Abstract
The immune system has evolved to respond to pathogens (nonself) and unresponsive to self-antigens (tolerance). During the development of T and B cells in the thymus and bone marrow, respectively, self-reactive T and B cells are deleted by a process of apoptosis (both T and B cells) and become unresponsive to self-antigen by receptor editing (for B cells). However, few self-reactive T cells are leaked into the periphery. A number of mechanisms are responsible to ensure that self-reactive T and B cells remain unresponsive to self-antigens. In the central tolerance, major mechanisms include apoptosis (for T cells) and receptor editing (for B cells), and in the peripheral tolerance, a major mechanism appears to be regulated by Treg cells. In T cell central tolerance, one of the most important molecules is a transcription factor, autoimmune regulator, which is selectively expressed in medullary thymic epithelial cells (mTECs) and constitutively regulates the transcription of hundreds of self-antigens in mTECs, thereby inducing central tolerance, negative selection, and Treg differentiation from some self-reactive thymocytes. Primary immunodeficiency diseases are a group of monogenic diseases where mutations of certain genes have resulted in the loss of central and/or peripheral tolerance. As a result autoimmunity and autoimmune diseases are common among patients with primary immunodeficiency diseases. Here, we have provided a comprehensive review of the mechanisms of central and peripheral tolerance and autoimmune manifestations and mechanisms of autoimmunity in primary immunodeficiency diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A key feature of the immune system is to control infections from pathogens (nonself) while maintaining tolerance to self-antigens. Central tolerance induces deletion of self-reactive T cells during development in the thymus, whereas peripheral tolerance ensures that self-reactive T cells that escape central tolerance checkpoints remain unresponsive in peripheral organs. Any breakdown of either central or peripheral tolerance can lead to autoimmunity. Primary immunodeficiency disorders present with a paradox of failure to respond to nonself pathogens resulting in an increased susceptibility to infections while reacting to self-antigens, therefore, high prevalence of autoimmunity [1].
Recent findings in several monogenic human immune disorders (where animal models exist) have provided evidence how immune deficiency can provoke breakdown in central and peripheral tolerance and, consequently, the development of autoimmunity [2, 3]. Here, we have briefly reviewed both central and peripheral tolerances and discuss autoimmunity and autoimmune diseases associated with primary immunodeficiency diseases (PID).
Central Tolerance
In the T cell central tolerance, one of the most important molecules is a transcription factor, autoimmune regulator (AIRE). This transcription factor is selectively expressed in medullary thymic epithelial cells (mTECs) and constitutively regulates the transcription of hundreds of self-antigens in mTECs, thereby inducing central tolerance, negative selection, and Treg differentiation from some self-reactive thymocytes [4–6]. mTEC can directly present endogenously expressed self-antigens to thymocytes. Furthermore, it is of interest that AIRE can concomitantly induce the apoptosis of mTEC itself. Then, dendritic cells (DCs) residing in the medulla can engulf them and cross-present self-antigen to the thymocytes to induce the negative selection and Treg differentiation.
Studies in PID patients and animal models of PID have further supported a critical role of Aire in deletion of self-reactive T cells in the thymus (central or recessive tolerance). Mice deficient in AIRE function develop autoimmune disorders, and humans with AIRE mutation [2] develop autoimmune polyendrocrinopathy candidiasis ectodermal dystrophy (APECED) syndrome.
Baba and associates [7] have shown that signal regulatory protein α + (Sirpa+) DCs in the thymus can efficiently capture blood-borne antigens across the blood–thymus barrier and subsequently induce the antigen-specific Treg cells and clonal deletion, depending on the intensity of antigen presentation. Therefore, it is suggested that thymic Sirpa+ cDCs may function as a generator of the AIRE-independent central tolerance against blood-borne antigens.
The central B cell tolerance is mediated by the deletion of self-reactive B cells and by receptor editing; however, receptor editing is a major mechanism of central tolerance in B cells [8]. Immature B cells reacting with high affinity to self-antigen undergo apoptosis (deletion). This deletion mechanism may be crucial when all receptor editing options are exhausted. In receptor editing, immature B cells in the bone marrow that encounter multivalent self-antigens revert to pre-B cell-like phenotype, reexpress Rag genes, and induce κ light chain gene rearrangements (if necessary, λ). New Vκ-Jκ rearrangements, involving upstream V segment and downstream J segment, delete the original Vκ-Jκ rearrangement that had produced self-reactive light chain, resulting in newly generated B cells that have a novel light chain that no longer reactive to self-antigen. These immature B cells then migrate to the periphery where they mature into IgM- and IgD-bearing B cells.
Peripheral Tolerance
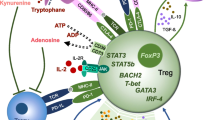
A number of mechanisms appear to play a role in peripheral tolerance, including Treg, Breg, natural autoantibodies, and tolerogenic myeloid and plasmacytoid DCs. Breg deficiency is associated with autoimmune diseases in both mice and humans [8, 9]. However, Treg (CD4+FoxP3+CD25+) appears to be the major cell type that is responsible for peripheral T cell tolerance [10], and the suppressive function is mediated by transcription factor FoxP3. FoxP3 mutations resulting in immunodysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome in humans and in Scurfy mice have provided evidence for the critical role of Tregs in peripheral tolerance and demonstrated that autoreactive T cells do escape central tolerance checkpoints in normal host [2, 11–13]. Furthermore, ectopic expression of FoxP3 is sufficient to induce suppressive function to conventional CD4+CD25− T cells. FoxP3 appears to induce a suppressive function through the association with other transcription factors (NFAT, NF-κB, and Runx1). Tregs form long-lasting synapses with antigen-primed DCs in vivo and compete with effector T cells for DC interaction.
Peripheral B cell tolerance is mediated by a number of mechanisms, including anergy, clonal deletion of mature B cells that interact with self-antigen with high affinity, antigen-specific inhibition by Treg, clonal ignorance mediated by Siglec/SIAE pathway, and inhibition of B cells by stimulation via FcγRIIb [8]. The role of Breg in peripheral B cell tolerance remains to be defined. Natural autoantibodies may play a minor role via their housekeeping function of removing self-antigen containing apoptotic cells and apoptotic bodies.
Autoimmune diseases in primary immunodeficiency can be classified as primary immunodeficiency defined by autoimmune manifestations, autoimmune diseases associated with primary immunodeficiency with well-defined single-gene defects, and autoimmune diseases associated with primary immunodeficiency diseases in which gene defects have not been clearly defined (Table 1). Recently, clinical features and molecular defects in primary immunodeficiencies have been reviewed [14].
Primary Immunodeficiency Defined by Autoimmune Manifestations
Autoimmune Polyendrocrinopathy Candidiasis Ectodermal Dystrophy
APECED is also known as autoimmune polyendocrine syndrome I and Whitaker syndrome. APECED is characterized by a triad of chronic mucocutaneous candidiasis, hypoparathyroidism, and adrenal insufficiency. Candidiasis is usually the first clinical manifestation (5 years of age), followed by hypoparathyroidism (10 years), and adrenocortical failure (15 years). Mutations in Aire gene appear to be the major mechanism for APECED [15, 16]. However, it is unclear why there is a long latent period for the development of autoimmune disease. Autoimmune diseases associated with APECED include hypothyroidism, hypogonadism, type I diabetes mellitus, autoimmune hepatitis, pernicious anemia, vitiligo, alopecia, primary biliary cirrhosis, and ectodermal dysplasia. Therapy includes immunosuppressive agents like corticosteroids and cyclosporine A, and hematopoietic stem cell transplantation.
Immunodysregulation Polyendocrinopathy Enteropathy X-Linked Syndrome
IPEX is a rare disorder, which is (and Scurfy mice) due to mutation in Foxp-3 gene resulting in defective development of CD4+CD25+Foxp-3+ regulatory T cells, and hyperactive CD4+ T cells, and an increased production of inflammatory cytokines [17]. As a consequence, patients develop multiorgan autoimmunity, eczema, and recurrent infections. Patients with IPEX usually die before the age of 2. Scurfy mice were described with mutation in Foxp-3 gene and autoimmunity [12, 13]. Autoimmune diseases include autoimmune enteritis and type I diabetes mellitus, which occur during first few months of life, autoimmune hemolytic anemia, hypothyroidism, and membranous glomerulonephritis. Stable hematopoietic cell engraftment following low-intensity non-myeloablative conditioning corrects immune deficiency (including Treg), enteropathy, and hemolytic anemia.
Autoimmune Lymphoproliferative Syndrome
Autoimmune lymphoproliferative syndrome (ALPS) is inherited as an autosomal dominant trait and is characterized by lymphadenopathy, splenomegaly, and polyclonal hyperimmunoglobulinemia, autoimmunity, and lymphocytosis of circulating polyclonal CD3+CD4−CD8− TCRαβ+ T cells (double negative). Double-negative T cells express dysregulated eomesodermin transcription factor.
ALPS is characterized by gene mutations involved in CD95–CD95L death receptor signaling pathway of apoptosis, including mutations in CD95, CD95L, caspase-8, and caspase-10 [18]. A variety of autoimmune manifestations are present in ALPS (Table 2). Autoimmune cytopenias are usually severe or become severe with age. Therapy of ALPS depends upon severity of the disease. A short course of steroids for immune cytopenias is usually effective. In severe cases, this includes steroids; high-dose IVIG; alemtuzumab (Campath-1H), a humanized monoclonal antibody against CD52 expressed on lymphocytes; or pentostatin (adenosine deaminase inhibitor). Splenectomy may be helpful in cases with hypersplenism. Finally, hematopoietic stem cell transplantation was used for severe cases that are refractory to multiple agents.
Autoimmune Manifestations in Primary Immunodeficiencies with Defined Gene Defects
Severe Combined Immunodeficiencies
Omenn Syndrome
Hypomorphic mutations in RAG1/RAG2 in SCID may allow residual T cell development. In these cases, there is an impairment of interaction between medullary thymic epithelial cells and thymocytes, resulting in an impaired central tolerance. As a result, there is a failure to delete self-reactive T cells and to generate Treg cells, and autoimmune manifestations are common [19]. Omenn syndrome is a prototype, which is characterized by expansion of oligoclonal T cells (mostly memory T cells), elevated IgE, erythroderma, lymphadenopathy, and inflammatory bowel disease [19].
STAT5b Deficiency
STAT5b plays an important role in cellular functions of proliferation, differentiation, and apoptosis. STAT5b is activation by cytokines and their cognate cytokine receptors via JAK/STAT5b signaling pathway. STAT5b deficiency is a rare autosomal recessive disorder, which is commonly associated with homozygous and heterozygous missense mutations of STAT5b gene [20]. Different genotypes contribute to STAT5b deficiency and associated with variable clinical features. The majority of patients present clinically with growth defects, eczema, chronic severe lung disease including lymphocytic interstitial pneumonitis and recurrent pulmonary infections, viral infection, and autoimmune diseases. Various autoimmune diseases include autoimmune thrombocytopenia, autoimmune thyroiditis, and systemic juvenile rheumatoid arthritis. Immunological abnormalities include moderate lymphopenia with very low numbers of natural killer (NK) and T cells (CD4+ and CD8+) and impaired T cell functions, including impaired IL-2 receptor signaling. The reduced numbers and impaired functions of Treg suggest that in humans, Treg cells require activation of STAT5b [21]. STAT5b deficiency has an impact on the coexpression by CD4+ T cells of CD25 and FOXP3.
IL-2Rα (CD25) Deficiency
The high-affinity IL-2 receptor is composed of three subunits: α (CD25), β (CD122), and γ (γ common chain). Β and γ chains are constitutively expressed on T cells; however, α chain expression is restricted in early stages of thymocyte development and on activated mature T cells. In older mice, CD25 deficiency is associated with T and B cells that are expanded as a result of impaired apoptosis and display an increased propensity to autoimmune disorders [22]. In humans, CD25 deficiency, which is caused by a 4-bp deletion resulting in complete lack of CD25, is much more severe and produces profound cellular deficiency, which presents early in life with recurrent infections, and dramatic lymphocyte infiltration in multiple tissues [23]. CD25 deficiency is a rare monogenic primary immune deficiency with autoimmune manifestations. Roifman [24] also reported that CD25 deficiency is associated with decreased apoptosis in the thymus and therefore impaired negative selection, resulting in the expansion of autoreactive T cell clones in multiple tissues, causing inflammation. Since CD25 molecule is critical for the generation, survival, and suppressive function of Treg cells, CD25 deficiency is also associated with impaired peripheral tolerance.
Immunodeficiency Due to Stromal Interacting Molecule 1 Mutation
Lymphocyte activation requires intracellular Ca++ entry through specialized Ca++ channels in the plasma membrane, the Ca++ release-activated Ca++ (CRAC) channels encoded by the ORAI1 gene. ORAI1 is activated by stromal interacting molecule (STIM)1 that is localized in the endoplasmic reticulum (ER), where it senses the concentration of stored Ca++. Following T cell activation via TCR, ER stores of Ca++ are depleted, resulting in the activation of STIM1 and opening of ORAI1 CRAC channel and store-operated Ca++ entry. Mutation in STIM1 and ORAI1 genes results in a unique clinical phenotype characterized by immunodeficiency, muscular hypotonia, and anhydrotic ectodermal dysplasia [25]. In addition, STIM1 deficiency is associated with autoimmune thrombocytopenia and hemolytic anemia and lymphoproliferation. A reduction in Treg has been observed, which might contribute to autoimmunity in patients with STIM1 deficiency [26].
Hyper-IgM Syndromes
Hyper-IgM syndromes (HIGMS) are a heterogeneous group of genetic disorders resulting from defects of Ig class switch recombination (CSR), with or without defects of somatic hypermutation (SHM), resulting in deficiency of IgG, IgA, and IgE with normal or increased levels of IgM [14]. Patients with HIGMS are susceptible to bacterial and/or opportunistic infections, autoimmunity, and autoimmune diseases, and increased frequency of lymphoma [27]. Most common mutational defect is in CD40L gene (X-linked HIGMS) followed by mutations in activation-induced cytidine deaminase (AID) gene, which plays an important role in CSR and SHM [14]. Other gene mutations associated with HIGMS include uracil-DNA glycosylase (UNG), NF-κB essential modulator (NEMO), and CD40. HIGMS associated with different gene mutations appear to have different autoimmune diseases (Table 3).
Type I HIGMS
It is an X-linked disorder due to CD40L mutation. CD40L is expressed on activated T cells, which allows them to interact with CD40 on B cells, DCs, and macrophages. The engagement of CD40L with CD40 on B cells results in initiation of downstream signaling resulting in NF-κB activation and nuclear translocation. AID and UNG are NF-κB responsive genes and are important in CSR and SHM. These patients are impaired in the generation of memory B cells. Patients present in early life with bacterial, viral, fungal, and opportunistic infections. Autoimmune manifestations are common. A number of autoantibodies, including anti-RNP, anti-Ro, antiplatelet, anticardiolipin, anti-smooth muscle, antithyroid, and other autoantibodies, may be present.
Type II HIGMS
Type II HIGMS is generally inherited as an autosomal recessive (sometimes autosomal dominant) trait and is due to mutations in AID gene. It is characterized by enlarged tonsils and lymph nodes, recurrent bacterial sinopulmonary infections, and no opportunistic infections. We have observed autoimmune thrombocytopenia due to surface bound IgM antiplatelet antibodies and systemic lupus erythematosus (SLE) in our patients.
Type III HIGMS
It is clinically similar to type II HIGMS, inherited as autosomal recessive trait, and is due to CD40 gene mutation. Both impaired CSR and SHM are observed.
Type IV HIGMS
It resembles a milder form of type II with some IgG production. There is defect in CSR, but normal SHM. No gene defect has been identified.
Type V HIGMS
It is inherited as an autosomal trait and characteristically present similar to type II HIGMS. It is due to mutations in UNG gene. Only few cases have been reported.
Type VI HIGMS
It is an X-linked disorder and characterized by hypohidrotic ectodermal dysplasia and immunodeficiency, which is associated with mutation of the IKBKG gene that encodes for the NF-κB essential modulator (NEMO), which plays an important role in NF-κB signaling pathway. Most patients present with hypogammaglobulinemia with or without elevated IgM
Wiskott–Aldrich Syndrome
Wiskott–Aldrich syndrome (WAS) is an X-linked disease characterized by a triad of increased susceptibility to infections due to immunodeficiency, eczema, and thrombocytopenia with small platelets. WAS is caused by mutations of the WASP gene, which encodes for a regulator of actin cytoskeleton [28]. WAS protein (WASP) is expressed on all nonerythroid hematopoietic cells and plays a role in actin polymerization and cytoskeleton remodeling. As a result, chemokine-induced migration, and trafficking of monocytes, DCs, PMN, T cells, and B cells is impaired in WAS. Both innate and adaptive immune responses are affected in WAS. WASP is essential for optimal signaling through the TCR and stabilizes synapses between T cells and APC. WASP-deficient DCs have reduced ability to form immune synapses with naïve CD8+ T cells. Furthermore, WAS is associated with deficient function of Treg cells (unrelated to IL-2). Patients with WAS display high prevalence of lymphoid malignancies and autoimmune diseases (up to 70 % of patients have at least one autoimmune disorder), which include hemolytic anemia, neutropenia, arthritis, cutaneous vasculitis, glomerulonephritis, and inflammatory bowel disease.
Hyper-IgE Syndromes
The hyper-IgE syndromes (HIES) are characterized by increased susceptibility to cutaneous and sinopulmonary infections, predominantly caused by Staphylococcus aureus and Candida species; eczema; and extremely elevated IgE levels [29]. Autosomal dominant, autosomal recessive, and sporadic forms have been reported. The autosomal dominant form is due to dominant-negative heterozygous mutations of the STAT3 gene and is associated with characteristic facial appearance, teeth and bone changes, and vascular abnormalities, including aneurysms. Autosomal recessive form of HIES is different from autosomal dominant form; they do not present with teeth or bony abnormalities and might be susceptible to viral diseases; vasculitis and autoimmunity are common.
Chronic Granulomatous Disease
Chronic granulomatous disease (CGD) is a result of deficient oxidative burst and impaired generation of reactive oxygen species (ROS) due to mutations in genes encoding for NADPH oxidase complex [30]. The X-linked CGD is most common and is due to mutation in CYBB gene encoding for NOX-2 (gp91phox). Autosomal recessive forms are caused by defects of the p22phox, p47phox, and p67phox. Patients present with recurrent bacterial and fungal infections and often with noninfectious granulomata. In addition, patients with X-linked CGD are prone to autoimmune diseases, including Crohn’s disease, rheumatoid arthritis, discoid lupus, systemic lupus erythematosus, idiopathic thrombocytopenia, and Behçet’s disease. Apoptosis of neutrophils is impaired in neutrophils from patients with CGD as well as in experimental models of X-linked CGD. Kraaij et al. [31] reported that macrophage-derived ROS induce Tregs. They showed that rat or human CGD with mutated p47phox or gp91phox displayed impaired macrophage-induced Treg and T cell suppression. However, basal Treg numbers in these subjects were comparable to those in controls, indicating a role for ROS in the induction of peripheral Tregs. Therefore, it appears that NAPDH oxidase activity in macrophages is important for the induction of Tregs to regulate T cell-mediated inflammation. Hematopoietic stem cell transplantation remains the curative therapy for CGD.
Dendritic Cell Deficiency
Recently, three different genetically defined DC deficiency syndromes have been described [32]. The largest group is associated with GATA-2 mutation and termed as DC, monocyte, B, and NK lymphoid (DCML) deficiency; others are due to mutations in interferon regulatory factor 8 (IRF-8) gene, and human autosomal recessive DCML resembles to IRF-8 deficiency in mice. Two distinct mutations (K108E and T80A) in IRF-8 gene have been described. Patients with DCML present clinically with infections, leukemic transformation (myelodysplasia and acute myeloid leukemia), and pulmonary alveolar proteinosis. Approximately 25 % of patients with DCML present with associated autoimmune diseases including erythema nodosum, panniculitis, and arthritis. Patients with GATA-2 (DCML) and IRF-8 (K108E) mutations have reduced numbers of Treg cells; however, it is unclear whether loss of Treg cells is the predominant mechanism for autoimmunity.
Autoimmunity Associated with Hereditary Complement Deficiencies
Complement component deficiencies, especially of early components of classical pathway (C1q, C1r, C1s, C4, and C2), are associated with autoimmune diseases, especially systemic lupus erythematosus [33, 34].
The complete deficiency of C1q, C1r, and C1s are rare; however, this is associated with a high risk of developing pediatric SLE. C1q deficiency is more than 90 % homozygous, and C1q-deficient patients present with “SLE-like” syndrome. There is a high frequency of glomerulonephritis and CNS disease, marked photosensitivity, and impaired clearance of apoptotic debris, and dissolution of immune complexes. In C1r/C1s deficiency, >50 % of patients develops SLE. Multiorgan involvement, including glomerulonephritis, occurs in 30 % of patients. One of the mechanisms appears to be an impaired clearance of immune complexes.
C4 deficiency is very rare (25 cases); however, 75 % of patients develop SLE, and glomerulonephritis, in 30 %. Association is observed between homozygous C4A (not C4B) deficiency and SLE. Impaired clearance of immune complexes appears to be the major mechanism for autoimmunity.
Homozygous C2 deficiency is the most frequent hereditary deficiency in classical complement pathway (1/10,000–1/30,000 Caucasian). SLE is as severe as SLE occurring among patients without inherited complement deficiency and usually starts in adulthood. Photosensitivity and articular involvement are prominent. There is mild or no renal or CNS involvement. There is low frequency of ANA and high frequency of anti-Ro antibodies. Other manifestations may include ANCA + vasculitis. Impaired dissolution of immune complexes is considered a mechanism for autoimmunity.
Autoimmunity in Primary Immunodeficiency with not Well-Defined Mechanisms for Autoimmunity
Common Variable Immunodeficiency
Common variable immunodeficiency (CVID) is the most frequent symptomatic PID in adults. Autoimmune manifestations occur in approx 22 % of patients at the time of diagnosis of CVID (Table 4); however, this prevalence has been 36 % when patients with CVID were followed for several years. Autoimmune diseases are more common in women and among patients with granuloma [35]. Furthermore, autoimmune diseases are more commonly organ specific. ITP frequently precedes other manifestations; other cytopenias may occur later in the course of disease. Genetic defects include mutations in inducible costimulator, transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), B-cell-activating factor of the tumor necrosis factor family receptors, and CD19 [36–40]. However, the role of some of these gene mutations in the pathogenesis of CVID remains uncertain. For example, TACI mutations are also observed in healthy population. The pathogenesis of autoimmunity in CVID remains unknown. A number of hypothesis have been put forward, which include lack of removal of autoreactive B cells due to ineffective BCR signaling, abnormal ligand interaction (reduced CD40 expression on B cells), accelerated expansion of autoreactive B cells, and increased levels of BAFF/April.
Yu et al. [41] have reported that patients with CVID and autoimmune diseases have decreased activation of STAT5b through decreased phosphorylation of STAT5b protein. This decreased activation of STAT5b was correlated with impaired Treg cell function. STAT5b appears to influence the expression of FOXP3 on CD4+CD25+ T cells.
Selective IgA Deficiency
Autoimmunity is one of the most frequent manifestations of selective IgA deficiency (SIgAD) (beside infections), with many displaying autoantibodies including Jo1, ANA, antithyroid peroxidase, anti-thyroglobulin, anti-cardiolipin, anti-phosphatidylserine, and antibodies to collagen without autoimmune diseases [42]. Autoimmunity is more prevalent in adults and in females. Both systemic and organ-specific autoimmune diseases are observed (Table 5). Ten percent of first-degree relatives of patients with selective IgA deficiency develop autoimmunity as compared to 5 % in general population. Though mechanism(s) of autoimmunity in SIgAD is not known, several possible mechanisms proposed include (1) absorption of environmental antigen with cross reaction to self-antigen (molecular mimicry), (2) decreased antigen clearance resulting in immune complex deposition, and (3) mutation in TACI gene.
Summary
Studies of several monogenic human immune disorders and their animal models have been instrumental in defining a paradox of immunodeficiency and autoimmunity. Aire gene and apoptosis of self-reactive T cells, and receptor editing and deletion of self-reactive B cells, play an important role in T and B cells central tolerance, respectively, whereas Treg (and possibly Breg and natural autoantibodies) and apoptosis appear to play a role in peripheral tolerance. Break in central and/or peripheral tolerance appears to play a role in autoimmunity and autoimmune diseases associated with primary immunodeficiencies. Patients with autoimmune diseases and a history of recurrent or unusual infections may suggest underlying primary immunodeficiency.
References
Nortarangelo LD, Gambineri E, Badolato R (2006) Immunodeficiencies with autoimmune consequences. Adv Immunol 89:321–370
Westerberg LS, Klein C, Snapper SB (2008) Breakdown of T cell tolerance and autoimmunity in primary immunodeficiency—lessons learned from monogenic disorders in mice and men. Curr Opin Immunol 20:646–654
Ulmanen I, Halonen M, Ilmarinen T, Peltonen L (2005) Monogenic autoimmune diseases—lessons of self-tolerance. Curr Opin Immunol 17:609–615
Derbinski J, Schulte A, Kyewski B, Klein L (2001) Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol 2:1032–1039
Mathis D, Benoist C (2009) Aire. Ann Rev Immuno 27:287–312
Hogquist KA, Baldwin TA, Jameson SC (2005) Central tolerance: learning self-control in the thymus. Nat Rev Immunol 5:772–782
Baba T, Nakamoto Y, Mukaida N (2009) Crucial contribution of thymic Sirp alpha + conventional dendritic cells to central tolerance against blood-borne antigens I a CCR2-dependent manner. J Immunol 183:3053–3063
Pillai S, Mattoo H, Cariappa A (2011) B cells and autoimmunity. Curr Opin Immunol 23:721–731
Mauri C, Bosma A (2012) Immune regulatory function of B cells. Ann Rev Immunol 30:221–241
Sakaguchi S, Yamaguchi T, Nomura T, Ono R (2008) Regulatory T cells and immune tolerance. Cell 133:775–787
Moraes-Vasconcelos D, Costa-Carvalho BT, Torgerson TR, Ochs HD (2008) Primary immune deficiency disorders presenting as autoimmune diseases: IPEX and APECED. J Clin Immunol 28(Suppl 1):S11–S19
Brunkow ME, Jeffrey EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA et al (2001) Disruption of a new forkhead/winged-helix protein, scurfin, results in fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 27:68–73
Notarangelo JD (2010) Primary immunodeficiencies. J Allergy Clin Immunol 125:S182–S194
Finish-German APECED Consortium (1997) An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zing-finger domains. Nat Genet 17:399–403
Villaseñor J, Benoist C, Mathis D (2005) AIRE and APECED: molecular insights into an autoimmune disease. Immunol Rev 204:156–164
Wildin RS, Smyk-Pearson S, Filipovich AH (2002) Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Gent 39:537–545
Bosco JB, Gupta S (2008) Disorders of apoptosis: mechanisms for autoimmunity in primary immunodeficiency diseases. J Clin Immunol 28:S20–S28
Poliani PL, Facchetti F, Ravanini M, Gennery AR, Villa A, Roifman CM et al (2009) Eraly defects in T cell development severely affect distribution and maturation of thymic stromal cells: possible implications for the pathophysiology of Omenn syndrome. Blood 114:105–108
Nadeau K, Hwa V, Rosenfeld RG (2011) STAT5b deficiency: an unsuspected cause of growth failure, immunodeficiency, and sever pulmonary disease. J Ped 158:701–708
Cohen AC, Nadeau KC, Tu W, Hwa V, Dionis K, Dezrodnik I et al (2006) Decreased accumulation and regulatory function of CD4+ CD25 (high) T cells in human STAT5b deficiency. J Immunol 177:2770–2774
Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW (1995) Interleukin 2 receptor alpha chain regulate the size and content of the peripheral lymphoid compartment. Immunity 3:521–530
Sharfe N, Dadi HK, Shahar M, Roifman CM (1997) Human immune disorder arising from mutation of the α chain of the interleukin-2 receptor. Proc Natl Acad Sci (USA) 94:3168–3171
Roifman CM (2000) Human IL-2 receptor alpha chain deficiency. Pediatr Res 48:6–11
Feske S, Picard C, Fischer A (2010) Immunodeficiency due to mutations in ORAI1 and STIM1. Clin Immunol 135:162–182
Picard C, McCarl CA, Papolos A, Khalil S, Luthy K, Hivroz C, LeDeist F et al (2009) STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Eng J Med 360:1971–1980
Jesus AA, Duarte AJ, Oliveira JB (2008) Autoimmunity in hyper-IgM syndrome. J Clin Immunol 28(Suppl 1):S62–S66
Notarangelo LD, Miao CH, Ochs HD (2008) Wiskott–Aldrich syndrome. Curr Opin Hematol 15:30–36
Freeman AF, Holland SH (2008) The hyper-IgE syndromes. Immunol Allergy Clin North Am 28:277–291
De Ravin SS, Naumann N, Cowen EW et al (2008) Chronic granulomatous disease as a risk for autoimmune disease. J Allergy Clin Immunol 122:1097–1103
Kraaij MD, Savageb NDL, van der Kooija SW, Koekkoeka K, Wangc J, van den Bergd JM et al (2010) Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc Natl Acad Sci (USA) 107:17686–17691
Collin M, Bigley V, Haniffa M, Hambleton S (2011) Human dendritic cell deficiency: the missing ID? Nature Rev Immunol 11:575–583
Barilla-LaBarca MI, Atkinson JP (2003) Rheumatic syndromes associated with complement deficiency. Curr Opin Rheumatol 15:55–60
Unsworth DJ (2008) Complement deficiency and disease. J Clin Pathol 61:1013–1017
Knight AK, Cunningham-Rundles C (2006) Inflammatory and autoimmune complications of common variable immune deficiency. Autoimmune Rev 5:156–159
Baccheli C, Buckridge S, Thrasher AJ, Gasper HB (2007) Molecular defects in common variable immunodeficiency. J Exp Immunol 149:401–409
Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R et al (2003) Homozygous loss of ICOS is associated with adult onset common variable immunodeficiency. Nat Immunol 4:261–268
Van Zelm MC, Reisli I, van der Burg M, Castano D, van Noesel CJ, van Tol MJ et al (2006) An antibody deficiency syndrome due to mutation in the CD19 gene. N Eng J Med 354:1901–1912
Castigli E, Wilson SA, Garibyan I, Rachid R, Bonilla F, Schneider L et al (2005) TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Gen 37:829–834
Losi CG, Silini A, Fiorini C, Soresina A, Meini A, Meini A, Ferrari S et al (2005) Mutational analysis of human BAFF receptor TNFRSF13C (BAFF-R) in patients with common variable immunodeficiency. J Clin Immunol 25:496–502
Yu GP, Chiang D, Song SJ, Hoyte EG, Huang J, Vanisharn C et al (2009) Regulator T cell dysfunction in subjects with common variable immunodeficiency complicated by autoimmune disease. Clin Immunol 131:240–253
Yel L (2010) Selective IgA deficiency. J Clin Immunol 30:10–16
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, S., Louis, A.G. Tolerance and Autoimmunity in Primary Immunodeficiency Disease: a Comprehensive Review. Clinic Rev Allerg Immunol 45, 162–169 (2013). https://doi.org/10.1007/s12016-012-8345-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-012-8345-8