Abstract
Fucosyltransferase 2 (FUT2) mediates the inclusion of fucose in sugar moieties of glycoproteins and glycolipids. ABO blood group antigens and host-microbe interactions are influenced by FUT2 activity. About 20 % of the population has a “non-secretor” status caused by inactivating variants of FUT2 on both alleles. The non-sense mutation G428A and the missense mutation A385T are responsible for the vast majority of the non-secretor status in Caucasians, Africans, and Asians, respectively. Non-secretor individuals do not secrete fucose-positive antigens and lack fucosylation in epithelia. They also appear to be protected against a number of infectious diseases, such as Norovirus and Rotavirus infections. In recent years, genome-wide association studies (GWAS) identified inactivating variants at the FUT2 locus to be associated with primary sclerosing cholangitis (PSC), Crohn’s disease (CD), and biochemical markers of biliary damage. These associations are intriguing given the important roles of fucosylated glycans in host-microbe interactions and membrane stability. Non-secretors have a reduced fecal content of Bifidobacteria. The intestinal bacterial composition of CD patients resembles the one of non-secretors, with an increase in Firmicutes and decreases in Proteobacteria and Actinobacteria. Non-secretor individuals lack fucosylated glycans at the surface of biliary epithelium and display a different bacterial composition of bile compared to secretors. Notably, an intact biliary epithelial glycocalix is relevant for a stable ‘biliary HCO3 − umbrella’ to protect against toxic effects of hydrophobic bile salt monomers. Here, the biology of FUT2 will be discussed as well as hypotheses to explain the role of FUT2 in the pathophysiology of PSC and Crohn’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fucosyltransferases are a group of enzymes that catalyse the transfer of the sugar fucose to acceptor substrates present on oligosaccharides, glycoproteins and glycolipids [1]. The expression of fucosyltransferases is found in a variety of eukaryotic and prokaryotic cells [1, 2], and fucosylated glycans have been shown to participate in numerous biological and pathological processes, including signal transduction, host-microbe interactions, tissue development, cancer progression and metastasis [2, 3]. In humans, the FUT2 locus encodes a specific fucosyltransferase enzyme, which has been extensively studied for its role in the synthesis of ABO blood group antigens [4, 5]. In recent years, new interest in the biology of FUT2 has been raised by genome-wide association studies (GWAS), which consistently identified inactivating polymorphisms of the gene to be associated with the development of primary sclerosing cholangitis (PSC) and Crohn’s disease (CD) among other pathophysiological conditions. These studies have thus unveiled a novel risk locus and prompted new hypotheses aiming to connect the genetic discoveries to their biological relevance, hence fostering novel research lines. With this current review, we aim to present a concise overview on FUT2 biology and on the emerging putative roles of FUT2 polymorphisms in the pathophysiology of PSC and CD. As an outlook, additional associations between FUT2 dysfunction and other human diseases will be briefly discussed.
Fucose Biosynthesis and Fucosyltransferases
Fucose, also know as 6-deoxy-l-galactose, is a deoxy hexose monosaccharide, with the chemical formula C6H12O5, and is found as a common component of many N- and O-linked glycans. It has two peculiar characteristics compared to other six-carbon monosaccharides normally present in mammals, namely the l-configuration and the absence of a hydroxyl group on the carbon at position 6 of the molecule [3].
Fucose can be incorporated in glycan chains by specific members of the glycosyltransferase superfamily, the fucosyltransferases. These enzymes are expressed in a variety of different organisms, including vertebrates, invertebrates, plants and bacteria, and transfer fucose using its activated monomeric form, the guanosine diphosphate (GDP)-fucose, as substrate. Two distinct pathways for the formation of GDP-fucose have been described, the so-called de novo and salvage pathways [6, 7]. In the de novo pathway, GDP-fucose is formed from GDP-mannose through the concerted action of two enzymes, the GDP-mannose 4,6-dehydratase and the FX protein, an enzyme with epimerase-reductase activity [6, 8]. In the salvage pathway, GDP-fucose is directly synthesized from the fucose derived either from the extracellular compartment [9] or released from the metabolism of fucosylated glycans in the lysosomes [10]. The salvage pathway accounts for a minor fraction (around 10 %) of GDP-fucose biosynthesis [11]. Independently of the specific biosynthetic pathway followed, GDP-fucose eventually reaches the Golgi apparatus where fucosyltransferases catalyse the transfer of GDP-fucose to acceptor sugar substrates present on glycoproteins and glycolipids [1]. Schematically, fucosyltransferases are divided in α-1,2-, α-1,3/4-, α-1,6-, and O-fucosyltransferases according to the specific site of fucose addition on substrates. The α-1,3/4-, α-1,6-, and O-fucosylation linkages are mediated by a variety of different enzymes recently reviewed by Ma et al. [2].
FUT1 and FUT2 Mediate α-1,2-Fucosylation
Only two gene products account for the α-1,2-fucosylation of the terminal galactose of glycan chains in humans: the enzymes encoded by FUT1 and FUT2 [12, 13]. The acceptor specificity of FUT1 and FUT2 is slightly different. FUT1 prefers type II (Galβ1,4GlcNAc) chains, and FUT2 is more active on type III (Galβ1,3GalNAc) chains. Type I (Galβ1,3GlcNAc) chains are, however, used with equal efficiency by both enzymes [14, 15]. FUT1 and FUT2 play a pivotal role in the formation of ABO blood group antigens. The ABO system consists of complex carbohydrate molecules that are present not only on the surface of erythrocytes but also on the membranes of epithelial cells and in their mucoid secretions [16]. Red blood cell precursors express FUT1, which is also known as H transferase and is responsible for the formation of the H antigen by the addition of fucose to the terminal galactose of acceptor substrates [17]. The basic H antigen remains unchanged on the cell surface of individuals of blood group O, while it is subsequently modified by different glycosyltransferases via the addition of N-acetylgalactosamine and/or d-galactose in individuals of blood group A and/or B, respectively [18]. Epithelial tissues predominantly express FUT2, also known as secretor (Se) transferase. This enzyme mediates the formation of the basic H antigen in epithelial cells. The epithelial H antigen may also be further modified according to the ABO blood group status [4, 5].
“Non-secretors” and Natural Selection
Notably, it has been long known that the capacity to secrete A, B, or H antigens in body fluids is absent in about 20 % of the population [16]. These individuals, who have been named “non-secretors,” are homozygous for non-functional FUT2 alleles, and ABO antigens are undetectable in their epithelial tissues and body secretions. Normal levels of these antigens are present on the membranes of erythrocytes, in line with the tissue distribution of FUT1 and FUT2 [13]. The molecular defects responsible for the non-secretor status have been extensively studied after the cloning of the FUT2 gene [4]. FUT2 is encoded on chromosome 19 and consists of two exons (of which the first one is a non-coding exon). Two single nucleotide polymorphisms (SNPs) occurring at the second exon are responsible for the majority of the non-secretor status. The non-sense mutation G428A, giving rise to a premature stop codon at position 143 (p.W143X), is predominant in Caucasians (Europeans and Iranians) and Africans [13, 19, 20]. In Asian populations, the most common non-secretor variant is the missense mutation A385T, causing the substitution of isoleucine by phenylalanine at position 129 (p.I120P), resulting in a deficient FUT2 activity [21–23]. In the past two decades, many other FUT2 gene variants such as SNPs, deletions of the coding region, and a fusion gene, have been described (reviewed by Koda et al. [24]). Apart from the various specific SNPs occurring in the FUT2 gene and giving rise to a dysfunctional protein, it is interesting to note that FUT2-inactivating variants are actively maintained in the genome by natural selection processes [25–27]. Beneficial effects of the non-secretor status are a possible explanation for this phenomenon [24]. Indeed, as discussed later, the inactivation of FUT2 has been shown to protect against a number of infectious diseases and to result in higher vitamin B12 levels in plasma [28, 29].
FUT2 and Crohn’s Disease
The pathogenesis of idiopathic inflammatory bowel disease (IBD), clinically presenting as Crohn’s disease (CD) or ulcerative colitis (UC), remains largely elusive to date [30, 31]. In the past two decades, however, genome-wide association studies (GWAS) have provided an important insight in the genetic factors underlying IBD development [32]. Several SNPs are associated with significantly different risks for IBD, implying contributions of a total of 163 loci to disease etiology [33].
A number of SNPs suggestive for a FUT2 involvement in the genetic susceptibility to IBD have been recently identified (Table 1). A previous meta-analysis of 10 genome scans of IBD patients recognized a suggestive peak of linkage for CD on chromosome 19, where the FUT2 gene is encoded [34]. McGovern et al. subsequently performed a GWAS in 896 CD cases and 3,204 healthy controls of Caucasian origin and pinpointed a clear association with a total of four SNPs in the FUT2 locus. Of the SNPs discovered, two are in the 3’ untranslated region (UTR) of the gene (rs504963 and rs676388), one represents a synonymous polymorphism (rs485186), and one (rs602662) is a missense SNP (G739) that results in the substitution of glycine with serine (p.G247S) and gives rise to an inactive FUT2 enzyme [35, 36]. Moreover, in an independent replication cohort of 1,174 CD patients and 357 controls, the authors could confirm the association between CD and the previous four SNPs and identified two additional risk SNPs in the FUT2 gene (rs601338 and rs492602) [35]. Notably, the SNP rs601338 is responsible for the non-sense G428A substitution that accounts for the non-secretor status in the majority of Caucasians as previously discussed [13, 19, 20]. These results are supported by a genome-wide meta-analysis that identified the association between CD and the SNP rs281379 at 19q13 [37]. All the SNPs in the FUT2 gene discovered in the previous studies are strongly associated with the presence of the more common rs601338, thus suggesting a possible functional role of the non-secretor status in the context of CD [37]. It is important to note, however, that the FUT2 secretor status has also been associated specifically with the colonic localization of CD in a small Japanese cohort [38]. The discrepancy between this study and the GWAS previously discussed, conducted predominantly in Caucasian individuals, might be due to specific ethnic differences in the genetic susceptibility to CD, in a similar fashion to what is reported for NOD2 [39]. Moreover, the low frequency of the colonic localization of the disease might have overshadowed the importance of the secretor status in randomly recruited cohorts of CD patients, such as those used in the GWAS [38].
An improper immune response against commensal gut bacteria has been proposed as a possible pathogenetic mechanism of CD [30, 40]. The identification of FUT2 as a risk gene appears particularly interesting considering the well-known importance of fucosylated glycans in host-microbe interactions. For example, the adhesion of Helicobacter pylori to the gastric epithelium has been shown to depend on the expression by the host of the Lewisb antigen, a structure synthesized by the action of FUT2 and FUT3 [41, 42]. Similarly, fucosylated structures are important in the adhesion of Escherichia coli F18 to the epithelium of the small intestine [43]. Conversely, the expression of FUT2 is modulated by the intestinal microbiota, indicating a bidirectional relationship. The expression of Fut2 mRNA in the small intestine of mice is driven by the presence of a normal gut flora, while it is absent before weaning and in germ-free mice. Fut2 expression is rescued by the colonization of either an adult fecal microbiota or of a single component of it, namely Bacteroides thetaiotaomicron [44, 45], an organism that can use fucose as a carbon source for its metabolism. In microbiota-deficient mice, the restoration of Fut2 expression in the intestine can be achieved also by colonization by Bacteroides fragilis. In these mice, the re-colonization with B. fragilis ensures better recovery rates after DSS-induced mucosal injury compared to mice re-colonized with a mutant B. fragilis unable to induce Fut2 expression [46].
Given these observations, it comes as no surprise that the intestinal bacterial community in non-secretor individuals (homozygous for G428A) is different from the one of secretors [47]. Diversity and abundance of Bifidobacteria are significantly reduced in fecal samples from non-secretor individuals. Moreover, the secretor status determined by FUT2 genotype has been recently shown to influence the intestinal bacterial composition, diversity, and structure of CD patients [48, 49]. Rausch et al. demonstrated an increase in Firmicutes and a corresponding decrease in Proteobacteria and Actinobacteria in CD patients compared to controls. Notably, these differences could only be identified among secretor control individuals, indicating that the bacterial composition of non-secretors resembles more closely the one of the CD patients [48]. In addition, by analyzing the inter-individual differences (beta diversity) of the bacterial communities, the authors could assess that both disease status and disease-by-genotype interactions contribute to the variation of the bacterial community composition between individuals [48]. Taken together, the importance of fucosylated glycans in shaping the bacterial community of the gut appears to sustain the genetic association between FUT2 dysfunction and CD.
FUT2 and Ulcerative Colitis
The contribution of FUT2 to the pathogenesis of UC remains, to date, less clear. While McGovern et al. reported no association between FUT2 and UC [35], one study identified a total of three different inactivating SNPs in the FUT2 gene (rs281377, rs1047781, and rs601338) to be associated with UC in a small sample population of the Han and Uyghur ethnical groups in China [50]. Conversely, an additional report based on a Finnish population showed that the homozygous G428A SNP, responsible for the non-secretor status, did not correlate with UC. On the other hand, the secretor status was associated with an increased risk of UC, suggesting a protective role for the mutated allele [51]. Although both diseases cause a chronic inflammation of the intestine, UC and CD display profound differences in tissue damage and progression [52]. A dissimilar genetic susceptibility might underline a separate etiopathogenesis of the two diseases. Moreover, the contribution of FUT2 inactivation might differ between UC and CD and have complex interactions with the additional genetic and environmental factors that contribute to disease development.
FUT2 and Primary Sclerosing Cholangitis
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease frequently associated with IBD. Chronic inflammation of the biliary tree in PSC leads to the development of strictures and biliary cirrhosis over time, with patients often suffering from recurrent cholangitis and ultimately needing liver transplantation [53]. The pathogenesis of PSC is still debated, but it is likely to be multifactorial. The importance of a genetic predisposition is underlined by the fact that first-degree relatives of PSC patients have an about 100-fold increased risk of developing PSC compared to the general population [54]. The most important genetic risk factors for PSC have been located in the major histocompatibility complex (MHC), suggesting the involvement of the immune system in disease development [55]. In genetically susceptible individuals, environmental factors could trigger an aberrant or inadequate immune response eventually leading to liver damage and fibrosis [56]. Given the strong association between PSC and inflammatory bowel disease (IBD), some groups have started to investigate the role of intestinal microbiota and/or of increased intestinal permeability as pathogenetic determinants of PSC development and progression in the context of an inflamed leaky gut [57]. PSC patients do not appear to have bacterial overgrowth or increased intestinal permeability measured by differential urinary excretion of lactulose/l-rhamnose [58]. It is interesting to note, however, that enteric bacteria have been detected in the bile of PSC patients [59, 60]. Also, microbial products known as pathogen-associated molecular patterns (PAMPs) are present in portal blood and in bile. PAMPS are capable to establish complex interactions with cholangiocytes and immune cells and have been implied in the pathogenesis of PSC [57, 61, 62]. In this complex scenario, a recent GWAS identified, among others, three different SNPs in the FUT2 gene as novel risk loci for PSC, analyzing a population composed of 715 PSC cases and 2,962 healthy controls [63]. Of the three SNPs in the FUT2 gene discovered, rs281377 is a synonymous polymorphism; rs602662 is a missense SNP (G739A) giving rise to an inactive FUT2 enzyme [36]; and rs601338 introduces the non-sense “non-secretor” point mutation G428A [13, 19, 20]. All three SNPs described are in strong linkage disequilibrium with each other. In staining liver biopsies with the α-1,2-fucose-specific lectin Ulex europaeus agglutinin-I (UEA-I), it was demonstrated that α-1,2-fucosylated glycans are present on the apical membrane of the cholangiocytes of individuals carrying the GG variant of the rs601338 SNP (being secretors), while they are absent in the apical side of the bile ducts of patients with the AA variant (carrying a homozygous G428A mutation and being non-secretors) [63]. Moreover, the bacterial composition of bile appears to be profoundly influenced by the secretor status of the individual. Given the effect of FUT2 polymorphisms on the bacterial community structure in the context of Crohn’s disease [48], Folseraas et al. analyzed bile samples from a total of 39 PSC patients, of which 8 were homozygous for the functional GG variant, 10 were homozygous for the dysfunctional AA variant, and 21 were heterozygous. The authors found that Firmicutes are significantly more abundant in non-secretor bile, while Proteobacteria are decreased, in a similar fashion of what is described for the colon [63]. In addition, heterozygous individuals display significantly lower diversity in the bacterial species composition (alpha diversity) and also lower inter-individual variability (beta diversity), as compared to both GG and AA homozygous genotypes [63].
In the context of liver injury, it is worth mentioning that FUT2 polymorphisms have been linked with the plasma levels of alkaline phosphatase (ALP) and γ-glutamyl transferase (GGT) [64], common markers of biliary damage [65].
Taken together, the expression of fucosylated glycans along the biliary tree, according to the secretor status of the patients, might determine the adhesion of specific bacterial species to the biliary epithelium and consequently influence the development and/or severity of recurrent cholangitis, a well-known complication in the natural history of PSC [66]. In addition, the lack of fucosylated glycans in the mucus layer of non-secretor individuals may alter the permeability of the intestinal epithelium and favor an enhanced translocation of PAMPs reaching the liver through the portal circulation.
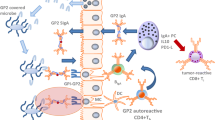
In the pathogenesis of PSC and other cholangiopathies, an important role has been attributed to the intrinsic toxicity of bile acids towards biliary epithelial cells [56]. Our group has recently proposed the “biliary HCO3 − umbrella hypothesis” indicating that cholangiocytes protect their integrity against hydrophobic bile acids by secreting high amounts of HCO3 − close to the apical membrane; the biliary HCO3 − umbrella would then lead to the deprotonation of toxic hydrophobic bile acids to bile salts, rendering them unable to diffuse across cellular membranes and cause apoptosis [67] or cholangiocyte senescence. Functional impairment of the biliary HCO3 − umbrella, or of its regulation, may therefore predispose to cholangiocyte damage and contribute to the development of chronic cholangiopathies [68]. Polymorphisms in the FUT2 gene might affect the stability of the biliary HCO3 − umbrella (Fig. 1). We have recently shown that cholangiocytes express a 20–30 nm thick glycocalyx layer on the outer leaf of the apical membrane [68]. Notably, the modification of the glycocalyx by the removal of sialic acid residues (derivatives of neuraminic acid, a monosaccharide commonly found in the glycocalyx [69, 70]) has profound effects on pH-dependent cholangiocyte toxicity induced by chenodeoxycholic and glycochenodeoxycholic acid in vitro [68]. These results support the idea that the specific composition of the glycocalyx in terms of sugar residues may stabilize the pH nanoenvironment close to the apical membrane as earlier described for melanoma cells [71, 72] and might thus contribute to the stabilization of the biliary HCO3 − umbrella. In this context, it is attractive to speculate that non-secretor individuals, lacking α-1,2-fucosylated glycans on the apical glycocalyx of cholangiocytes [63], may have an impaired capacity to effectively maintain an alkaline pH microclimate close to the apical surface of cholangiocytes and may, therefore, be more susceptible to the toxic effect of bile acids. Of note, preliminary experiments of our group indicate that Fut2 −/− mice might have an increased susceptibility to hepatobiliary damage (Maroni et al., manuscript in preparation). Further studies aiming to dissect in detail the role of FUT2 in the biology of the biliary epithelium and its possible role in the pathophysiology of PSC are warranted.
FUT2 in cholangiocytes and enterocytes (schematic overview). FUT2-positive individuals (FUT2+; secretors; top part of the figure) have fucose moieties at the cell surface of cholangiocytes (left) and enterocytes (right), whereas non-secretor (FUT2-) individuals lack this fucosylation. This results in differences in the mucus layer in epithelia and possibly alteration in barrier function and pathogen adhesion. In the liver, the constitution and thickness of the mucus layer will affect the diffusion rate of HCO3 −, secreted by cholangiocytes, and thus the local pH. Increased pH in close proximity of the cell surface (biliary HCO3 − umbrella) results in more deprotonated, charged bile salts which do not enter the cell in a carrier-independent fashion. FUT2-negative individuals might have an altered (thinner) mucus layer, rendering the cholangiocytes more susceptible to bile acid-induced cell damage
FUT2 Involvement in Other Human Diseases and Conditions
The status (non-secretor versus secretor) of the FUT2 gene has been associated with a variety of pathophysiological processes other than IBD and PSC (Table 2). In particular, bacterial adhesion and plasma levels of vitamin B12 appear to be strongly influenced by the secretor status of the individual. An altered bacterial adhesion and consequently a different susceptibility to infections are possible reasons why the non secretor-status is actively maintained in about 20 % of the population [16, 24]. For example, different studies have identified that non-secretor individuals are effectively protected against the susceptibility to H. pylori (HP) infection and the severity of the gastric lesions caused by it [73–75]. Indeed, the presence of fucosylated glycans in the gastric epithelium promotes the attachment of HP to the gastric mucosa [41, 42]. Its adhesion is mediated by the bacterial expression of the BabA adhesin, which recognizes Leb and H type 1 structures expressed on gastric mucins [76]. Notably, it has long been known that the non-secretor status plays a role in the risk of bleeding and perforation of duodenal ulcer [77]. Inactivation of the FUT2 gene has been also shown to confer protection against nosocomial and sporadic outbreaks of Norovirus infection [28, 78, 79], one of the major causes of acute gastroenteritis worldwide [80]. In a similar fashion to what is described for HP, Norovirus is thought to bind to H type 1 antigens expressed along the gastrointestinal tract [81]. Consistently, FUT2 non-secretor individuals have a lower prevalence and titer of immunoglobulin G antibody to Norovirus [82]. Of note, despite the lack of evidence for a specific pattern of antibodies in CD patients [83], some authors have hypothesized that asymptomatic Norovirus infections in non-secretors may alter the homeostasis of the gut microbiota, and therefore contribute to CD development [84]. Recently, non-secretor individuals have been also described to be resistant to Rotavirus of the P8 genotype [85]. Along the same line, the susceptibility to different other infectious diseases, such as recurrent urinary tract infection in women [86, 87], acute pyelonephritis [88], development of oral and vaginal candidiasis [89–91], and infections with Haemophilus influenzae [92], Neisseria meningitidis, and Streptococcus pneumoniae [93], have been linked to the secretor status of affected individuals, confirming a pivotal role of FUT2 in host-microbe interactions.
The association between FUT2 polymorphisms and serum levels of vitamin B12 has been identified in three different GWAS and one meta-analysis [29, 94–96]. In particular, the levels of vitamin B12 appear to be higher in individuals carrying the non-functional SNPs (or other polymorphisms in strong linkage disequilibrium with the latter). Some authors have hypothesized that atrophic gastritis developing after HP infection, towards which non-secretor individuals are protected, could be a possible functional explanation for the genetic association [29]. Atrophic gastritis might indeed lead to a decrease of the intrinsic factor, a glycoprotein secreted by gastric cells and required for vitamin B12 absorption [97]. Recently, however, Oussalah et al. demonstrated that the HP serological status has no influence on the association between FUT2 SNPs and vitamin B12 levels [98], and subsequent work confirmed that FUT2 secretor variants lower the vitamin B12 levels through an impaired intrinsic factor secretion, independently of HP-induced gastritis [99].
GWAS and meta-analyses of GWAS have identified important associations between FUT2 polymorphisms and a number of other pathophysiological conditions (Table 3). Indeed, FUT2 variants have been recently recognized to predispose to psoriasis [100, 101] and also to Behcet's disease development [102]. Plasma concentrations of total homocysteine are also influenced by FUT2 [103]. Conversely, no link with the occurrence of venous thrombosis could be demonstrated [104], despite a previous study that identified FUT2 to be associated with the levels of fibrinogen A-α [105].
Finally, fucosylated glycans have been shown to contribute to cancer progression and metastasis. Indeed, α-1,2-fucosyltransferase activity appears to be higher in colonic carcinoma tissues than in normal epithelium [106, 107] and to be correlated with the progression of colonic adenocarcinoma [108]. The secretor status has been shown to predict the risk of axillary lymph node metastasis in the context of breast cancer [109]. Given the altered glycosylation pattern of cancerous tissues, fucosylated glycans have also attracted interest as possible tumor markers [110]. In this context, serum levels of cancer antigen 19–9 (CA19-9) clearly correlate with the secretor status of the individuals, both in colorectal cancer patients [111] and in PSC patients diagnosed with cholangiocarcinoma [112]. Moreover, a recent GWAS identified the locus of FUT2 to be associated with plasma concentrations of both CA19-9 and carcinoembryonic antigen (CEA) [113].
Conclusions
Growing evidence supports an involvement of FUT2 in the pathogenesis of a number of human diseases and conditions. Various inactivating polymorphisms in the FUT2 gene, responsible for the non-secretor status occurring in about 20 % of the population, have been described in literature. Inactivating variants of the FUT2 gene are maintained in the human genome, possibly due to beneficial effects under evolutionary pressure of infectious diseases. However, inactivating SNPs at the FUT2 locus have also been identified as possible risk factors in a variety of human diseases, in particular CD and PSC in recent GWAS. As for many other loci identified by GWAS, the biological relevance of the genetic associations in the pathophysiological processes still remains to be completely unraveled. Nonetheless, the involvement of fucosylated glycans in host-microbe interaction and the finding that the secretor status of individuals play a crucial role in shaping the intestinal microbiota in health and disease appear particularly interesting in the study of diseases such as CD and PSC, in which a complex dysregulation of the immune system is thought to contribute to disease development. Future studies aiming to selectively investigate the role of FUT2, in the context of the genetic associations uncovered by the GWAS, will undoubtedly deepen the knowledge on the biology of different diseases and, possibly, open doors to new therapeutic strategies.
References
Oriol R, Mollicone R, Cailleau A, Balanzino L, Breton C (1999) Divergent evolution of fucosyltransferase genes from vertebrates, invertebrates, and bacteria. Glycobiology 9:323–334
Ma B, Simala-Grant JL, Taylor DE (2006) Fucosylation in prokaryotes and eukaryotes. Glycobiology 16:158R–184R
Becker DJ, Lowe JB (2003) Fucose: biosynthesis and biological function in mammals. Glycobiology 13:41R–53R
Rouquier S, Lowe JB, Kelly RJ, Fertitta AL, Lennon GG, Giorgi D (1995) Molecular cloning of a human genomic region containing the H blood group alpha(1,2)fucosyltransferase gene and two H locus-related DNA restriction fragments. Isolation of a candidate for the human secretor blood group locus. J Biol Chem 270:4632–4639
Avent ND (1997) Human erythrocyte antigen expression: its molecular bases. Br J Biomed Sci 54:16–37
Tonetti M, Sturla L, Bisso A, Benatti U, De Flora A (1996) Synthesis of GDP-L-fucose by the human FX protein. J Biol Chem 271:27274–27279
Kaufman RL, Ginsburg V (1968) The metabolism of L-fucose by HeLa cells. Exp Cell Res 50:127–132
Yamamoto K, Katayama I, Onoda Y, Inami M, Kumagai H, Tochikura T (1993) Evidence that the enzyme catalyzing the conversion of guanosine diphosphate D-mannose to a 4-keto sugar nucleotide intermediate requires nicotinamide adenine dinucleotide phosphate. Arch Biochem Biophys 300:694–698
Wiese TJ, Dunlap JA, Yorek MA (1994) L-fucose is accumulated via a specific transport system in eukaryotic cells. J Biol Chem 269:22705–22711
Michalski JC, Klein A (1999) Glycoprotein lysosomal storage disorders: alpha- and beta-mannosidosis, fucosidosis and alpha-N-acetylgalactosaminidase deficiency. Biochim Biophys Acta 1455:69–84
Yurchenco PD, Atkinson PH (1977) Equilibration of fucosyl glycoprotein pools in HeLa cells. Biochemistry 16:944–953
Larsen RD, Ernst LK, Nair RP, Lowe JB (1990) Molecular cloning, sequence, and expression of a human GDP-L-fucose: beta-D-galactoside 2-alpha-L-fucosyltransferase cDNA that can form the H blood group antigen. Proc Natl Acad Sci U S A 87:6674–6678
Kelly RJ, Rouquier S, Giorgi D, Lennon GG, Lowe JB (1995) Sequence and expression of a candidate for the human secretor blood group alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J Biol Chem 270:4640–4649
Kyprianou P, Betteridge A, Donald AS, Watkins WM (1990) Purification of the blood group H gene associated alpha-2-L-fucosyltransferase from human plasma. Glycoconj J 7:573–588
Sarnesto A, Kohlin T, Thurin J, Blaszczyk-Thurin M (1990) Purification of H gene-encoded beta-galactoside alpha 1–2 fucosyltransferase from human serum. J Biol Chem 265:15067–15075
Hoskins LC (1967) The ABO blood group antigens and their secretion by healthy and diseased gastric mucosa. Ann N Y Acad Sci 140:848–865
Oriol R, Danilovs J, Hawkins BR (1981) A new genetic model proposing that the Se gene is a structural gene closely linked to the H gene. Am J Hum Genet 33:421–431
Lowe JB (1993) The blood group-specific human glycosyltransferases. Baillieres Clin Haematol 6:465–492
Liu YH, Koda Y, Soejima M et al (1998) Extensive polymorphism of the FUT2 gene in an African (Xhosa) population of South Africa. Hum Genet 103:204–210
Svensson L, Petersson A, Henry SM (2000) Secretor genotyping for A385T, G428A, C571T, C628T, 685delTGG, G849A, and other mutations from a single PCR. Transfusion 40:856–860
Henry S, Mollicone R, Fernandez P, Samuelsson B, Oriol R, Larson G (1996) Molecular basis for erythrocyte Le(a + b+) and salivary ABH partial-secretor phenotypes: expression of a FUT2 secretor allele with an A- > T mutation at nucleotide 385 correlates with reduced alpha(1,2)fucosyltransferase activity. Glycoconjugate J 13:985–993
Yu LC, Yang YH, Broadberry RE, Chen YH, Chan YS, Lin M (1995) Correlation of a missense mutation in the human secretor alpha-1,2-fucosyl-transferase gene with the Lewis(A + B+) phenotype—a potential molecular-basis for the weak secretor allele (Se-W). Biochem J 312:329–332
Koda Y, Soejima M, Liu YH, Kimura H (1996) Molecular basis for secretor type alpha(1,2)-fucosyltransferase gene deficiency in a Japanese population: a fusion gene generated by unequal crossover responsible for the enzyme deficiency. Am J Hum Genet 59:343–350
Koda Y, Soejima M, Kimura H (2001) The polymorphisms of fucosyltransferases. Leg Med (Tokyo) 3:2–14
Soejima M, Pang H, Koda Y (2007) Genetic variation of FUT2 in a Ghanaian population: identification of four novel mutations and inference of balancing selection. Ann Hematol 86:199–204
Walsh EC, Sabeti P, Hutcheson HB et al (2006) Searching for signals of evolutionary selection in 168 genes related to immune function. Hum Genet 119:92–102
Ferrer-Admetlla A, Sikora M, Laayouni H et al (2009) A natural history of FUT2 polymorphism in humans. Mol Biol Evol 26:1993–2003
Thorven M, Grahn A, Hedlund KO et al (2005) A homozygous nonsense mutation (428G– > A) in the human secretor (FUT2) gene provides resistance to symptomatic Norovirus (GGII) infections. J Virol 79:15351–15355
Hazra A, Kraft P, Selhub J et al (2008) Common variants of FUT2 are associated with plasma vitamin B12 levels. Nat Genet 40:1160–1162
Xavier RJ, Podolsky DK (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature 448:427–434
Podolsky DK (2002) Inflammatory bowel disease. N Engl J Med 347:417–429
Brant SR (2013) Promises, delivery, and challenges of inflammatory bowel disease risk gene discovery. Clin Gastroenterol Hepatol 11:22–26
Jostins L, Ripke S, Weersma RK et al (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491:119–124
van Heel DA, Fisher SA, Kirby A, Daly MJ, Rioux JD, Lewis CM (2004) Inflammatory bowel disease susceptibility loci defined by genome scan meta-analysis of 1952 affected relative pairs. Hum Mol Genet 13:763–770
McGovern DP, Jones MR, Taylor KD et al (2010) Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn's disease. Hum Mol Genet 19:3468–3476
Serpa J, Mendes N, Reis CA et al (2004) Two new FUT2 (fucosyltransferase 2 gene) missense polymorphisms, 739G– > A and 839 T– > C, are partly responsible for non-secretor status in a Caucasian population from Northern Portugal. Biochem J 383:469–474
Franke A, McGovern DP, Barrett JC et al (2010) Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet 42:1118–1125
Miyoshi J, Yajima T, Okamoto S et al (2011) Ectopic expression of blood type antigens in inflamed mucosa with higher incidence of FUT2 secretor status in colonic Crohn's disease. J Gastroenterol 46:1056–1063
Inoue N, Tamura K, Kinouchi Y et al (2002) Lack of common NOD2 variants in Japanese patients with Crohn’s disease. Gastroenterology 123:86–91
Loh G, Blaut M (2012) Role of commensal gut bacteria in inflammatory bowel diseases. Gut Microbes 3:544–555
Boren T, Falk P, Roth KA, Larson G, Normark S (1993) Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 262:1892–1895
Hooper LV, Gordon JI (2001) Glycans as legislators of host-microbial interactions: spanning the spectrum from symbiosis to pathogenicity. Glycobiology 11:1R–10R
Meijerink E, Neuenschwander S, Fries R et al (2000) A DNA polymorphism influencing alpha(1,2)fucosyltransferase activity of the pig FUT1 enzyme determines susceptibility of small intestinal epithelium to Escherichia coli F18 adhesion. Immunogenetics 52:129–136
Bry L, Falk PG, Midtvedt T, Gordon JI (1996) A model of host-microbial interactions in an open mammalian ecosystem. Science 273:1380–1383
Nanthakumar NN, Dai D, Newburg DS, Walker WA (2003) The role of indigenous microflora in the development of murine intestinal fucosyl- and sialyltransferases. FASEB J 17:44–46
Nanthakumar NN, Meng D, Newburg DS (2013) Glucocorticoids and microbiota regulate ontogeny of intestinal fucosyltransferase 2 requisite for gut homeostasis. Glycobiology 23:1131–1141
Wacklin P, Makivuokko H, Alakulppi N et al (2011) Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PLoS ONE 6:e20113
Rausch P, Rehman A, Kunzel S et al (2011) Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (secretor) genotype. Proc Natl Acad Sci U S A 108:19030–19035
Franks I (2012) Gut microbiota: FUT2 genotype influences the gut microbiota in patients with Crohn's disease and healthy individuals. Nat Rev Gastroenterol Hepatol 9:2
Aheman A, Luo HS, Gao F (2012) Association of fucosyltransferase 2 gene variants with ulcerative colitis in Han and Uyghur patients in China. World J Gastroenterol 18:4758–4764
Parmar AS, Alakulppi N, Paavola-Sakki P et al (2012) Association study of FUT2 (rs601338) with celiac disease and inflammatory bowel disease in the Finnish population. Tissue Antigens 80:488–493
Fiocchi C (1998) Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 115:182–205
Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD (2013) Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology 145:521–536
Bergquist A, Lindberg G, Saarinen S, Broome U (2005) Increased prevalence of primary sclerosing cholangitis among first-degree relatives. J Hepatol 42:252–256
Karlsen TH, Franke A, Melum E et al (2010) Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology 138:1102–1111
Hirschfield GM, Karlsen TH, Lindor KD, Adams DH (2013) Primary sclerosing cholangitis. Lancet 382:1587–1599
Tabibian JH, Talwalkar JA, Lindor KD (2013) Role of the microbiota and antibiotics in primary sclerosing cholangitis. Biomed Res Int 2013:389537
Bjornsson E, Cederborg A, Akvist A, Simren M, Stotzer PO, Bjarnason I (2005) Intestinal permeability and bacterial growth of the small bowel in patients with primary sclerosing cholangitis. Scand J Gastroenterol 40:1090–1094
Pohl J, Ring A, Stremmel W, Stiehl A (2006) The role of dominant stenoses in bacterial infections of bile ducts in primary sclerosing cholangitis. Eur J Gastroenterol Hepatol 18:69–74
Olsson R, Bjornsson E, Backman L et al (1998) Bile duct bacterial isolates in primary sclerosing cholangitis: a study of explanted livers. J Hepatol 28:426–432
O'Mahony CA, Vierling JM (2006) Etiopathogenesis of primary sclerosing cholangitis. Semin Liver Dis 26:3–21
O'Hara SP, Tabibian JH, Splinter PL, LaRusso NF (2013) The dynamic biliary epithelia: molecules, pathways, and disease. J Hepatol 58:575–582
Folseraas T, Melum E, Rausch P et al (2012) Extended analysis of a genome-wide association study in primary sclerosing cholangitis detects multiple novel risk loci. J Hepatol 57:366–375
Chambers JC, Zhang W, Sehmi J et al (2011) Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat Genet 43:1131–1138
Pratt DS, Kaplan MM (2000) Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 342:1266–1271
Chapman R, Fevery J, Kalloo A et al (2010) Diagnosis and management of primary sclerosing cholangitis. Hepatology 51:660–678
Beuers U, Hohenester S, de Buy Wenniger LJ, Kremer AE, Jansen PL, Elferink RP (2010) The biliary HCO(3)(−) umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology 52:1489–1496
Hohenester S, Wenniger LM, Paulusma CC et al (2012) A biliary HCO3- umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology 55:173–183
Meesmann HM, Fehr EM, Kierschke S et al (2010) Decrease of sialic acid residues as an eat-me signal on the surface of apoptotic lymphocytes. J Cell Sci 123:3347–3356
Linnartz B, Kopatz J, Tenner AJ, Neumann H (2012) Sialic acid on the neuronal glycocalyx prevents complement C1 binding and complement receptor-3-mediated removal by microglia. J Neurosci 32:946–952
Krahling H, Mally S, Eble JA, Noel J, Schwab A, Stock C (2009) The glycocalyx maintains a cell surface pH nanoenvironment crucial for integrin-mediated migration of human melanoma cells. Pflugers Arch 458:1069–1083
Stock C, Mueller M, Kraehling H et al (2007) pH nanoenvironment at the surface of single melanoma cells. Cell Physiol Biochem 20:679–686
Azevedo M, Eriksson S, Mendes N et al (2008) Infection by Helicobacter pylori expressing the BabA adhesin is influenced by the secretor phenotype. J Pathol 215:308–316
Ikehara Y, Nishihara S, Yasutomi H et al (2001) Polymorphisms of two fucosyltransferase genes (Lewis and secretor genes) involving type I Lewis antigens are associated with the presence of anti-Helicobacter pylori IgG antibody. Cancer Epidemiol Biomarkers Prev 10:971–977
Lee HS, Choe G, Kim WH, Kim HH, Song J, Park KU (2006) Expression of Lewis antigens and their precursors in gastric mucosa: relationship with Helicobacter pylori infection and gastric carcinogenesis. J Pathol 209:88–94
Magalhaes A, Gomes J, Ismail MN et al (2009) Fut2-null mice display an altered glycosylation profile and impaired BabA-mediated Helicobacter pylori adhesion to gastric mucosa. Glycobiology 19:1525–1536
Evans DA, Horwich L, McConnell RB, Bullen MF (1968) Influence of the ABO blood groups and secretor status on bleeding and on perforation of duodenal ulcer. Gut 9:319–322
Carlsson B, Kindberg E, Buesa J et al (2009) The G428A nonsense mutation in FUT2 provides strong but not absolute protection against symptomatic GII.4 Norovirus infection. PLoS ONE 4:e5593
Kindberg E, Akerlind B, Johnsen C et al (2007) Host genetic resistance to symptomatic Norovirus (GGII.4) infections in Denmark. J Clin Microbiol 45:2720–2722
Kaplan JE, Gary GW, Baron RC et al (1982) Epidemiology of Norwalk gastroenteritis and the role of Norwalk virus in outbreaks of acute nonbacterial gastroenteritis. Ann Intern Med 96:756–761
Huang P, Farkas T, Marionneau S et al (2003) Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J Infect Dis 188:19–31
Larsson MM, Rydell GE, Grahn A et al (2006) Antibody prevalence and titer to Norovirus (genogroup II) correlate with secretor (FUT2) but not with ABO phenotype or Lewis (FUT3) genotype. J Infect Dis 194:1422–1427
Van Kruiningen HJ, Mayo DR, Vanopdenbosch E, Gower-Rousseau C, Cortot A, Colombel JF (2000) Virus serology in familial Crohn disease. Scand J Gastroenterol 35:403–407
Chamaillard M, Cesaro A, Lober PE, and Hober D (2013) Decoding Norovirus infection in Crohn’s disease. Inflamm Bowel Dis
Imbert-Marcille BM, Barbe L, Dupe M, et al. (2013), A FUT2 gene common polymorphism determines resistance to Rotavirus A of the P [8] genotype. J Infect Dis
Kinane DF, Blackwell CC, Brettle RP, Weir DM, Winstanley FP, Elton RA (1982) ABO blood group, secretor state, and susceptibility to recurrent urinary tract infection in women. Br Med J (Clin Res Ed) 285:7–9
Hooton TM (2000) Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother 46(Suppl 1):1–7, discussion 63–5
Ishitoya S, Yamamoto S, Mitsumori K, Ogawa O, Terai A (2002) Non-secretor status is associated with female acute uncomplicated pyelonephritis. BJU Int 89:851–854
Ben-Aryeh H, Blumfield E, Szargel R, Laufer D, Berdicevsky I (1995) Oral Candida carriage and blood group antigen secretor status. Mycoses 38:355–358
Thom SM, Blackwell CC, MacCallum CJ et al (1989) Non-secretion of blood group antigens and susceptibility to infection by Candida species. FEMS Microbiol Immunol 1:401–405
Chaim W, Foxman B, Sobel JD (1997) Association of recurrent vaginal candidiasis and secretory ABO and Lewis phenotype. J Infect Dis 176:828–830
Blackwell CC, Jonsdottir K, Hanson MF, Weir DM (1986) Non-secretion of ABO blood group antigens predisposing to infection by Haemophilus influenzae. Lancet 2:687
Blackwell CC, Jonsdottir K, Hanson M et al (1986) Non-secretion of ABO antigens predisposing to infection by Neisseria meningitidis and Streptococcus pneumoniae. Lancet 2:284–285
Tanaka T, Scheet P, Giusti B et al (2009) Genome-wide association study of vitamin B6, vitamin B12, folate, and homocysteine blood concentrations. Am J Hum Genet 84:477–482
Lin X, Lu D, Gao Y et al (2012) Genome-wide association study identifies novel loci associated with serum level of vitamin B12 in Chinese men. Hum Mol Genet 21:2610–2617
Hazra A, Kraft P, Lazarus R et al (2009) Genome-wide significant predictors of metabolites in the one-carbon metabolism pathway. Hum Mol Genet 18:4677–4687
Watanabe F (2007) Vitamin B12 sources and bioavailability. Exp Biol Med (Maywood) 232:1266–1274
Oussalah A, Besseau C, Chery C et al (2012) Helicobacter pylori serologic status has no influence on the association between fucosyltransferase 2 polymorphism (FUT2 461 G- > A) and vitamin B-12 in Europe and West Africa. Am J Clin Nutr 95:514–521
Chery C, Hehn A, Mrabet N et al (2013) Gastric intrinsic factor deficiency with combined GIF heterozygous mutations and FUT2 secretor variant. Biochimie 95:995–1001
Tang H, Jin X, Li Y et al (2014) A large-scale screen for coding variants predisposing to psoriasis. Nat Genet 46:45–50
Ellinghaus D, Ellinghaus E, Nair RP et al (2012) Combined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility loci. Am J Hum Genet 90:636–647
Xavier JM, Shahram F, Sousa I, et al. (2013) FUT2: filling the gap between genes and environment in Behcet’s disease? Ann Rheum Dis
van Meurs JB, Pare G, Schwartz SM et al (2013) Common genetic loci influencing plasma homocysteine concentrations and their effect on risk of coronary artery disease. Am J Clin Nutr 98:668–676
Tregouet DA, Sabater-Lleal M, Bruzelius M et al (2012) Lack of association of non-synonymous FUT2 and ALPL polymorphisms with venous thrombosis. J Thromb Haemost 10:1693–1695
Suhre K, Shin SY, Petersen AK et al (2011) Human metabolic individuality in biomedical and pharmaceutical research. Nature 477:54–60
Orntoft TF, Greenwell P, Clausen H, Watkins WM (1991) Regulation of the oncodevelopmental expression of type 1 chain ABH and Lewis(b) blood group antigens in human colon by alpha-2-L-fucosylation. Gut 32:287–293
Yazawa S, Nakamura J, Asao T et al (1993) Aberrant alpha 1– > 2fucosyltransferases found in human colorectal carcinoma involved in the accumulation of Leb and Y antigens in colorectal tumors. Jpn J Cancer Res 84:989–995
Sun J, Thurin J, Cooper HS et al (1995) Elevated expression of H type GDP-L-fucose:beta-D-galactoside alpha-2-L-fucosyltransferase is associated with human colon adenocarcinoma progression. Proc Natl Acad Sci U S A 92:5724–5728
Teresa DB, Santos RA, Takahashi CS et al (2010) Polymorphisms of Lewis and secretor genes are related to breast cancer and metastasis in axillary lymph nodes. Tumour Biol 31:401–409
Kannagi R, Fukushi Y, Tachikawa T et al (1986) Quantitative and qualitative characterization of human cancer-associated serum glycoprotein antigens expressing fucosyl or sialyl-fucosyl type 2 chain polylactosamine. Cancer Res 46:2619–2626
Narimatsu H, Iwasaki H, Nakayama F et al (1998) Lewis and secretor gene dosages affect CA19-9 and DU-PAN-2 serum levels in normal individuals and colorectal cancer patients. Cancer Res 58:512–518
Wannhoff A, Hov JR, Folseraas T et al (2013) FUT2 and FUT3 genotype determines CA19-9 cut-off values for detection of cholangiocarcinoma in patients with primary sclerosing cholangitis. J Hepatol 59:1278–1284
He M, Wu C, Xu J et al (2014) A genome wide association study of genetic loci that influence tumour biomarkers cancer antigen 19–9, carcinoembryonic antigen and alpha fetoprotein and their associations with cancer risk. Gut 63:143–151
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maroni, L., van de Graaf, S.F.J., Hohenester, S.D. et al. Fucosyltransferase 2: A Genetic Risk Factor for Primary Sclerosing Cholangitis and Crohn's Disease—A Comprehensive Review. Clinic Rev Allerg Immunol 48, 182–191 (2015). https://doi.org/10.1007/s12016-014-8423-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-014-8423-1