Abstract
1,25-Dihydroxyvitamin D displays immunoregulatory and anti-inflammatory properties, and the cells involved in innate and adaptive immune response express the vitamin D receptor and can both produce and respond to this hormone. This article aims at describing the complex immune regulatory role of vitamin D and depicting whether a correlation exists between atrophic type A gastritis and hypovitaminosis. We studied 62 autoimmune gastritis (AIG) patients and compared them to 54 lymphocytic gastritis patients, 21 Helicobacter pylori gastritis patients and 212 healthy subjects. We also statistically analyzed vitamin D concentration in 36,384 outpatients referred to our clinical laboratories. 25-Hydroxyvitamin D levels, the measurable metabolite used to determine vitamin D status in plasma, were measured by a chemiluminescent method. Average level of 25-OHD in AIG subjects was 9.8 ± 5.6 ng/mL (95% confidence interval (CI) 8.4–11.2), 11.1 ± 8.4 (CI 7.5–14.7) in H. pylori gastritis patients, 22.2 ± 13.5 (CI 18.6–25.8) in nonspecific lymphocytic gastritis patients, 21.3 ± 12.2 (CI 19.7–22.9) in healthy subjects, and 21.8 ± 13.1 (CI 21.7–21.9) in the 36,384 outpatients. Vitamin D levels in AIG patients were significantly lower than in patients with nonspecific gastritis or in the general population, supporting the hypothesis that hypovitaminosis D might be a risk factor for the development of autoimmune diseases. The low vitamin D concentration in H. pylori gastritis patients might act as predisposing factor for a more severe Th1-type aggression to the stomach epithelium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
1,25-Dihydroxyvitamin D (1,25(OH)2D) or calcitriol is a steroid hormone derived from vitamin D, which plays an important role in maintaining an adequate level of serum calcium and phosphorus [1]. The two major precursors of vitamin D are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). Both vitamin D precursors, resulting from exposure to the sunshine (D3) and the diet (D2), are converted to 25-hydroxyvitamin D (25(OH)D) (calcidiol) when they enter the liver [2].
More than 95% of 25(OH)D measurable in serum is typically 25(OH)D3, while 25(OH)D2 reaches measurable concentrations only in patients taking vitamin D supplementation [3]. Calcidiol, an inactive form of the hormone synthesized by 25-hydroxylase CYP2R1, is carried in the blood as a complex with a vitamin D binding protein (DBP), transformed into 1,25(OH)2D mainly in the kidneys by means of megalin-mediated endocytosis into the cells of the proximal nephrons, and again hydroxylated by the enzyme 1α-hydroxylase CYP27B1 [3, 4]. The renal production of 1,25(OH)2D that is closely regulated by the plasma levels of parathyroid hormone (PTH), calcium, and phosphorus controls systemic endocrine actions such as the intestinal transport of calcium, the regulation of the bone metabolism, the increase in renal calcium reabsorption, the regulation of blood pressure, and the secretion of insulin [1].
Other types of cells are provided with enzymes apt to produce the active hormone on site: Extrarenal 1,25(OH)2 D appears to act firstly as an autocrine/paracrine factor with specific cellular functions, such as the inhibition of proliferation, the promotion of differentiation, and the regulation of the immune response [1, 5]. 1α-Hydroxylase is present in at least ten tissues other than the proximal nephron (Table 1), but its regulation in extrarenal sites is very different and aligned with autocrine/paracrine functions carried out in situ by 1,25(OH)2D [6–15].
The synthesis and catabolism of calcitriol are controlled by local factors, cytokines, and growth factors which optimize the hormone levels for specific cellular actions by means of yet unknown mechanisms. This hormone performs its action by binding to the vitamin D receptor (VDR), part of the superfamily of steroid hormone receptors, acting as a transcription factor for a target gene [4]. The presence of VDR in tissues not taking part in mineral homeostasis led to the discovery of a myriad of other functions controlled by the D hormone (Table 2). The ability of calcitriol to inhibit growth and promote the differentiation of some cellular types suggests that the D endocrine system can have a very important role in the prevention of tumors, in regulating the immune system, and in modulating other endocrine systems; these actions were recently demonstrated by observational studies which associated vitamin D deficiency with cancer, autoimmune diseases, hypertension, and diabetes and which detected that this hormone can act in some tissues also with autocrine and/or paracrine mechanisms [16, 17].
The catabolism of 25(OH)D and 1,25(OH)2D in calcitroic acid, biologically inactive and soluble in water, is performed by the enzyme 24-hydroxylase (CYP24A1), almost ubiquitously expressed. 1,25(OH)2D itself is a very strong inducer of the expression of 24-hydroxylase, by means of the presence of two sequences of vitamin D responding elements (VDRE) in the promoter of this gene, inducing its own catabolism. This negative feedback is an internal security to avoid excessive levels of 1,25(OH)2D [4, 18, 19].
While the best known action of vitamin D is on bone metabolism, it is clear today that this hormone displays other functions, mainly the regulation of immune response, with potential therapeutic effects on autoimmune diseases, and an anti-proliferative action, useful in treatment of psoriasis and neoplasms and in prevention of cardiovascular diseases [20].
The Vitamin D Hormonal System
The D hormonal system is modulated by intrinsic mechanisms which regulate its activity and effectiveness for the bone metabolism and many other tissue functions in which it is involved. The first regulation takes place with the close control of the circulating concentration of 1,25(OH)2D, the production of which is modulated according to the body requirements for calcium and other hormones and is inhibited by the same active compounds. Other regulating factors are the transport in blood, the capture in renal tubules, the activity of renal and extrarenal 1α-hydroxylases, and the interaction with the specific receptor.
The circulating metabolites of vitamin D, particularly 25(OH)D, bind with high affinity to the DBP, which has plasma levels 20 times higher than the metabolites. This has an impact on their pharmacokinetics since bound compounds have limited access to target cells and are less susceptible to the hepatic metabolism; consequently, their average circulating life is increased [21]. DBP therefore acts as carrier as well as a modulator of vitamin D metabolism with respect to its blood concentration, optimizing the cellular catabolism and/or regulating its biological activity, related to the concentration of free hormones, protecting the tissues from toxic levels of vitamin D [22]. The concentration of DBP is decreased in the presence of hepatic diseases, nephrotic syndromes, and malnutrition, conditions where the risk of vitamin D intoxication is high; on the other hand, it increases during pregnancy and during estrogen therapy [5].
25(OH)D bound to DBP is actively transported inside the brush border cells of the proximal renal tubule, containing 1α-hydroxylase, by means of a megalin-mediated endocytosis process and not by means of diffusion through their basolateral surface [23]. Megalin is part of a group of complex proteins (protein associated to the cubilin receptor) located on the surface of the cells of the proximal renal tubule, capable of reabsorbing the calcidiol–DBP complex filtered by the kidney. DBP is broken up in the lysosomes (legumin) [24], and 25(OH)D is directed to 1α-hydroxylase by IDBP-3, or, depending on the concentration of active hormone, it returns in the blood, where it binds again with DBP [25, 26]. Megalin levels are increased by 1,25(OH)2D, through a positive control mechanism for the production of active hormones [27].The regulation of renal 1α-hydroxylase ensures the limited regular concentration range of 1,25(OH)2D. The activity of 1α-hydroxylase CYP27B1 is strongly regulated by the axis calcium–phosphorus–parathyroid hormone. Diet-deriving calcium regulates the enzyme directly by means of serum variations or through the action on proximal tubule cells and indirectly by altering the PTH levels: Hypocalcemia in fact increases the levels of circulating hormones capable of stimulating 1α-hydroxylase [25]. Phosphate dietetic restrictions also increase the activity of renal enzymes, independently from the calcium and PTH concentration, probably by means of the action of phosphatonins (fibroblast growth factor 23, frizzled-related protein 4, phosphoglycoproteins of the extracellular matrix), capable to act as mediators of phosphate regulation on the enzyme activity and/or on the gene controlling it [4, 28]. Calcitonin, GH, and IGF1 are also considered positive regulators of 1α-hydroxylase. Calcitriol limits its circulating levels by means of a negative feedback mechanism, through the inhibition of PTH-mediated induction of the activities of the promoter gene [29]. The product of the Klotho gene, induced by 1,25(OH)2D, is also considered a negative regulator of 1α-hydroxylase [30].
The regulation of 1α-hydroxylase located in extrarenal sites (Table 1) is very different compared to the renal enzyme and is in line with autocrine/paracrine functions performed by the locally produced 1,25(OH)2D, although the gene controlling the expression and activity of 1α-hydroxylase in extrarenal tissues is identical to that regulating it in the kidney (chromosomal locus 12q13.1–q13.3) [4]. The synthesis and catabolism are controlled by local factors, cytokines, and growth factors which optimize the levels of calcitriol for specific cellular actions by means of not yet fully understood mechanisms. In particular, the expression of 1α-hydroxylase in macrophages is significantly stimulated by immune signals mediated by interferon-γ (IFN-γ), lipopolysaccharides, and viral infections and in dendritic cells is associated with the p38 MAPK and NF-κB-dependent maturation of the same cells [31].
The local production of 1,25(OH)2D depends on the levels of circulating precursors (condition that potentially explains the association between vitamin D deficiency and autoimmune diseases/neoplasms), is not subjected to a negative feedback mediated by 1,25(OH)2D as it occurs for the renal enzyme, and does not increase the concentration of circulating active hormone.
Recent data demonstrate that T cells also express the enzymes responsible for the production of 1,25(OH)2D [32]. The binding of 1,25(OH)2D with a nuclear receptor, with a plasma membrane receptor or both in target organs, generates an appropriate biological response capable of regulating genetic transcription (genomic action) or of rapidly activating a variety of transduction signals on or near the plasma membrane (rapid nongenomic action) [33].
Calcitriol is one of the most important gene regulators, and their action is dependent to the binding of the high-affinity receptor VDR. VDR belongs to the superfamily of nuclear receptors for steroid hormones, which modulate the genetic expression of cells [34], and is present in many tissues (Table 2). The receptor is constituted by a single polypeptide of 427 amino acids, structured in three functional domains: the C terminus (ligand binding domain, LBD), which binds the hormone at the level of its A ring; the DNA binding domain, organized in two zinc finger DNA binding motifs, responsible for the high-affinity interactions with specific DNA sequences in the promoter regions of the target gene, called VDRE; and the N terminus for transcription processes [4].
VDR regulates the transcription of the DNA in mRNA, performed by the RNA polymerase II, which migrates in the cytoplasm, transducing the information to the ribosomes for the protein synthesis [35]; it controls four main and subsequent phases: VDR binds 1,25(OH)2D in the C terminus domain (LBD); this reaction causes conformational changes in the receptor which facilitate its heterodimerization with the retinoic acid X receptor (RXR) in LBD and the translocation to the nucleus; the newly formed complex 1,25(OH)2D–VDR/RXR binds, by means of the DNA binding domain, to specific sequences of VDRE and causes conformational changes of the genomic DNA [36, 37]; VDR recruits co-activator and/or co-repressor nuclear proteins [38, 39] that help it to modulate genetic transcription [4, 40, 41].
The most important co-activator proteins are part of the family of steroid hormone receptor co-activators (SRC-1, SRC-2, and SRC-3) and the CBP/p300 protein. They recruit enzymes which remodel chromatin [42, 43] and DNA transcription mediator complexes of the transcription gene [44, 45]. The main co-repressors are NcoR-1, NcoR-2, and Hairless which compact chromatin and inhibit gene transcription [44, 46]. The VDR co-modulator, NCoA-62/Ski, can act both as promoter or inhibitor of the transcription activity, depending on NcoR and CBP/p300 levels [47, 48]. Calcitriol itself can modulate the transduction activity by means of the selective induction of TIF2 (p160), co-activator, and/or SMRT, co-repressor of the NcoR family; the final balance between co-activators and co-repressors determines the control of genetic transcription in the presence of physiological or pathological stimuli [49].
The genomic actions of vitamin D are divided in classic and nonclassic (Table 3). With regard to the classic actions, the endocrine system of vitamin D acts as a fundamental actor in the interactions between kidney, bone, parathyroid, and intestine in order to maintain extracellular calcium levels within the normal levels, a vital process for the physiology of normal cells and the integrity of the skeleton. Among the nonclassic genomic actions, the recent discovery of VDR in the cells of the immune system and the fact that many of these cells have an enzyme collection capable of producing the hormone indicated that it carries out important immunoregulatory properties [4, 32].
The Vitamin D Hormonal System and the Immune Response
1,25(OH)2D modulates the immune system, causing direct regulatory effects on the functions of B and T lymphocytes and influencing the phenotype and function of antigen presenting cells (APCs) and dendritic cells (DCs), promoting tolerogenic properties that facilitate the induction of T regulators (Tregs) instead of T effectors [50]. This regulation is mediated by the action of vitamin D on nuclear transcription factors such as NF-AT and NF-κB or by the direct interaction with VDRE in the promoter regions of the genes of cytokines. The vitamin is produced by the macrophages, DCs, and T and B cells and is therefore capable of physiologically contributing, by means of the VDR expressed in their nucleus, to the autocrine and paracrine regulation both of innate and adaptive immunity [51, 52]. The strict control of the hormone production by the cells of the innate and adaptive immune system confirms the significance of the D endocrine system in modulating the immune response in healthy or sick subjects.
Strengthening of the Innate Immune System Response
The innate immune response involves the activation of Toll-like receptors (TLR) in the polymorphonuclear granulocytes, monocytes, macrophages, as well as in the epithelial cells of epidermis, gums, intestine, vagina, bladder, and lungs. TLRs are transmembrane receptors with the task of recognizing pathogens, which react to specific membrane patterns expressed by etiological agents and unleash the innate immune response of the host [53]. Calcitriol represents the key link between the activation of TLRs and the antibacterial response of the innate immune system, especially in the case of tuberculosis, where it is capable of causing the production of an antimicrobial peptide, cathelicidin, coded by a primary gene which responds and is strongly regulated by vitamin D [54]. This suggests that a sufficient quantity of active hormone contributes to decreasing the susceptibility to this infection. Other studies demonstrated that vitamin D allows keratinocytes to defend the wound repairing process from infections and confirmed that the hormone has a key role in the innate immune response against bacterial attacks [32, 55]. Furthermore, the autocrine production of 1,25(OH)2D by DCs is capable of programming the homing of T cells associated to the skin, including Tregs capable of fighting the proinflammatory effects caused in the tissue. The hormone also regulates the expression of the receptor for epidermotropic chemokines CCR10 in activated T cells, coded by a primary gene responding to vitamin D. This allows the CCR10+ T cells to migrate to the skin in response to epidermic chemokine CCL27 produced by keratinocytes, where they can perform their tissue action [56]. Hence, the innate immune system is largely distributed and operates not only in the cells of the lymphopoietic system but also within the epithelium of those tissues that face external factors, where it is a fundamental protective barrier.
Regulation of the Adaptive Immune System Response
1,25(OH)2D exercises an inhibitory action onto the adaptive immune system: It suppresses the proliferation and production of immunoglobulins and delays the differentiation of precursors B in plasma cells [57]; it inhibits the proliferation of Th1, capable of producing IFN-γ and interleukin 2 (IL-2), and activating macrophages [58, 59]; it also acts on the DCs/APCs facilitating the production of IL-4, IL-5, and IL-10 and moving the T differentiation in favor of phenotype Th2 [32].
The action of the hormone also increases the quantity of Tregs CD4+/CD25+ which produce IL10, by means of which they block the development of Th1, and inhibits the secretion of IL-17 by the T effectors [60]. These actions regulate subsequent presentations of antigens to the lymphocytes by the APC system and therefore the recruitment (facilitated by IFN-γ) and proliferation (facilitated by IL-2) of T effectors.
Induction of Tolerance Properties in Dendritic Cells
DCs are a highly specialized system of APC, critical for the initiation of the T cells CD4+ response. They are circulating in different maturation stages as well as being present in lymphoid and nonlymphoid organs, where they act as sentinels. After contact with the antigen, the DCs migrate toward T-dependent areas of afferent lymph nodes, where they can transform the native T cells in Th1 [61].
According to the expression of CD11c, two sub-populations of DCs are recognized: (a) M-DCs belonging to the myeloid line, characterized by a monocyte morphology, myeloid-like markers (CD13, CD33, β2-integrin, CD11c, immunoglobulin-like transcript (ILT)1 activation receptor) and low levels of CD123 receptor for IL-3 and (b) P-DCs belonging to the lymphoid line, characterized by plasma cell morphology, without myeloid markers, expressing high levels of CD4 and CD123.
M-DCs produce high levels of IL-12, while P-DCs produce high levels of IFN-α, with distinct effects on the activation and differentiation of T cells. Therefore, DCs can be immunogenic but can also induce tolerance both in the thymus and in the periphery and can modulate the development of T cells; in particular, immature DCs seem capable of inducing tolerance and proliferation of Tregs [61].
The VDR agonists facilitate the proliferation of DCs with tolerance properties [62], stopping the differentiation and maturation of DCs, decreasing the expression of CD40, CD80, and CD86 co-stimulatory molecules, significantly decreasing the production of IL-12, increasing the production of IL-10 [63], and finally inducing the expression of ILT3, an inhibitory molecule associated with tolerance induction [64].
Experimental studies demonstrated that DCs with tolerance properties induced by VDR agonists are capable of inducing differentiation of CD4+ CD25+ Tregs capable of stopping the development of autoimmune diabetes (T1DM) [62]. We can derive that the active synthesis of 1,25(OH)D within DCs not only inhibits the differentiation from monocyte precursors in immature DCs but also blocks their ability to undergo a final differentiation in response to maturation stimuli [65]. Recent studies highlighted that during inflammatory processes calcitriol negatively regulates, with a paracrine mechanism and by means of the ligand-induced recruitment of histone deacetylase, the expression of the nuclear factor κB, fundamental both for the differentiation and maturation of DCs and to trigger the inflammatory response. This explains the inhibitory effect of the active hormone on the maturation of DCs and on their production of proinflammatory mediators [66, 67]. Calcitriol and the VDR agonists perform their action exclusively on the M-DCs, regulating their production of cytokines and chemokines and modulating their ability to inhibit the development of T helpers and to increase the Treg function [61, 68]; on the contrary, P-DCs are not subjected to regulation by the active hormone in inducing tolerance [32].
Modulation of the Function of Effector Lymphocytes
The VDR agonists regulate the activation and differentiation of T cells by modulating the DC functions [32]. Furthermore, they can also have direct effects on B and T cells, inhibiting (a) selectively the development of Th1 and Th17 [58, 69] and (b) directly type 1 cytokines such as IL-2 and IFN-γ [70].
It was also demonstrated that 1,25(OH)2D increases the number of Th2 through a direct effect on native CD4s [71]. Therefore, both Th1 and Th2 are regulated by the action of VDR agonists, both with respect to their activation and differentiation [72]. Calcitriol has powerful and direct effects also on the B cell response, causing induction of apoptosis, inhibition of proliferation, generation of B memory cells, plasma cell differentiation, and immunoglobulin production [57].
The B cells, like the macrophages, DCs and T cells, express VDR and can synthesize the active hormone, which can perform autocrine/paracrine regulatory actions. Treatment with VDR agonists inhibits the production of IL-17 by T cells, a proinflammatory cytokine produced by pathogenic T cells in several organ-specific autoimmune diseases affecting the brain, heart, synovium, and intestine [60, 73]. The production of IL-17 is sustained by IL-23, a member of the IL-12 family, formed by p19 and p40 chains, the latter of which is strongly inhibited by VDR agonists [69].
Increase in Treg Functions
Tolerance-inducing DCs, produced after a short treatment with 1,25(OH)2D, lead to the development of CD4+CD25+ Tregs capable of mediating the tolerance to transplants and stopping the development of T1DM [74]. VDR agonists not only facilitate the induction of Tregs and increase their inhibiting activity but can also facilitate their recruitment in inflammatory sites [75]. Circulating M-DCs also produce high levels of chemokines CCL17 and CCL22, increased by binding with the CD40 ligand [61], capable of selectively recruiting CD4+CD25+ CCR4+Foxp3+ Tregs. In particular, the high production of CCL22 by immature M-DCs leads to a preferential attraction of such Tregs [76]. The production of this chemokine is significantly increased by 1,25(OH)2D in blood M-DCs, indicating that VDR agonists can facilitate the recruitment of Tregs by DC subgroups [61].
Hypovitaminosis D and Autoimmune Gastritis
There is no full agreement on the optimal serum levels of 25(OH)D3, the measurable metabolite to assess the level of vitamin D in man, but deficiency is defined when hormone levels are lower than 20 ng/mL [20, 77]. Studies carried out in the past decade indicated a high prevalence of deficiency and/or insufficiency of vitamin D in the general population, and this low concentration is correlated with an increased incidence of autoimmune diseases, bone pathologies, and cancer [20]. Epidemiological studies clearly showed that a sufficient concentration of hormone (>30 ng/mL) decreases the risk of multiple sclerosis, T1DM, chronic intestinal inflammatory diseases, rheumatoid arthritis, osteoarthritis, and systemic lupus erythematosus, confirming that the level of vitamin D has an effect on the prevalence of autoimmune diseases.
As no experiences are available on vitamin D levels in patients with autoimmune gastritis (AIG), we studied the concentration of the hormone in a group of AIG patients, diagnosed by histology, in order to verify if hypovitaminosis D can be a predisposing factor for this pathology as well. AIG is an organ-specific disease, found in 2% of the general population, characterized by the presence of circulating autoantibodies against the enzyme H+/K+-adenosintriphosphatase (H+/K+-ATPase), the protonic pump of the parietal cells (PCA), and against the intrinsic factor (IFA), another product of the parietal cells [78]; it is often an asymptomatic disease that may cause atrophy of the gastric mucosa and in 10% of cases develops into an adenocarcinoma or a carcinoid tumor.
The chronic autoimmune attack against H+/K+-ATPase takes place on the fundus and body of the stomach and does not affect the antrum; it is characterized histologically by a mononucleate inflammatory infiltrate in the mucosa, constituted by T CD4 and CD8 lymphocytes, macrophages, and B cells, which causes the loss of parietal cells and zymogenic cells and the subsequent decrease of gastric acid secretion, hypergastrinemia, and, finally, anemia due to iron deficiency. In the latter stages of the disease, pernicious anemia (AP) occurs, caused by a deficiency of vitamin B12 that is no longer absorbed by the intestine due to the presence in the serum and/or gastric juices of the patients of IFA; in this phase, the biopsy shows a more advanced degree of atrophy and metaplasia of the gastric body [78, 79].
The aim of our study was (a) to determine vitamin D concentration in patients affected by autoimmune gastritis, (b) to compare it with concentration in patients affected by nonautoimmune gastric pathologies, and (c) to compare it with the concentration in an Italian reference population. This allowed to verify whether the average concentration of vitamin D in these patients was lower than that in healthy subjects of the same age and sex, as well as in patients affected by nonautoimmune gastritis.
Materials and Methods
We determined vitamin D concentration in 62 patients with AIG (aged 37–80) and compared it with the concentration found in 54 PCA-positive patients with nonspecific lymphocytic gastritis [20–80], 21 patients with H. pylori gastritis [20–80], and with a control group formed by 36,384 subjects (29,401 women, 6,983 men aged 1–80); the latter were chosen among outpatients of the northeast of Italy (Veneto and Friuli). They had access in the last 2 years to laboratories at Cittadella, Vicenza, Udine, and Latisana and underwent a vitamin D test. All dosages of 25(OH)D3 were determined by a quantitative chemiluminescent competitive direct method (Liaison, Diasorin, Saluggia, Italy) using a specific anti-vitamin D antibody linked to a solid phase and a vitamin D tracer bound to isoluminol. In order to classify the vitamin D status of our population, we used Holick’s decision-making levels, which have been determined by putting them in relationship with calcium and PTH concentration [80].
To calculate the average concentration of vitamin D in the control group, representing the population of the northeast of Italy, we used the statistical indirect method of Kairisto and Poola [81] and processed 36,384 vitamin D concentration values obtained in subjects without metabolic diseases, not under vitamin D therapy, living indoors as the majority of the population, and with PTH values within reference limits. These were the same criteria used by Holick and Chen in selecting the individuals for the control group in their study [82]. The first three criteria were assessed by questionnaire administered to the patients before blood drawing and the fourth by automatic cross-matching performed by the Laboratory Information System.
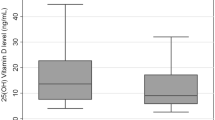
This indirect statistical method is based on the theory that most of the laboratory data regarding outpatients derive from screening and refer to individuals who do not have the disease or a correlated pathology. It assumes that the mode of the frequency distribution of all data is the same as that of the reference population if the outliers are eliminated. This objective model has a performance comparable with the International Federation of Clinical Chemistry standard when the asymmetry and kurtosis of frequency distribution are not very large. The data collected (Fig. 1) showed a scatter of values too high to calculate the reference range in the population; however, the statistical elaboration allowed us to calculate the average concentration in the subjects recruited in our territory. To verify whether the data obtained by means of the indirect statistical method of Kairisto and Poola were representative of vitamin D levels in the general population, we studied also 212 healthy subjects (181 females, 31 males), 49 aged 1–10, and 163 aged 20–80, selected with the same Holick’s criteria.
Results
Using the Kairisto and Poola indirect method, we were allowed to consider many samples. The statistical calculations on which this mathematical model is based hypothesize that the mode of frequency distribution of all data is the same as that of the reference population; each side of the frequency distribution is considered a half gaussian distribution, with the same mode and the same mode frequency but with a different standard deviation. By means of this method, we have processed data for 36,384 patients who were referred to our laboratories in the past 2 years for measurement of 25(OH)D3. By means of the statistical elaboration, we found that once the outliers were removed from the study population, the lower limit was 5.42 ng/mL and the upper limit was 43.8 ng/mL, with an average concentration of 21.8 ± 13.1 ng/mL. These data are a statistical–mathematical expression of the distribution of values in a control population, allowing us to calculate the average value of the hormone content in the study group; however, they do not allow us to establish a reference range since the values are extremely scattered and include deficient, insufficient, and optimal levels. As seen in Table 4, the average level of vitamin D found in 212 control subjects that is 21.3 ± 12.2 ng/mL (95% confidence interval (CI) 19.7–22.9) exactly corresponds with that found in the northeastern population obtained through the indirect statistical method that is 21.8 ± 13.1 ng/mL (CI 21.7–21.9); it was slightly higher in children aged <10 years (22.9 ± 11.3 ng/mL (CI 19.7–26.1)) than in the group aged 20–80 but still inadequate according to Holick’s. Vitamin D average concentration in subjects with AIG was 9.8 ± 5.6 ng/mL (CI 8.4–11.2), in nonspecific gastritis patients 22.2 ± 13.5 ng/mL (CI 18.6–25.8), and in Helicobacter pylori gastritis patients it was 11.3 ± 8.4 ng/mL (CI 7.5–14.7).
Discussion and Conclusions
Data from the present study show that patients with AIG have significantly lower levels of vitamin D than the control group and the outpatient population and than patients affected by nonautoimmune gastric pathologies. This confirms the hypothesis, as seen in subjects affected by systemic lupus erythematosus and multiple sclerosis that a deficiency of vitamin D is a risk factor for autoimmune diseases in genetically predisposed patients. The low level of 25(OH)D3 in northeastern Italy detected from as early as childhood could explain the high frequency of osteoporosis in our population and the fact that the dietetic administration of vitamin D in the first months of life eliminated only the onset of rickets but not vitamin D insufficiency [18]. Evidence emerging from literature, as well as from our study, suggests that if we want to prevent autoimmune aggression in genetically predisposed subjects with vitamin D deficiency, it is very important to supply them with this hormone before the aggression has already developed into the autoimmune disease [66]. Supplying medications after that is completely useless. This should be taken into consideration for a therapeutic strategy with first degree relatives of patients affected by these autoimmune diseases.
The unexpected result was the finding of an important hormone deficiency in patients with H. pylori gastritis; by studying the pathogenic mechanism, it becomes clear that the host response to a chronic H. pylori infection is of the Th1 type, exactly the same of that in an autoimmune disease, and it participates in the induction of damage to the gastric epithelium, thus having an integral role in the pathogenesis of this gastritis. Vitamin D deficiency may facilitate Th aggression because fails its ability to differentiate Th0 into Treg and regulate the secretion of nuclear factor-kappa B and the early-response transcription-factor activator protein 1, intracellular messengers involved in the initiation of this chronic inflammatory process that are negatively regulated by vitamin D. This point needs to be addressed by specific studies, designed to determine how much a significant hypovitaminosis D in subjects with H. pylori gastritis influences the severity of gastric mucosal immune aggression.
References
Norman AW (2008) From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr 88:491S–499S
Zhang R, Naughton DP (2010) Vitamin D in health and disease: current perspectives. Nutr J 9:65
Cappelletti P, Tozzoli R (2010) Laboratory and hypercalcemia. In: Lumachi F, Basso SMM (eds) Hypercalcemia pathophysiology and treatment. Bentham Science, Sharjah, pp 45–61
Rojas-Rivera J, De La Piedra C, Ramos A et al (2010) The expanding spectrum of biological actions of vitamin D. Nephrol Dial Transplant 25:2850–2865
Hewison M, Zehnder D, Chakraverty R et al (2004) Vitamin D and barrier function: a novel role for extra-renal 1a-hydroxylase. Mol Cell Endocrinol 215:31–38
Bises G, Kallay E, Weiland T et al (2004) 25-Hydroxyvitamin D3-1alpha-hydroxylase expression in normal and malignant human colon. J Histochem Cytochem 52:985–989
Adorini L, Penna G, Giarratana N et al (2004) Dendritic cells as key targets for immunomodulation by vitamin D receptor ligands. J Steroid Biochem Mol Biol 89–90:437–441
Zehnder D, Bland R, Chana RS et al (2002) Synthesis of 1, 25-dihydroxyvitamin D3 by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol 13:621–629
Eyles DW, Smith S, Kinobe R et al (2005) Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 29:21–30
Segersten U, Holm PK, Bjorklund P et al (2005) 25-Hydroxyvitamin D3 1alpha-hydroxylase expression in breast cancer and use of non-1-hydroxylated vitamin D analogue. Breast Cancer Res 7:980–986
Townsend K, Evans KN, Campbell MJ et al (2005) Biological actions of extra-renal 25-hydroxyvitamin D-1alpha-hydroxylase and implications for chemoprevention and treatment. J Steroid Biochem Mol Biol 97:103–119
Segersten U, Correa P, Hewison M et al (2002) 25-Hydroxyvitamin D(3)-1alpha-hydroxylase expression in normal and pathological parathyroid glands. J Clin Endocrinol Metab 87:2967–2972
Evans KN, Bulmer JN, Kilby MD et al (2004) Vitamin D and placental–decidual function. J Soc Gynecol Investig 11:263–271
Ma JF, Nonn L, Campbell MJ et al (2004) Mechanisms of decreased vitamin D 1alpha-hydroxylase activity in prostate cancer cells. Mol Cell Endocrinol 221:67–74
Bikle DD, Chang S, Crumrine D et al (2004) 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol 122:984–992
Griffin MD, Xing N, Kumar R (2003) Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr 23:117–145
Norman AW, Mizwicki MT, Okamura WH (2003) Ligand structure–function relationships in the vitamin D endocrine system from the perspective of drug development (including cancer treatment). Recent Results Cancer Res 164:55–82
Holick MF (2006) Resurrection of vitamin D deficiency and rickets. J Clin Invest 116:2062–2072
DeLuca HF (2004) Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80:1689–1696
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281
Cooke NE, Haddad JG (1989) Vitamin D binding protein (Gc-globulin). Endocr Rev 10:294–307
Dusso AS, Negrea L, Gunawardhana S et al (1991) On the mechanisms for the selective action of vitamin D analogs. Endocrinology 128:1687–1692
Takemoto F, Shinki T, Yokoyama K et al (2003) Gene expression of vitamin D hydroxylase and megalin in the remnant kidney of nephrectomized rats. Kidney Int 64:414–420
Yamane T, Takeuchi K, Yamamoto Y et al (2002) Legumain from bovine kidney: its purification, molecular cloning, immunohistochemical localization and degradation of annexin II and vitamin D-binding protein. Biochim Biophys Acta 1596:108–120
Adams JS, Chen H, Chun RF et al (2003) Novel regulators of vitamin D action and metabolism: lessons learned at the Los Angeles zoo. J Cell Biochem 88:308–314
Nykjaer A, Dragun D, Walther D et al (1999) An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96:507–515
Liu W, Yu WR, Carling T et al (1998) Regulation of gp330/megalin expression by vitamins A and D. Eur J Clin Investig 28:100–107
Brandi L (2008) 1alpha(OH)D3 One-alpha-hydroxy-cholecalciferol—an active vitamin D analog. Clinical studies on prophylaxis and treatment of secondary hyperparathyroidism in uremic patients on chronic dialysis. Dan Med Bull 55:186–210
Brenza HL, Kimmel-Jehan C, Jehan F et al (1998) Parathyroid hormone activation of the 25-hydroxyvitamin D3-1alpha-hydroxylase gene promoter. Proc Natl Acad Sci USA 95:1387–1391
Tsujikawa H, Kurotaki Y, Fujimori T et al (2003) Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol 17:2393–2403
Dong X, Lutz W, Schroeder TM et al (2005) Regulation of relB in dendritic cells by means of modulated association of vitamin D receptor and histone deacetylase 3 with the promoter. Proc Natl Acad Sci USA 102:16007–16012
Adorini L, Penna G (2008) Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol 4:404–412
Norman AW, Mizwicki MT, Norman DPG (2004) Steroid hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov 3:27–41
Pinette KV, Yee YK, Amegadzie BY et al (2003) Vitamin D receptor as a drug discovery target. Min Rev Med Chem 3:193–204
Brown AJ, Dusso A, Slatopolsky E (1999) Vitamin D. Am J Physiol Renal Physiol 277:F157–F175
Sutton ALM, MacDonald PN (2003) Vitamin D: more than a “bone-a-fide” hormone. Mol Endocrinol 17:777–791
Strugnell SA, Deluca HF (1997) The vitamin D receptor—structure and transcriptional activation. Proc Soc Exp Biol Med 215:223–228
Kliewer SA, Umesono K, Mangelsdorf DJ et al (1992) Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature 355:446–449
Cheskis B, Freedman LP (1994) Ligand modulates the conversion of DNA-bound vitamin D3 receptor (VDR) homodimers into VDR-retinoid X receptor heterodimers. Mol Cell Biol 14:3329–3338
Dusso AS, Brown AJ (2009) Vitamin D: molecular biology and gene regulation. In: Singh AJ, Williams GH (eds) Textbook of nephro-endocrinology. Elsevier, San Diego, pp 69–93
Nagpal S, Na S, Rathnachalam R (2005) Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 26:662–687
Oñate SA, Tsai SY, Tsai MJ et al (1995) Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354–1357
Chen HW, Lin RJ, Schiltz RL et al (1997) Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90:569–580
Rachez C, Suldan Z, Ward J et al (1998) A novel protein complex that interacts with the vitamin D3 receptor in a ligand-dependent manner and enhances VDR transactivation in a cell-free system. Genes Dev 12:1787–1800
Chiba N, Suldan Z, Freedman LP et al (2000) Binding of liganded vitamin D receptor to the vitamin D receptor interacting protein coactivator complex induces interaction with RNA polymerase II holoenzyme. J Biol Chem 275:10719–10722
Carvallo L, Henríquez B, Paredes R et al (2008) 1alpha, 25-Dihydroxy vitamin D3-enhanced expression of the osteocalcin gene involves increased promoter occupancy of basal transcription regulators and gradual recruitment of the 1alpha, 25-dihydroxy vitamin D3 receptor-SRC-1 coactivator complex. J Cell Physiol 214:740–749
Baudino TA, Kraichely DM, Jefcoat SC Jr et al (1998) Isolation and characterization of a novel coactivator protein, NCoA-62, involved in vitamin D-mediated transcription. J Biol Chem 273:16434–16441
Zhang C, Dowd DR, Staal A et al (2003) Nuclear coactivator-62 kDa/Ski-interacting protein is a nuclear matrix-associated coactivator that may couple vitamin D receptor-mediated transcription and RNA splicing. J Biol Chem 278:35325–35336
Dunlop TW, Väisänen S, Frank C et al (2004) The genes of the coactivator TIF2 and the corepressor SMRT are primary 1alpha, 25(OH)2D3 targets. J Steroid Biochem Mol Biol 89–90:257–260
Adorini L, Penna G (2009) Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol 70:345–352
Cutolo M (2009) Vitamin D and autoimmune rheumatic diseases. Rheumatology 48:210–212
van Etten E, Mathieu C (2005) Immunoregulation by 1, 25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol 97:93–101
Liu PT, Stenger S, Li H et al (2006) Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773
Wang TT, Nestel FP, Bourdeau V et al (2004) Cutting edge: 1, 25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173:2909–2912
Schauber J, Dorschner RA, Coda AB et al (2007) Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest 117:803–811
Sigmundsdottir H, Pan J, Debes GF et al (2007) DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol 8:285–293
Chen S, Sims GP, Chen XX et al (2007) Modulatory effects of 1, 25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 179:1634–1647
Mattner F, Smiroldo S, Galbiati F et al (2000) Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1, 25-dihydroxyvitamin D(3). Eur J Immunol 30:498–508
Cippitelli M, Santoni A (1998) Vitamin D3: a transcriptional modulator of the interferon-gamma gene. Eur J Immunol 28:3017–3030
Penna G, Amuchastegui S, Cossetti C et al (2006) Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J Immunol 177:8504–8511
Penna G, Amuchastegui S, Giarratana N et al (2007) 1, 25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol 178:145–153
Adorini L, Giarratana N, Penna G (2004) Pharmacological induction of tolerogenic dendritic cells and regulatory T cells. Semin Immunol 16:127–134
Penna G, Adorini L (2000) 1 alpha, 25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 164:2405–2411
Penna G, Roncari A, Amuchastegui S et al (2005) Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4 + Foxp3+ regulatory T cells by 1, 25-dihydroxyvitamin D3. Blood 106:3490–3497
Hewison M, Freeman L, Hughes SV et al (2003) Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol 170:5382–5390
Adorini L (2005) Intervention in autoimmunity: the potential of vitamin D receptor agonists. Cell Immunol 233:115–124
Griffin MD, Dong X, Kumar R (2007) Vitamin D receptor-mediated suppression of RelB in antigen presenting cells: a paradigm for ligand-augmented negative transcriptional regulation. Arch Biochem Biophys 460:218–226
Liu YJ (2005) IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol 23:275–306
Lemire JM, Archer DC, Beck L et al (1995) Immunosuppressive actions of 1, 25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr 125:1704S–1708S
D’Ambrosio D, Cippitelli M, Cocciolo MG et al (1998) Inhibition of IL-12 production by 1, 25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest 101:252–262
Boonstra A, Barrat FJ, Crain C et al (2001) 1alpha, 25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol 167:4974–4980
Mahon BD, Wittke A, Weaver V et al (2003) The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem 89:922–932
Steinman L (2007) A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med 13:139–145
Gregori S, Giarratana N, Smiroldo S et al (2002) A 1alpha, 25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 51:1367–1374
Gregori S, Casorati M, Amuchastegui S et al (2001) Regulatory T cells induced by 1 alpha, 25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol 167:1945–1953
Curiel TJ, Coukos G, Zou L et al (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949
Adams JS, Hewison M (2010) Update in vitamin D. J Clin Endocrinol Metab 95:471–478
Antico A (2008) Autoimmune gastritis. It J Lab Med 4:125–133
De Block CE, De Leeuw IH, Van Gaal LF (2008) Autoimmune gastritis in type 1 diabetes: a clinically oriented review. J Clin Endocrinol Metab 93:363–371
Holick MF (2006) High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81:353–373
Kairisto V, Poola A (1995) Software for illustrative presentation of basic clinical characteristics of laboratory tests—GraphROC for Windows. Scand J Clin Lab Invest 222:43–60
Holick MF, Chen TC (2008) Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 87:1080–1086
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Antico, A., Tozzoli, R., Giavarina, D. et al. Hypovitaminosis D as Predisposing Factor for Atrophic Type A Gastritis: a Case–Control Study and Review of the Literature on the Interaction of Vitamin D with the Immune System. Clinic Rev Allerg Immunol 42, 355–364 (2012). https://doi.org/10.1007/s12016-011-8255-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-011-8255-1