Abstract
The pathogenesis of accelerated cardiovascular damage commonly characterizing patients affected by systemic chronic inflammatory and autoimmune rheumatic disorders is quite complex and still not fully clarified. However, it is well accepted that a strong relationship between multiple factors, including both traditional cardiovascular risk factors and disease-related inflammatory and autoimmune mechanisms, may in part explain the precocious atherosclerotic vessel damage and the increased incidence of cardiovascular events. Nevertheless, although several recent studies focused their attention on the investigation of these complex mechanisms, data regarding possible preventive strategies aimed to reduce long-term cardiovascular risk in these subjects are still lacking and not conclusive. In this setting, the early introduction of evidence-based preventive measures for the correct management of patients with systemic autoimmune disorders would be of extreme importance to reduce subclinical atherosclerosis incidence and possible major cardiovascular events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite continuous and remarkable improvement in the treatment of chronic inflammatory and autoimmune systemic disorders, there is well accepted evidence that the mortality gap between these patients and the general population is actually increasing, especially in the first period following disease diagnosis [1]. Indeed, it is widely documented that patients with chronic inflammatory articular disorders, such as rheumatoid arthritis (RA), and to a lesser extent, with systemic autoimmune connective tissue diseases, experience a significant increased mortality and morbidity with respect to the general population, mainly due to an enhanced risk of myocardial infarction and other manifestations of ischemic heart disease (IHD) [2–4]. In this setting, an acceleration of the physiological and age-related atherosclerotic vessel wall damage has been regarded as the main pathogenetic mechanism leading to the enhanced cardiovascular (CV) risk in these patients [5–7]. However, the increased risk cannot be fully attributed to traditional CV risk factors for IHD, such as diabetes mellitus, smoking, hypertension, obesity and dyslipidaemia. Indeed, the intriguing and not yet fully understood relationship between inflammatory and autoimmune mechanisms, underlying the pathogenesis of these disorders, has been demonstrated to take part also in the complex process leading to endothelial damage and subsequent plaque formation [8, 9]. Nevertheless, it is interesting to note that the same inflammatory milieu and immune system dysregulation, known to antedate disease onset, may play a relevant role also in the induction of subclinical atherosclerosis (ATS) in these patients, as recently shown in early RA patients with a disease duration lower than 1 year [10]. Newly diagnosed RA patients, independent from previous CV events or risk factors, display vascular impairment of both endothelial and smooth muscle cell function. In addition, inflammatory mediators seem to play a relevant role in the pathophysiology of vascular damage, as assessed by indirect markers of subclinical ATS [10]. However, although early RA patients may have an increased risk of subclinical ATS, at present there is no clear evidence of an increased incidence of IHD-related events before the onset of disease symptoms, as recently reported in a large population-based case–control study [11]. Nevertheless, an enhanced occurrence of CV events after disease diagnosis was confirmed.
These findings deserve some observations. First of all, the absence of overt CV events before the onset of RA symptoms in a population with a documented enhanced risk of IHD after disease diagnosis supports the idea that RA itself may represent an independent risk factor for CV disease, as widely shown in several studies. Despite genetic susceptibilities and shared risk factors for RA and ATS which may have a role in the induction of preclinical ATS damage in the earliest phases of rheumatoid disease [10], they are likely to contribute less than RA-related mechanisms to the increased occurrence of CV events and IHD in these patients. On the other hand, the lack of a clear demonstration of enhanced incidence of CV events in the earliest disease stages suggests a possible remarkable role of preventive measures to reduce the probability of overt CV manifestations in these patients. Inflammatory and immune-mediated mechanism interaction has a key role in determining precocious atherosclerotic wall damage, even in the absence of traditional CV risk factors and before treatment introduction. Structural vascular remodelling, assessed by carotid intima-media thickness (IMT) and plaque, may subsequently occur with disease and inflammatory burden progression. However, it is intriguing to note that RA patients do not display at clinical presentation an arterial IMT already indicative of vessel wall structural damage, thereby suggesting that preventive measure introduction, early therapeutic intervention and rapid control of inflammation may reverse the initial ATS wall damage [10]. Nevertheless, the lack of a unifying pathogenetic mechanism able to explain accelerated ATS in RA is reflected by the confusion that still exists regarding possible preventive measures aimed at decreasing atherogenic risk. There is still considerable uncertainty, indeed, on how to manage patients with RA in order to reduce their risk for future CV events, even because some of the medications used to treat RA, including corticosteroids (CS), may have dual effects on CV morbidity risk. CV disease does not only impact mortality in RA, but also leads to significant morbidity. CV events occur approximately a decade earlier in RA than in the general population. Moreover, subjects with RA, in particular younger patients without known prior CV events, are twice as likely to suffer a myocardial infarction with respect to matched controls [12, 13]. Thus, since factors promoting ATS and premature CV mortality are present early in RA course, it would be conceivable to believe that preventive strategies aimed at reducing CV risk should start promptly after disease diagnosis. However, despite specific guidelines addressing the management of CV risk factors in RA have been recently published [14], several aspects regarding prevention remain unanswered. For instance, it is unknown which levels of lipids or arterial blood pressure should prompt therapeutic intervention in these patients and whether these interventions could modify CV risk. Similarly, it is unknown which patients with RA or other connective tissue diseases should be considered as candidates for screening of subclinical and clinical coronary and carotid artery disease. To further support such considerations, it interesting to note that, although a multidisciplinary European League Against Rheumatism Expert Committee recently recommended statin use in the CV risk management in RA, lipid lowering drugs have been shown to be underutilized in these patients [14, 15].
Nevertheless, the introduction of preventive measures in the management of CV risk in patients with chronic inflammatory and autoimmune disorders is of outstanding importance in the multidisciplinary approach to these disorders. The aim of the present review is to summarize the available data about preventive pharmacological and non-pharmacological strategies, including physical activity and treatment with lipid lowering and anti-hypertensive drugs, oral hypoglycaemic agents and anti-rheumatic therapy, that may be carried out in order to minimize CV risk in rheumatic autoimmune disorders.
Physical Activity
Physical activity is considered as one of the most important non-pharmacological interventions in the prevention of CV diseases, both in the general population and in patients with chronic diseases. Physical exercise and aerobic activity, indeed, have been demonstrated to exert significant effects on the endothelial system, both acutely and in the long term [16]. Exercise, indeed, reverses endothelial dysfunction by improving anti-oxidative mechanisms and by increasing endothelial progenitor cell, prostaglandin, endothelial nitric oxide synthase (eNOS) and vascular endothelial growth factor production, thus enhancing local blood flow, angiogenesis and endothelial growth. The increased activity of eNOS is associated with a reduced up-regulation of adhesion molecules, endothelin-1 and monocyte chemoattractant protein-1, all of which have been demonstrated to favour the recruitment of inflammatory cells, in particular monocytes and T cells, to endothelial wall, thus promoting atherosclerotic wall damage [17]. Finally, regular physical activity has been demonstrated to have a relevant systemic anti-inflammatory effect. Indeed, moderate muscular exercise reduces adipose tissue size, which is able to produce pro-inflammatory molecules, such as interleukin (IL)-6 and C reactive protein (CRP). During the last decade, evidence derived from randomized trials supports the fact that regular physical training exerts several beneficial effects in patients with chronic inflammatory disorders [18]. In RA, muscular exercise improves muscle coordination and hypertrophy, reduces adipose tissue and is beneficial for immune response, in particular in patients with structural joint damage. Moreover, physical exercise has been demonstrated to improve disease activity and severity and to be beneficial for various disease outcomes [16]. However, although CV benefits of physical exercise are well recognized, few studies with contradictory results have investigated the relationship between exercise and subclinical markers of ATS, as carotid IMT or plaques, or the effects of exercise on CV outcomes in patients with chronic inflammatory and autoimmune disorders. Recently, low physical activity has been associated with an increased risk of subclinical ATS, assessed by increased carotid IMT and plaque formation, in a cohort of women with systemic lupus erythematosus (SLE) [19]. Moreover, decreased physical exercise was associated, in the same population, with the presence of pro-inflammatory high density lipoprotein (HDL), a molecule recently shown to be involved in the induction of subclinical ATS in SLE [20].
Previous findings suggest that physical exercise may play a relevant role in reducing inflammation associated with ATS and modifying subclinical markers of ATS in these patients. It is to note, however, that it is recognized that patients with RA and other chronic inflammatory diseases have a reduced level of physical activity due to articular pain and joint deformity. In this setting, given the established evidence of the role of physical activity in inhibiting disease activity and improving disease outcomes, regular physical exercise should be included in the current management of patients with chronic autoimmune rheumatic disorders. However, further studies are needed to investigate and analyse the impact of physical activity and muscular exercise on CV events in these patients.
Lipid Lowering Drug Treatment
It has been widely demonstrated that patients with chronic autoimmune rheumatic diseases display an altered pro-atherogenic lipid profile characterized by low levels of HDL-cholesterol (c) and high levels of total cholesterol (TC), low density lipoprotein (LDL-c) and triglycerides [4, 7]. Moreover, untreated active RA patients present increased levels of native oxidized LDL (oxLDL) and higher levels of small, dense LDL-c [21], which represents an emerging CV risk factor associated with higher atherosclerotic risk. Although an altered lipid profile seems to be marginal in the acceleration of ATS in some autoimmune systemic disease, such as Sjögren’s syndrome (SS) [22], the unfavourable TC/HDL-c ratio characterizing these patients, particularly during the active phase of the disease, may contribute to the increased CV risk and may be an important prognostic marker of future CV disease.
Several experimental studies have clearly demonstrated that lipid lowering drugs exert anti-inflammatory and immunomodulating effects [23–25]. Statins induce apoptosis in RA synoviocytes and regulate T helper 1 cytokine production, namely IL-2 and interferon-γ, in inflamed joints. As a result of statin treatment, endothelial cells exhibit increased eNOS with reduced expression of endothelin and production of reactive oxygen species, therefore decreasing endothelial cell activation, an early event in atherogenesis. Moreover, statins reduce circulating levels of CRP and related pro-inflammatory molecules, suppress inflammatory cytokine release and exert a plaque-stabilizing effect [25].
On the basis of the above cited data, recent studies evaluated the effect of statin in patients with chronic autoimmune rheumatic disorders, in particular RA. Subjects with hyperlipaemia treated with lipid lowering drugs were at lower risk of developing RA compared to subjects not receiving statins, thus suggesting a potential protective role of this class of drugs against RA development in subjects with altered lipid profile [26]. Together with an immunomodulatory mechanism, evidence is mounting in support of a favourable effect of statins on disease activity. In the past, several studies demonstrated that lipid lowering drug treatment is associated with a significant reduction of tender and swollen joints as well as of inflammatory markers in RA [27]. Such results have been supported by the first, largest, double-blind, randomized, placebo-controlled trial (TARA), which evaluated the effects of 6 months of statin treatment (atorvastatin 40 mg/day) on disease activity measures in RA patients [28]. In this cohort of patients with active disease, the addition of statin therapy was accompanied by improvement of disease activity, as assessed by DAS28 score and EULAR response criteria, as well as by a significant reduction of systemic inflammation parameters [28]. In spite of these beneficial effects, employment of statins was not associated with a significant reduction of corticosteroid treatment in a large cohort of RA patients, suggesting that their effect in reducing disease activity may be much smaller than the effect of drugs commonly used to manage the disease [29].
Administration of atorvastatin and simvastatin has been demonstrated to modify indirect measures of subclinical ATS in RA patients. In particular, a significant improvement of endothelium-dependent vasodilation and systemic arterial stiffness, both considered indirect measures of yet reversible endothelial dysfunction, has been demonstrated in small RA cohorts following a short period of statin administration [27]. On the other hand, in a recent placebo-controlled study investigating the effect of atorvastatin on markers of ATS in SLE patients, there was no significant treatment effect on carotid artery IMT and coronary calcium score [30]. The deep dissimilarities in disease pathogenesis and the plausible different role of altered lipid profile in the induction of atherosclerotic damage in these patients may explain, at least in part, such contradictory results.
Of interest, the degree of improvement in endothelial function was more evident in RA patients with higher level of inflammation, thereby demonstrating a direct relationship between systemic inflammation and endothelial dysfunction. However, although endothelial function improvement was associated with a reduction of LDL-c and oxLDL levels in many studies, clear evidence about the effects of statin therapy on clinically manifested CV events and the risk–benefit ratio of long-term use of lipid lowering drugs in these patients is currently lacking. Moreover, it is still unclear which levels of lipids are the ideal target for CV prevention in RA. Such area of uncertainty can be in part explained by the paucity of data derived from the few published trials investigating the effects of statins on RA and other systemic autoimmune diseases and by the small number of patients included in these studies. Nevertheless, it is relevant to consider that lipid metabolism in chronic inflammatory diseases can be regulated by several factors. In this setting, TC and LDL-c are generally lower in RA patients with no complete control of systemic inflammation than in the general population and inversely correlated with markers of inflammation [31]. The demonstrated rise in lipid levels following anti-tumor necrosis factor (TNF)-α therapy reflects the reduction of inflammatory burden and may not necessarily translate into increased CV risk. A lower risk of CV events has been demonstrated in clinical responders to TNF-α blockade [32], suggesting that the mechanisms underlying CV risk in chronic inflammatory diseases are rather complex. Indeed, lipid metabolism in these patients may be influenced by several factors, including inflammatory state, disease activity and concomitant immunosuppressive treatment. Since randomized trials specifically addressing the effect of statins in the prevention of CV events in patients with chronic inflammatory autoimmune disorders are lacking, levels of LDL-c lower than 100 mg/dL and of HDL over 40 mg/dL, similar to those recommended for patients with other CV risk factors, may be recommended in these patients. However, based on the current available data, decision to start primary prevention with lipid lowering drugs in these patients should be individualized. A careful evaluation of risk–benefit ratio of long-term statin use should be always considered. Moreover, patient age and concomitant CV risk factors, comorbidity, concurrent drugs, disease activity and long-term prognosis should be accurately evaluated before statin introduction.
Anti-hypertensive Strategy
Hypertension is a common problem in patients with systemic autoimmune rheumatic diseases, in particular in RA subjects. Nevertheless, the observation that SS patients display lower frequency of hypertension with respect to age and sex-matched control subjects may suggest that traditional CV risk factors have a different pathogenetic role in the induction of atherosclerotic damage in patients with chronic inflammatory and autoimmune diseases [33].
Several factors may contribute to hypertension in these patients. Among these, obesity, low physical activity associated with joint deformity, long-term CS therapy and systemic inflammation have been proposed as main factors associated with the increased blood pressure observed in these subjects, in particular in RA [34]. In addition, some gene polymorphisms have been identified to increase the risk of hypertension in RA patients with respect to the general population. In particular, transforming growth factor-β 869T/C and endothelin-1 gene polymorphism have been demonstrated to be associated with elevated blood pressure independent of other hypertension risk factors in RA cohorts [35].
As assessed for lipid lowering drugs, there is evidence for undertreatment of hypertension in RA population, although elevated blood pressure values have been demonstrated to be the major determinant of target organ damage in these patients [34, 36]. In a recent retrospective study on a large cohort of Greek RA patients with at least 2 years of follow-up, hypertension has been revealed to be the strongest variable related to the development of CV disease, defined as cardiac fatal and non-fatal ischemic events, stroke, peripheral artery disease and hypertensive heart disease [37]. Similar results have been further strengthened in a recent cross-sectional study aimed to identify risk factors associated with thrombosis and pregnancy in a cohort of primary or SLE-associated anti-phospholipid syndrome patients [38]. Hypertension was associated with a 2.4-fold increase in the risk of arterial thrombosis and resulted to be the strongest risk factor for this complication at multivariate analysis. This suggests that an optimal arterial blood pressure control should be achieved in this population to potentially prevent arterial events.
Angiotensin-converting enzyme (ACE) inhibitors may be considered a good anti-hypertensive strategy in RA patients. A recent study suggested that 10 mg/day of ramipril for 8 weeks in combination with standard care markedly improved endothelial function in a small cohort of RA patients, underscoring the need of large, randomized prospective trials to investigate the effect of ACE inhibitors in the prevention and reduction of clinical CV events in these patients [39]. Similar considerations are further supported by the observation that RA patients on ACE inhibitors or angiotensin receptor blockers display significantly lower erythrocyte sedimentation rate levels with respect to controls, after adjusting for potential confounder such as statin use [40]. A potential anti-inflammatory effect of this class of drugs, therefore, may be postulated and ACE inhibitors at the moment should be recommended as the first choice anti-hypertensive agent in these patients. In addition, current evidence supports the beneficial role of this class of drugs in patients with autoimmune diseases. It is well accepted, indeed, that ACE inhibitor use delays the development of renal involvement and is associated with a decreased risk of disease activity in SLE patients. In addition, it has a primary role in the early control of blood pressure and in the prevention of scleroderma renal crisis in systemic sclerosis [41, 42].
Oral Hypoglycaemic Agents
Diabetes mellitus (DM) is an established risk factor for ATS both in the general population and in patients with autoimmune diseases, in particular RA and SLE. The prevalence of CV disease in RA is comparable to that of type II DM, emphasizing the need for a correct CV risk management in RA as currently recommended for DM [43]. A relatively high prevalence of metabolic syndrome, a cluster of CV risk factors associated with increased insulin resistance (IR) and higher risk of developing type II DM, has been demonstrated in SLE and has been suggested to contribute to ATS development in these patients [44]. Indeed, SLE patients with metabolic syndrome display increased aortic stiffness, a well recognized marker of subclinical ATS and independent predictor of CV mortality, with respect to patients without such comorbidity [45]. This suggests that pharmacological intervention and lifestyle changes aimed at modifying metabolic syndrome may have a role in the prevention of accelerated ATS in these patients. Interestingly, IR has been recently recognized to characterize more than 70% of untreated early RA patients as well as patients with established disease and to be associated with accelerated coronary ATS [46, 47]. A significant higher prevalence of DM has been recently demonstrated in SS patients with respect to the general population, although the contribution of DM in determining CV disease in these patients has not been assessed [33].
Weight loss and increased exercise are first-line strategies for reducing IR and hyperglycaemia and should be always addressed in conjunction with a proper control of the systemic inflammatory burden characterizing autoimmune disorders. The role of oral hypoglycaemic agents in the prevention of CV disease in these patients has not been clarified. Peroxisome proliferator activator receptor gamma (PPARgamma) agonists (rosiglitazone and pioglitazone) have been demonstrated to reduce blood pressure and to improve endothelial-dependent vasorelaxation of thoracic aortas and endothelial progenitor cell function in murine SLE models [48, 49]. Interestingly, pioglitazone-treated mice showed improvement in IR, adipokine and lipid profile, suggesting a potential role of these drugs in the prevention of premature CV disease in SLE [49]. However, considering the pleiotropic effects of these drugs on the endothelium, prospective studies on diabetic patients with systemic autoimmune diseases are needed to demonstrate the putative effect of hypoglycaemic drugs in improving IR and in preventing CV risk.
In this context, interesting results come from a recent cross-sectional study aimed to evaluate the relationship between current hydroxychloroquine (HCQ) use and glycaemia control in non-diabetic SLE and RA women [50]. After adjusting for traditional CV risk factors, disease activity and CS use, HCQ use was independently associated with lower fasting glucose and IR in both populations. Antimalarials have been demonstrated to have other potential beneficial effects on the reduction of CV risk factors. HCQ has been associated with improvement of lipid levels in RA patients and reduction of the risk of thrombovascular events in an inception cohort of SLE patients, thereby suggesting a potential role of antimalarials in decreasing CV disease risk and in preventing DM onset in these patients [51, 52].
Anti-rheumatic Therapy
On the basis of the strong relationship between inflammation, immune dysregulation and ATS, in recent years, attention has focused on the potential beneficial effects of conventional disease-modifying drugs and biologic agents on different CV risk factors, including lipid profile, metabolic syndrome and surrogate markers of subclinical ATS in these patients. In fact, in order to prevent ATS in subjects with autoimmune rheumatic diseases, early and rapid suppression of inflammatory milieu and tight control of disease activity are now considered important preventive measures of CV disease risk.
Among drugs commonly employed for the rapid control of inflammation in these patients, long-term high-dose CS therapy has been associated with obesity, hypertension, glucose intolerance and lipid profile alteration [53]. Moreover, high-dose treatment with CS has been demonstrated to exert adverse effects on the CV system, including higher incidence of endothelial dysfunction, arterial stiffness and plaque formation [54]. On the other hand, current evidence for similar clinical effects in patients treated with low-dose CS (prednisone ≤7.5 mg/day) is lacking. Indeed, a protective effect from CV disease of the lowest effective CS dosage, based on control of disease activity and severity, has been postulated [53].
In RA patients, current evidence supports that methotrexate (MTX) use is associated with a reduced risk of CV events and mortality ranging from 40% to 70%, mainly associated with decreased risk of acute coronary events and hospitalization due to heart failure [55]. Similar beneficial effects have been also demonstrated in a cohort of early RA patients following the precocious introduction of MTX therapy [56]. Mechanisms underlying this beneficial effect on CV manifestations in RA have not been yet fully clarified. Certainly, the drug-induced suppression of systemic inflammation and control of disease activity may explain, at least in part, the reduced subclinical ATS progression in these patients. To support this hypothesis, MTX use has recently been associated with a significant carotid IMT decrease both in long-standing RA patients free from traditional CV risks and in patients with a disease duration lower than 1 year [57, 58]. On the other hand, the effect of MTX treatment on traditional CV risk factors, including lipid profile, IR and hypertension, is currently uncertain.
In recent years, treatment with anti-TNF-α agents has been demonstrated to effectively suppress systemic inflammation and control disease activity in large cohorts of RA patients. The effect of TNF-α blockade on vascular function in these patients is, however, rather complex to understand. In fact, these drugs can promote heart failure and reduce cardiac compliance, in particular at high doses, in patients with moderate to severe chronic heart failure. On the other hand, anti-TNF-α agents appear to exert a favourable effect on vascular function, in particular on aortic stiffness and endothelial function, while conflicting results have been obtained on carotid IMT improvement [59]. However, it is interesting to observe that such beneficial effect on vascular function is transient and reversible and generally observed only in responders to anti-rheumatic treatment. These observations suggest that prospective, long-term, longitudinal studies are required to evaluate the precise role of anti-TNF-α blockade in ATS prevention [60, 61]. It is of interest, however, that anti-TNF-α treatment can also improve other risk factors for accelerated ATS, since it seems to be able to decrease IR, CRP and IL-6 and increase HDL-c [62]. Interestingly, anti-TNF-α agents have been demonstrated also to exert a selective action on T-cell subsets that are thought to be involved in plaque formation. CD4+ cells lacking the co-stimulatory receptor CD28 (CD4+CD28null T cells) are present in atherosclerotic plaques of patients with unstable angina and are expanded in the peripheral blood of these patients and in a group of patients with RA. Their expansion is associated with higher carotid IMT in RA, thus representing a possible marker of subclinical ATS [63, 64]. It has been shown, in this setting, that infliximab is able to reduce the expansion of these potentially dangerous T cells in RA peripheral blood [65].
Finally, preliminary data suggest that anti-CD20 monoclonal antibody infusion may exert a short-term beneficial effect on flow-mediated vasodilation and plasma lipids in a small cohort of RA patients [66]. However, studies with a longer follow-up and involving a larger number of patients are needed to confirm such findings.
Conclusions
Despite the high number of studies carried out on the topic, CV disease remains a major problem for patients with chronic inflammatory and autoimmune diseases. Moreover, the concept of accelerated ATS in these diseases is still under investigation. Most of the evidence, coming from studies employing non-invasive assessments of vascular function or morphology, demonstrate that such functional and/or morphological changes reflect early, preclinical stages of ATS. However, no clear evidence actually suggests that preclinical ATS is associated with long-term unfavourable CV outcome in these patients. Moreover, the majority of studies performed are cross-sectional, with very few, short-term, longitudinal, randomized controlled clinical trials. This does not allow to correctly define the relative contribution of inflammatory and autoimmune mechanisms with respect to traditional CV risk factors in ATS damage. Effective RA treatment appears to be associated with some, often transient, improvements in vascular function, but there is no clear and consistent relationship between these dysfunctions and disease activity.
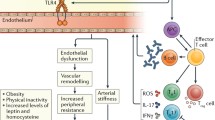
In this context, indeed, advice based on literature evidence about a preventive strategy to reduce CV disease in these patients is quite difficult, and no definitive recommendations can be drawn. Moreover, several questions remain unanswered [67]. Effective control of traditional risk factors is necessary, but considering the importance of disease-related inflammatory milieu in the induction of atherosclerotic damage may be insufficient to reduce CV risk in these patients. In fact, tight control of systemic inflammation and a deep knowledge of the intriguing and complex autoimmune mechanisms involved in ATS are likely to be required to achieve CV disease prevention in systemic autoimmune diseases. Surely, long-term, randomized trials including CV events as primary end points and guidelines for the management of CV risk factors are needed to improve patient management and to prevent CV disease. Future studies should also focus on the development of effective screening methods for the identification of those patients who are at the highest CV risk and who would benefit from early intervention. However, as summarized in Table 1 and Fig. 1, some preventive strategies may be suggested to be employed, for the meantime, in the management of CV disease in patients with systemic autoimmune rheumatic diseases.
References
Holmqvist ME, Wedrén S, Jacobsson LTH et al (2010) Rapid increase in myocardial infarction risk following diagnosis of rheumatoid arthritis among patients diagnosed between 1995 and 2006. J Intern Med 268:578–585
Shoenfeld Y, Gerli R, Doria A et al (2005) Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation 112:3337–3347
Nurmohamed MT (2009) Cardiovascular risk in rheumatoid arthritis. Autoimmun Rev 8:663–667
Bartoloni Bocci E, Luccioli F, Angrisani C, Moscatelli S, Alunno A, Gerli R (2007) Accelerated atherosclerosis in systemic lupus erythematosus and other connective tissue diseases. Expert Rev Clin Immunol 3:531–541
Sarzi-Puttini P, Azteni F, Gerli R et al (2010) Cardiac involvement in systemic rheumatic diseases: an update. Autoimmun Rev 9:849–852
Gerli R, Vaudo G, Bocci EB et al (2010) Functional impairment of arterial wall in primary Sjögren’s syndrome: combined action of immunological and inflammatory factors. Arthritis Care Res 62:712–718
Vaudo G, Bocci EB, Shoenfeld Y et al (2005) Precocious intima-media thickening in patients with primary Sjögren’s syndrome. Arthritis Rheum 52:3890–3897
Bartoloni E, Shoenfeld Y, Gerli R (2010) Inflammatory and autoimmune mechanisms in the induction of atherosclerotic damage in systemic rheumatic diseases: two faces of the same coin. Arthritis Care Res. doi:10.1002/acr.20322
Bartoloni E, Alunno A, Luccioli F et al (2010) Atherosclerotic vascular damage and rheumatoid arthritis: a complex but intriguing link. Expert Rev Cardiovasc Ther 8:1309–1316
Bartoloni E, Alunno A, Bistoni O, Gerli R (2010) How early is the atherosclerotic risk in rheumatoid arthritis? Autoimmun Rev 9:701–707
Holmqvist ME, Wedrèn S, Jacobsson LT et al (2010) No increased occurrence of ischemic heart disease prior to the onset of rheumatoid arthritis. Results from two Swedish population-based rheumatoid arthritis cohorts. Arthritis Rheum 60:2861–2869
Gabriel SE, Crowson CS, Kremers HM et al (2003) Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis Rheum 48:54–58
Solomon DH, Goodson NJ, Katz JN et al (2006) Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis 65:1608–1612
Peters MJ, Symmons DP, McCarey D et al (2010) EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 69:325–331
Toms TE, Panoulas VF, Douglas KM et al (2010) Statin use in rheumatoid arthritis in relation to actual cardiovascular risk: evidence for substantial undertreatment of lipid-associated cardiovascular risk? Ann Rheum Dis 69:683–688
Metsios G, Stavropoulos-Kalinoglou A, Sandoo A et al (2010) Vascular function and inflammation in rheumatoid arthritis: the role of physical activity. Cardiovasc Med J 4:89–96
Libby P, Okamoto Y, Rocha V, Folco E (2010) Inflammation in atherosclerosis: transition from theory to practice. Circ J 74:213–220
Turesson C, Matteson EL (2007) Cardiovascular risk factors, fitness and physical activity in rheumatic diseases. Curr Opin Rheumatol 19:190–196
Volkmann ER, Grossman JM, Sahakian LJ et al (2010) Low physical activity is associated with proinflammatory high-density lipoprotein and increased subclinical atherosclerosis in women with systemic lupus erythematosus. Arthritis Care Res 62:258–265
McMahon M, Grossman I, Skaggs B et al (2009) Dysfunctional proinflammatory high density lipoproteins confer increased risk for atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum 60:2428–2437
Steiner G, Urowitz M (2009) Lipid profiles in patients with rheumatoid arthritis: mechanisms and the impact of treatment. Arthritis Rheum 38:372–381
Gerli R, Bocci EB, Vaudo G, Marchesi S, Vitali C, Shoenfeld Y (2006) Traditional cardiovascular risk factors in primary Sjögren’syndrome: role of dyslipidemia [letter]. Rheumatology 45:1580–1581
Abeles AM, Pillinger MH (2006) Statins are anti-inflammatory and immunomodulatory agents: a future in rheumatologic therapy. Arthritis Rheum 54:393–407
Tristano AG, Fuller K (2006) Immunomodulatory effects of statins and autoimmune rheumatic diseases: novel intracellular mechanism involved. Int Immunopharmacol 6:1833–1846
Bisoendial RJ, Stroes ES, Kastelein JJ, Tak PP (2010) Targeting cardiovascular risk in rheumatoid arthritis: a dual role for statins. Nat Rev Rheumatol 6:157–164
Jick SS, Choi H, Li L, McInnes IB, Sattar N (2009) Hyperlipidaemia, statin use and the risk of developing rheumatoid arthritis. Ann Rheum Dis 68:546–551
Paraskevas KI (2008) Statin treatment for rheumatoid arthritis: a promising novel indication. Clin Rheumatol 27:281–287
McCarey D, McInnes IB, Madhok R et al (2004) Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet 363:2015–2021
Lodi S, Evans SJ, Egger P, Carpenter J (2010) Is there an anti-inflammatory effect of statins in rheumatoid arthritis? Analysis of a large routinely collected claims database. Br J Clin Pharmacol 69:85–94
Petri M, Kiani AN, Post W et al (2006) Lupus Atherosclerosis Prevention Study (LAPS): randomized double blind placebo controlled trial of atorvastatin versus placebo. Arthritis Rheum 54:S520
Choy E, Sattar N (2009) Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis 68:460–469
Dixon WG, Watson KD, Lunt M et al (2007) Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis α therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 56:2905–2912
Pérez-De-Lis M, Akasbi M, Sisó A et al (2010) Cardiovascular risk factors in primary Sjögren’s syndrome: a case–control study in 624 patients. Lupus 19:941–948
Kitas G, Gabriel SE (2011) Cardiovascular disease in rheumatoid arthritis: state of the art and future perspectives. Ann Rheum Dis 70:8–14
Panoulas VF, Douglas KM, Smith JP et al (2009) Transforming growth factor-beta1 869T/C, but not interleukin-6-174G/C, polymorphism associates with hypertension in rheumatoid arthritis. Rheumatology 48:113–118
Panoulas VF, Toms TE, Metsios GS et al (2010) Target organ damage in patients with rheumatoid arthritis: the role of blood pressure and heart rate. Atherosclerosis 209:255–260
Serelis J, Panagiotakos DB, Mavrommati M, Skopouli FN (2010) Cardiovascular disease is related to hypertension in patients with rheumatoid arthritis: a Greek Cohort Study. J Rheumatol. doi:10.3899/jrheum.100564
Danowski A, Leitão N, de Azevedo M, de Souza Papi JA, Petri M (2009) Determinants of risk for venous and arterial thrombosis in primary antiphospholipid syndrome and in antiphospholipid syndrome with systemic lupus erythematosus. J Rheumatol 36:1195–1199
Flammer AJ, Sudano I, Hermann F et al (2008) Angiotensin-converting enzyme inhibition improves vascular function in rheumatoid arthritis. Circulation 117:2262–2269
Perry ME, Chee MM, Ferrell WR, Lockhart JC, Sturrock RD (2008) Angiotensin receptor blockers reduce erythrocyte sedimentation rate levels in patients with rheumatoid arthritis. Ann Rheum Dis 67:1646–1647
Durán-Barragán S, McGwin G Jr, Vilá LM, Reveille JD, Alarcón GS (2008) Angiotensin-converting enzyme inhibitors delay the occurrence of renal involvement and are associated with a decreased risk of disease activity in patients with systemic lupus erythematosus—results from LUMINA (LIX): a multiethnic US cohort. Rheumatology 47:1093–1096
Bussone G, Bérezné A, Pestre V, Guillevin L, Mouthon L (2010) The scleroderma kidney: progress in risk factors, therapy, and prevention. Curr Rheumatol Rep. doi:10.1007/s11926-010-0145-7
van Halm VP, Peters MJ, Voskuyl AE et al (2009) Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: a cross-sectional study, the CARRE investigation. Ann Rheum Dis 68:1395–1400
Parker B, Bruce IN (2010) The metabolic syndrome in systemic lupus erythematosus. Rheum Dis Clin North Am 36:81–97
Sabio JM, Vargas-Hito J, Zamora-Pasadas M et al (2009) Metabolic syndrome is associated with increased arterial stiffness and biomarkers of subclinical atherosclerosis in patients with systemic lupus erythematosus. J Rheumatol 36:2204–2211
Shahin D, Eltoraby E, Mesbah A, Houssein M (2010) Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem 43:661–665
Chung CP, Oeser A, Solus JF et al (2008) Inflammation associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum 58:2105–2112
Venegas-Pont M, Sartori-Valinotti JC, Maric C et al (2009) Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 296:R1282–R1289
Zhao W, Thacker SG, Hodgin JB (2009) The peroxisome proliferator-activated receptor gamma agonist pioglitazone improves cardiometabolic risk and renal inflammation in murine lupus. J Immunol 183:2729–2740
Kaprove Penn S, Kao AH, Schott LL et al (2010) Hydroxychloroquine and glycemia in women with rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol 37:1136–1142
Morris SJ, Wasko MC, Antohe JL et al (2010) Hydroxychloroquine use is associated with improvement in lipid profiles in rheumatoid arthritis patients. Arthritis Rheum. doi:10.1002/acr.20393
Jung H, Bobba R, Su J et al (2010) The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum 62:863–868
Turesson C, Jacobsson LT, Matteson E (2008) Cardiovascular co-morbidity in rheumatic diseases. Vasc Health Risk Manag 4:605–614
del Rincon I, O’Leary DH, Haas RW et al (2004) Effect of glucocorticoids on the arteries in rheumatoid arthritis. Arthritis Rheum 50:3813–3822
Westlake SL, Colebatch AN, Baird J (2010) The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology 49:295–307
Hochberg MC, Johnston SS, John AK (2008) The incidence and prevalence of extra-articular and systemic manifestations in a cohort of newly-diagnosed patients with rheumatoid arthritis between 1999 and 2006. Curr Med Res Opin 24:469–480
Ristic G, Lepic T, Glisic B et al (2010) Rheumatoid arthritis is an independent risk factor for increased carotid intima-media thickness: impact of anti-inflammatory treatment. Rheumatology 49:1076–1081
Georgiadis AN, Voulgari PV, Argyropoulou MI et al (2008) Early treatment reduces the cardiovascular risk factors in newly diagnosed rheumatoid arthritis patients. Semin Arthritis Rheum 38:13–19
Angel K, Aarrestad Provan S, Lovahl Gulseth H, Mowinckel P, Kvien K, Atar D (2010) Tumor necrosis factor-α antagonists improve aortic stiffness in patients with inflammatory arthropathies. A controlled study. Hypertension 55:333.38
Kerekes G, Soltsèz P, Dèr H et al (2009) Effects of biologics on vascular function and atherosclerosis associated with rheumatoid arthritis. Ann N Y Acad Sci 1173:814–821
Gallaraga B, Belch JJ, Pullar T, Ogston S, Khan F (2010) Clinical improvement in rheumatoid arthritis is associated with healthier microvascualr function in patients who respond to antirheumatic therapy. J Rheumatol 37:521–528
Gonzalez-Gay MA, Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Miranda-Filloy JA, Llorca J (2010) Insulin resistance in rheumatoid arthritis: the impact of the anti-TNF-alpha therapy. Ann N Y Acad Sci 1193:153–159
Gerli R, Schillaci G, Giordano A et al (2004) CD4+CD28null T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients. Circulation 109:2744–2748
Gerli R, Vaudo G, Bartoloni Bocci E, Schillaci G, Bistoni O, Shoenfeld Y (2009) Different roles for anti-cyclic citrullinated peptide antibodies and CD4+CD28null cells in the acceleration of atherosclerosis in rheumatoid arthritis: comment on the article by Farragher et al. Arthritis Rheum 60:631–632
Gerli R, Bocci EB, Shoenfeld Y (2006) Effect of tumor necrosis factor α inhibition on CD28 surface expression on CD4+ T cells. Arthritis Rheum 54:3060–3061
Kerekes G, Soltsez P, Der H et al (2009) Effects of rituximab treatment on endothelial dysfunction, carotid atherosclerosis and lipid profile in rheumatoid arthritis. Clin Rheumatol 28:705–710
Bruce IN (2005) Cardiovascular disease in lupus patients: should all patients be treated with statins and aspirin? Best Pract Res Clin Rheumatol 19:823–838
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bartoloni, E., Alunno, A., Bistoni, O. et al. Cardiovascular Risk in Rheumatoid Arthritis and Systemic Autoimmune Rheumatic Disorders: a Suggested Model of Preventive Strategy. Clinic Rev Allerg Immunol 44, 14–22 (2013). https://doi.org/10.1007/s12016-010-8251-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-010-8251-x