2014) The sex bias in systemic sclerosis: on the possible mechanisms underlying the female disease preponderance. Clin Rev Allergy Immunol 47:334–343
Moutsopoulos HM (2014) Sjogren’s syndrome: a forty-year scientific journey. J Autoimmun 51:1–9
Yu C, Gershwin ME, Chang C (2014) Diagnostic criteria for systemic lupus erythematosus: a critical review. J Autoimmun 48-49:10–13
Valim V, Trevisani VF, de Sousa JM, Vilela VS, Belfort R, Jr (2014) Current approach to dry eye disease. Clin Rev Allergy Immunol 49:288–297
Borchers AT, Gershwin ME (2015) Fibromyalgia: a critical and comprehensive review. Clin Rev Allergy Immunol 49:100–151
Gunawardena H (2015) The clinical features of myositis-associated autoantibodies: a review. Clin Rev Allergy Immunol (in press)
Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK (2015) A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol (in press)
Connors GR, Christopher-Stine L, Oddis CV, Danoff SK (2010) Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest 138:1464–1474
Cavagna L, Gonzalez-Gay MA, Castaneda-Sanz S, Franceschini F, Airo P, Cavazzana I (2014) Clinical and temporal characterization of anti-Jo-1 positive antisynthetase syndrome: preliminary results of an international multicentre study. Arthritis Rheum 66:550
Song JW, Chung KC (2010) Observational studies: cohort and case-control studies. Plast Reconstr Surg 126:2234–2242
Mann CJ (2003) Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J 20:54–60
Stone KB, Oddis CV, Fertig N, Katsumata Y, Lucas M, Vogt M et al (2007) Anti-Jo-1 antibody levels correlate with disease activity in idiopathic inflammatory myopathy. Arthritis Rheum 56:3125–3131
Cavagna L, Monti S, Grosso V, Boffini N, Scorletti E, Crepaldi G et al (2013) The multifaceted aspects of interstitial lung disease in rheumatoid arthritis. Biomed Res Int 2013:759760
Ishikawa Y, Yukawa N, Ohmura K, Hosono Y, Imura Y, Kawabata D et al (2010) Etanercept-induced anti-Jo-1-antibody-positive polymyositis in a patient with rheumatoid arthritis: a case report and review of the literature. Clin Rheumatol 29:563–566
Musial J, Undas A, Celinska-Lowenhoff M (2003) Polymyositis associated with infliximab treatment for rheumatoid arthritis. Rheumatology (Oxford) 42:1566–1568
Urata Y, Wakai Y, Kowatari K, Nitobe T, Mizushima Y (2006) Polymyositis associated with infliximab treatment for rheumatoid arthritis. Mod Rheumatol 16:410–411
Tansley S, Gunawardena H (2014) The evolving spectrum of polymyositis and dermatomyositis—moving towards clinicoserological syndromes: a critical review. Clin Rev Allergy Immunol 47:264–273
Iaccarino L, Ghirardello A, Bettio S, Zen M, Gatto M, Punzi L et al (2014) The clinical features, diagnosis and classification of dermatomyositis. J Autoimmun 48-49:122–127
Marie I, Josse S, Decaux O, Dominique S, Diot E, Landron C et al (2012) Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun Rev 11:739–745
Johnson C, Connors GR, Oaks J, Han S, Truong A, Richardson B et al (2014) Clinical and pathologic differences in interstitial lung disease based on antisynthetase antibody type. Respir Med 108:1542–1548
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Cavagna, L., Nuño, L., Scirè, C.A. et al. Serum Jo-1 Autoantibody and Isolated Arthritis in the Antisynthetase Syndrome: Review of the Literature and Report of the Experience of AENEAS Collaborative Group. Clinic Rev Allerg Immunol 52, 71–80 (2017). https://doi.org/10.1007/s12016-016-8528-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-016-8528-9