Abstract
There are two main families of polyunsaturated fatty acids (PUFAs), the n−6 and the n−3 families. It has been suggested that there is a causal relationship between n−6 PUFA intake and allergic disease, and there are biologically plausible mechanisms, involving eicosanoid mediators of the n−6 PUFA arachidonic acid, that could explain this. Fish and fish oils are sources of long-chain n−3 PUFAs and these fatty acids act to oppose the actions of n−6 PUFAs. Thus, it is considered that n−3 PUFAs will protect against atopic sensitization and against the clinical manifestations of atopy. Evidence to examine this has been acquired from epidemiologic studies investigating associations between fish intake in pregnancy, lactation, infancy, and childhood, and atopic outcomes in infants and children and from intervention studies with fish oil supplements in pregnancy, lactation, infancy, and childhood, and atopic outcomes in infants and children. All five epidemiological studies investigating the effect of maternal fish intake during pregnancy on atopic or allergic outcomes in infants/children of those pregnancies concluded protective associations. One study investigating the effects of maternal fish intake during lactation did not observe any significant associations. The evidence from epidemiological studies investigating the effects of fish intake during infancy and childhood on atopic outcomes in those infants or children is inconsistent, although the majority of the studies (nine of 14) showed a protective effect of fish intake during infancy or childhood on atopic outcomes in those infants/children. Fish oil supplementation during pregnancy and lactation or during infancy or childhood results in a higher n−3 PUFA status in the infants or children. Fish oil provision to pregnant women is associated with immunologic changes in cord blood and such changes may persist. Studies performed to date indicate that provision of fish oil during pregnancy may reduce sensitization to common food allergens and reduce prevalence and severity of atopic dermatitis in the first year of life, with a possible persistence until adolescence with a reduction in eczema, hay fever, and asthma. Fish oil provision to infants or children may be associated with immunologic changes in the blood but it is not clear if these are of clinical significance and whether they persist. Fish oil supplementation in infancy may decrease the risk of developing some manifestations of allergic disease, but this benefit may not persist as other factors come into play. It is not clear whether fish oil can be used to treat children with asthma as the two studies conducted to date give divergent results. Further studies of increased long-chain n−3 PUFA provision in during pregnancy, lactation, and infancy are needed to more clearly identify the immunologic and clinical effects in infants and children and to identify protective and therapeutic effects and their persistence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are two main families of polyunsaturated fatty acids (PUFAs), the omega-6 (n−6) and omega-3 (n−3) families [1]. These are generally considered to act antagonistically to one another, such that relative imbalances may be associated with physiological dysfunction and increased risk of disease [2]. Intake of the n−6 PUFA linoleic acid (LA) increased over the latter part of the twentiath century and this has been said to be causally related to increased prevalence and incidence of atopic diseases in children [3, 4]. There is a plausible biological explanation for this relationship involving eicosanoid mediators produced from the long-chain n−6 PUFA arachidonic acid (AA), a derivative of LA [5, 6]. Because long-chain n−3 PUFAs act to counter the effect of n−6 PUFAs, a higher intake of these fatty acids should reduce the risk of atopic diseases [3, 4]. The richest food source of long-chain n−3 PUFAs is fish, especially oily fish, and they are also found in fish oil supplements. Therefore, it may be anticipated that higher consumption of fish or oily fish or use of fish oil supplements would be associated with lowered risk of atopy and its clinical manifestations. The aim of this article is to collate, describe, and interpret the current literature describing associations between early exposure to fish or to fish oil supplements and infant or childhood atopy or immune markers relevant to atopy; data from cohort, cross-sectional, case-control, and intervention studies involving pregnant or lactating women, infants, or children are considered. Prior to presenting this information, relevant background information on PUFAs and eicosanoids is presented.
Fatty acids: structure, nomenclature, sources, roles, and intakes
PUFAs contain two or more double bonds in their hydrocarbon (acyl) chain. A commonly used shorthand notation for describing and naming fatty acids relies upon identifying the number of carbon atoms in the chain, and the number of double bonds and their position. Thus, the 18-carbon fatty acid with two double bonds in its acyl chain and with the first double bond on carbon number 6 from the methyl terminal carbon is described as 18:2ω−6. The ω−x nomenclature is sometimes referred to as omega x (e.g. 18:2 omega 6) or n−x (e.g. 18:2n−6). In addition, fatty acids are often described by their common names. The main fatty acids of relevance to the current article are:
-
Linoleic acid (LA; 18:2n−6)
-
Arachidonic acid (AA; 20:4n−6)
-
α-Linolenic acid (ALA; 18:3n−3)
-
Eicosapentaenoic acid (EPA; 20:5n−3)
-
Docosahexaenoic acid (DHA; 22:6n−3).
There are two principal families of PUFAs, the n−6 (or omega-6) and the n−3 (or omega-3) families. The simplest members of each family, LA and ALA, cannot be synthesized by mammals. LA is found in significant quantities in many vegetable oils, including corn, sunflower, and soybean oils, and in products made from such oils, such as margarines [1]. ALA is found in green plant tissues, in some common vegetable oils, including soybean and rapeseed oils, in some nuts, and in flaxseed (also known as linseed) and flaxseed oil. Between them, LA and ALA contribute over 95%, and perhaps as much as 98% of dietary PUFA intake in most Western diets [1], with LA intake being in excess of that of ALA. The intake of LA in Western countries increased greatly over the second half of the twentieth century, following the introduction and marketing of cooking oils and margarines [1]. ALA intake probably changed little over this time. Typical intakes of both essential fatty acids are in excess of requirements [1]. However, the changed pattern of consumption of LA has resulted in a marked increase in the ratio of n−6 to n−3 PUFAs in the diet. This ratio is currently between 5 and 20 in most Western populations [7, 8].
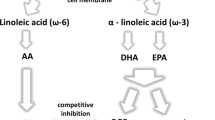
Although LA and ALA cannot be synthesized by humans, they can be metabolized to other fatty acids (Fig. 1). This is achieved by the insertion of additional double bonds into the acyl chain (i.e., unsaturation) and by elongation of the acyl chain. Thus, LA can be converted via γ-linolenic acid (18:3n−6) and di-homo-γ-linolenic acid (20:3n−6) to AA (Fig. 1). By an analogous set of reactions catalyzed by the same enzymes ALA can be converted to EPA. Both AA and EPA can be further metabolized, EPA giving rise to docosapentaenoic acid (22:5n−3; DPA) and DHA (Fig. 1). Dietary intakes of the longer chain, more unsaturated PUFAs are much lower than of LA and ALA [1, 8, 9]. AA is found in meat and offal and intakes are estimated at 50 to 500 mg/day. EPA, DPA, and DHA are found in fish, especially so-called “oily” fish (tuna, salmon, mackerel, herring, and sardine). One oily fish meal can provide between 1.5 and 3.5 g of these long-chain n−3 PUFAs [9]. The commercial products known as fish oils also contain these long-chain n−3 PUFAs, which typically will contribute about 30% of the fatty acids present. Thus, consumption of a typical 1-g fish oil capsule per day can provide about 300 mg of these fatty acids. In the absence of oily fish or fish oil consumption, intake of long-chain n−3 PUFAs is likely to be <100 mg/day, although foods fortified with these fatty acids are now available in many countries.
PUFAs, eicosanoids, inflammatory processes, and atopy
PUFAs play roles assuring the correct environment for membrane protein function, maintaining membrane fluidity and regulating cell signaling, gene expression and cellular function [1]. Through these actions PUFAs can influence the functioning of immune cells [10, 11] and so could impact on the development and manifestations of atopy [5, 6]. However, the key link between PUFAs and immunological processes related to atopy is that the eicosanoid family of mediators is derived from 20-carbon PUFAs [12, 13]. Because immune cells typically contain a high proportion of the n −6 PUFA AA and low proportions of other 20-carbon PUFAs [10, 11, 14], AA is usually the major substrate for eicosanoid synthesis. Eicosanoids, which include prostaglandins (PGs), thromboxanes (TXs), leukotrienes (LTs) and other oxidized derivatives, are generated from AA by the action of cyclooxygenase (COX) and lipoxygenase (LOX) enzymes (Fig. 2). These enzymes are expressed in inflammatory and epithelial cells and give rise to a mix of mediators depending upon the nature of cell types present and the nature, timing and duration of the stimulus [12, 13, 15, 16]. Eicosanoid mediators are involved in modulating the intensity and duration of inflammatory responses [see [15, 16] for reviews]. Through actions on dendritic cells, T cell differentiation and immunoglobulin (Ig) class switching in B cells, some eicosanoids (e.g. PGE2) play a role in promoting sensitization to allergens. Through their actions on inflammatory cells, smooth muscles and epithelial cells, some eicosanoids are strongly implicated in different immunologic features and clinical manifestations of atopic disease (Table 1). Indeed, allergic inflammation in animal models is associated with increased PG and LT production. However, inhibition of COX-1 or COX-2 or knockout of either COX results in augmented allergic inflammation with increased Th2 type cytokine production and increased airway reactivity (see [17, 18]). This suggests that the overall effect of PGs is to restrain allergic inflammation. However, individual PGs might enhance or inhibit allergic inflammation depending upon their specific action. One current view is that PGD2, PGF2α, and TXA2 increase allergic inflammation, whereas PGE2 and PGI2 inhibit it (see [17, 18]). PGD2 is produced mainly by mast cells and activated macrophages. It is a potent bronchoconstrictor, promotes vascular permeability, and activates eosinophils and a Th2-type response. TXA2 is a bronchoconstrictor and stimulates acetylcholine release. PGE2 is a vasodilator, increases vascular permeability, inhibits the production of Th1-type cytokines and primes naïve T cells to produce interleukin (IL)-4 and IL-5. PGE2 also promotes Ig class switching in uncommitted B cells towards the production of IgE. Despite these effects of PGE2, it is now considered that this eicosanoid is protective towards airway inflammation [17, 18]. It is possible that PGE2 promotes sensitization via its effects on T cell phenotype and B cells, but is protective against the subsequent manifestations of inflammation upon re-exposure to allergen. PGI2 appears to suppress Th2 lymphocyte activity and eosinophil recruitment. LTB4 is chemotactic for leukocytes, increases vascular permeability, induces the release of lysosomal enzymes and reactive oxygen species by neutrophils and of inflammatory cytokines (e.g., tumor necrosis factor (TNF)-α) by macrophages, and promotes IgE production by B cells. The cysteinyl LTs (LTC4, D4, and E4) may be either vasoconstrictors or vasodilators depending upon the situation and the location of their synthesis. They cause smooth muscle contraction and bronchoconstriction, increase vascular permeability and eosinophil recruitment, and promote mucus secretion. PGE2 inhibits 5-LOX activity, down-regulating LT production [19]. Furthermore, PGE2 induces 15-LOX leading to production of lipoxin A4 which is anti-inflammatory [20, 21]. These effects highlight the antagonist nature of eicosanoids and may underlie, at least in part, the protective effect of PGE2 in allergic inflammation.
Animal feeding studies have shown a strong positive relationship between the amount of AA in inflammatory cells and the ability of those cells to produce eicosanoids such as PGE2 [22]. A recent human study also reported a strong positive correlation between the amount of AA in mononuclear cells and their ability to produce PGE2 when stimulated by lipopolysaccharide [23].
Increased consumption of long-chain n−3 PUFAs such as EPA and DHA (usually given as fish oil) results in increased proportions of those fatty acids in inflammatory cell phospholipids (see [10, 11, 14, 23]). The incorporation of EPA and DHA into human inflammatory cells occurs in a dose-related fashion [23, 24] and is partly at the expense of AA. Since there is less substrate available for synthesis of eicosanoids from AA, fish oil supplementation of the human diet has been shown to result in decreased production of a range of AA-derived eicosanoids by inflammatory cells (see [14] for references). EPA is also able to act as a substrate for COX and LOX enzymes, giving rise to eicosanoids with a slightly different structure to those formed from AA (e.g., 5-series LTs; see [14]). The functional significance of this is that the mediators formed from EPA are believed to be less potent than those formed from AA. For example, LTB5 is ten to 100-fold less potent as a neutrophil chemotactic agent than LTB4 (see [14]).
In addition to long-chain n−3 PUFAs modulating the generation of eicosanoids from AA and to EPA acting as substrate for the generation of alternative eicosanoids, recent studies have identified a novel group of mediators, termed E and D-series resolvins, formed from EPA and DHA, respectively, that appear to exert anti-inflammatory and inflammation resolving actions (see [25] for a review). In recent studies in ovalbumin-sensitized Balb/C mice administration of resolvin E1 was found to decrease airway eosinophil and lymphocyte recruitment, production of the Th2 cytokine IL-13, circulating ovalbumin-specific IgE, and airway hyperresponsiveness to inhaled methacholine [26] and to promote the resolution of inflammatory airway responses by directly suppressing the production of IL-23 and IL-6 in the lung [27].
The above considerations have led to the idea that a high exposure to n−6 PUFAs (and/or low exposure to n−3 PUFAs) will promote atopy (both sensitization and manifestations) and that high exposure to n−3 PUFAs will be protective [3–6].
Epidemiological studies relating early fish exposure to atopic outcomes in infancy or childhood
The aim of this section is to describe and interpret studies relating maternal fish intake during pregnancy or lactation to atopic outcomes in the offspring of those mothers and relating fish intake during infancy or childhood to atopic outcomes in those infants or children. Studies were identified through Ovid Medline (1950–2009) and Embase (1980–2009) databases performing and combining searches using appropriate keywords.
Studies investigating the effect of maternal fish intake during perinatal life on atopic outcomes in infants or children
Table 2 summarizes all identified studies that investigate the association between maternal fish intake in perinatal life (five studies of fish intake during pregnancy and one study of fish intake during lactation) and atopic outcomes in the offspring of those mothers.
Out of the five studies which examined the effect of maternal fish intake during pregnancy three studies were prospective cohort [28–30], one study was case–control [31], and one study was retrospective cohort [32]. The age range of children taking part in these studies was between 2 and 16 years. Children who took part in the three prospective cohort studies were followed-up for 6, 2, and 5 years [28–30] for each study, respectively. Table 2 presents only statistically significant effects of maternal fish intake in relation to atopic outcomes in the infants or children. Other results reported by these studies that were either not significant or not related to fish intake are not included here. Regarding the quality and the method of assessing fish intake during pregnancy, three of the five studies used a food frequency questionnaire (FFQ) [29, 31, 32] and two used a semi-quantitative FFQ [28, 30]. FFQs varied in frequency categories and also in time point of administration (during pregnancy [30], shortly after birth [28, 29] or retrospectively some time after birth [31, 32]) and way of administration (self-administered [30] or interviewer-administered [28, 29, 31]). All of the studies adjusted for most recognized confounding factors. There was great heterogeneity among studies with regard to the outcome measures and their assessment. Salam et al. [31] focused on asthma, Calvani et al. [32] focused on allergic sensitizations, while Sausenthaler et al. [28], Romieu et al. [29], Willers et al. [30] included various outcome measures such as eczema, atopic wheeze, allergic sensitization, or hay fever. Each of the studies assessing clinical outcomes used a parental questionnaire which most of the time asked for doctor diagnosis and gave clear definitions of each outcome. Although doctor-diagnosed atopic diseases may be more valid, limited access to health care may result in under-diagnosis of atopic disease, especially when these are mild or at early stages [31].
There is consistency between the findings of these five studies since each of them identified beneficial associations between maternal fish intake during pregnancy and atopic or allergic outcomes in children (Table 2). In the study of Salam et al. [31] the association between maternal oily fish intake and children's risk of developing asthma was greater in children whose mothers had asthma compared to children of non-asthmatic mothers (p for interaction = 0.02). In contrast, Calvani et al. [32] observed stronger and more significant beneficial effects of increased oily fish intake during pregnancy for children of non-allergic mothers compared to those of allergic mothers for food sensitizations (p for trend = 0.002) but not for inhalant sensitizations. It is not clear why these findings are different. In the prospective cohort study conducted by Romieu et al. [29], although a beneficial association was observed initially for the whole sample, after stratifying by breast-feeding, increased fish consumption during pregnancy decreased the risk of persistent wheeze at 6 years of age among the non-breastfed infants whereas no protective effect was observed among the breastfed infants. The large cohort studies conducted by Sausenthaler et al. [28] and Willers et al. [30] concluded similar associations: high (≥1 time/week) vs. low maternal fish intake during pregnancy was associated with decreased doctor-diagnosed eczema. However, the decrease in the study of Willers et al. [30] was greater than that in the study of Sausenthaler et al. [28] (43% vs. 25%, respectively). This might be related to the fact that the study of Willers et al. [30] followed-up children for a longer period, allowing for manifestations of atopic disease to be revealed at the stage of clinical assessment. Willers et al. [30] also showed that there was 72% less doctor-diagnosed hay fever in children born to mothers with higher oily fish intake (but not total fish intake) during pregnancy. Across these five studies, the extent of the protective effect of maternal fish intake was highly variable. Fish intake resulted in a decrease in infant or childhood atopy which ranged between 25% and 95%. However, most decreases in atopic risk ranged between 40% and 80%.
The last study listed in Table 2 conducted by Hoppu et al. [33] assessed maternal dietary intake 1 month after birth (during lactation) and atopic dermatitis development in the infant at four timepoints. The primary objective of the study was to examine the effect of breast milk fatty acid composition on atopic dermatitis during the first year of life. Although a higher percentage of EPA in breast milk was related to lower risk of atopic dermatitis, fish consumption frequency during lactation was not associated with breast milk EPA content. This may be explained by the fact that breast milk fatty acid composition is determined more by fatty acids accumulated in maternal adipose tissue during pregnancy rather than dietary intake of fatty acids during lactation [34, 35]. The authors stated that maternal fish intake during pregnancy would have been more appropriate to investigate in relation to breast milk composition [33].
Summary and discussion of the findings of these studies
All five epidemiological studies investigating the effect of maternal fish intake during pregnancy on atopic or allergic outcomes in infants/children of those pregnancies concluded protective associations. The protective effect varied widely between 25% and 95% and this might be attributed to differences in study design, i.e., confounding factor adjustments, statistical analysis, definition of atopic outcome in infants/children and their mothers, method of atopy evaluation, method of collecting dietary information, oily and/or total fish definition as well as categories of consumption frequencies used for comparisons. The one study investigating the effects of maternal fish intake during lactation did not observe any significant associations.
Studies investigating the effects of fish intake during infancy or childhood on atopic outcomes in those infants or children
Table 3 summarizes all identified studies that investigate the association between fish intake during infancy or childhood and atopic or allergic outcomes in those infants or children; 14 studies were identified. Nine studies observed a beneficial effect of fish intake during infancy/childhood and atopic outcomes in those infants/children [36–44]. Two of the studies observed a negative effect of fish intake on childhood atopy [45, 46], and three studies observed no associations [47–49].
Of the studies that found a beneficial association between fish intake during infancy/childhood and atopic outcomes, three were prospective cohort [38, 41, 49], two were case–control [36, 37], and four were cross-sectional [39, 40, 42, 43]. The age range of children taking part in these studies was between 1 and 18 years (at the time point that outcomes were measured). Because of the wide age range of the study population, differences in the extent of the beneficial effect can be expected. Some of the studies measured exposure and outcome at a much older age [36, 37, 39, 40] than others [38, 41, 42, 49]. Two of the prospective cohort studies followed-up infants to age 4 years [38, 41], whereas Alm et al. [49] followed-up infants to age 1 year. Atopic outcome definitions and assessment methods differed between studies. Three of the studies performed skin prick testing (SPT) [36, 39, 42] and one study determined specific IgE to identify sensitization [41]. In the study of Alm et al. [49] no clinical test or biochemical measurement of allergy was conducted. Clinical outcomes in all of the studies were assessed with parental questionnaires. However, parents were not always asked for doctor diagnosis. The different studies controlled for different confounding factors.
The reduction in atopy/allergy risk due to fish intake among these nine studies ranged between 22% and 80% (Table 3). However, the risk reduction in most cases was between 50% and 60%, providing consistent evidence for the protective effects of fish consumption during infancy/childhood on atopy/allergy. The study of Antova et al. [43] showed that low fish intake, compared with higher intake, increased the risk of respiratory symptoms by 21% (current wheeze). However, studies were inconsistent as far as exposure assessment is concerned. All three prospective cohort studies [38, 41, 49] determined the time point of fish introduction during the first year of life using a parental questionnaire. In addition, Kull et al. [41] collected information on fish consumption frequencies. The cross-sectional studies of Kim et al. [40] and Antova et al. [43] used a parental FFQ, that of Chatzi et al. [42] used a semi-quantitative FFQ and the other cross-sectional study used parental report of fish consumption without frequencies [39]. The retrospective case–control study of Dunder et al. [37] used a 48-h recall of intake and the case–control study of Hodge et al. [36] used a parental FFQ (although consumption frequency categories were not used in their analysis). The ideal method of collecting information on fish consumption would be a FFQ with various food categories/items reflecting intake during the past 12 months, including time of introduction of fish into diet and different types of fish consumed (oily, non-oily). Oily fish consumption was recorded in only some of the studies [36, 42, 49]. The rest of the studies recorded only total fish intake without specifying the types of fish. In the study of Hodge et al. [36], fresh oily fish consumption had a greater beneficial effect on current asthma than total fish consumption (74% versus 48% reduction, respectively). The ‘Infants of Western Sweden’ study of Alm et al. [49] collected information on the fish usually consumed and this was categorized into two different types of fish (lean and oily). The vast majority of the infants consumed lean fish, and at the univariate analysis it was shown that eating lean fish reduced eczema risk by 19% at 1 year of age, but the effect was lost at the multivariate analysis [49]. However, Chatzi et al. [42] did not identify any significant association for any of the subgroups of fish included in their FFQ. Nafstad et al. [38] saw a protective effect of introduction of fish early into diet on allergic rhinitis for the whole study population, but this protective effect remained significant only among children who were breastfed for more than 6 months, without parental hay fever or asthma, with early life atopic eczema, or without an episode of lower respiratory tract infection during the first year of life. Similarly, in the other prospective cohort study of Kull et al. [41], although there was a dose-dependent reduction in risk of atopic outcomes with increased fish consumption frequency, the protective effect was significant only for children without parental allergy. Additionally and in contrast to the study of Nafstad et al. [38], in the study of Kull et al. [41] the results remained significant only for children without eczema and/or recurrent wheeze during the first year of life. Interestingly, introducing fish early during the first year of life (age 3–8 months) was more beneficial than introducing fish later on (age ≥ 9 months) and this was associated with a lower risk of eczema at 4 years of age [41]. This agrees with the findings of Alm et al. [49] who found that the introduction of fish before 9 months of age had a protective effect on eczema in infants at 1 year, lowering the risk by 24% at this age. The rest of the studies which identified a protective effect of fish conducted their analysis for the study population as a whole.
Two studies were identified that found a negative effect of fish consumption on atopic/allergic outcomes in infants/children [44, 45]. Both studies had a cross-sectional design, measuring exposure and outcome at the same time point. This study design is not strong enough to infer causality. Compared to the studies that identified a positive effect of fish consumption, these studies were conducted in older children. Both studies used a FFQ. The FFQ in the study of Takemura et al. [45] was completed by parents. The study of Huang et al. [44], apart from the FFQ, used a 24-h recall and compared the effect between quartiles of intake rather than consumption frequencies. Also, the study conducted by Huang et al. [44] included oily fish in the questionnaire. As far as the atopic outcome measures are concerned, in the study of Takemura et al. [45] questionnaires about atopy were answered by parents whereas in the study of Huang et al. [44] questionnaires were answered by children. Both studies were focused on clinical outcomes and included a component of doctor diagnosis in their questionnaires. The study of Huang et al. [44] excluded subjects with atopic symptoms who were not doctor-diagnosed. Although some confounders were adjusted for in each study, neither adjusted for socio-economic factors. In the study of Takemura et al. [45], comparing children with current asthma and healthy children there were no differences in fish consumption frequencies. However, the increase in risk (12% for current asthma) was significant when comparing the effect of fish intake 1–2 times/month with that of fish intake 1–2 times/week. Also, the authors showed that the risk of current asthma increased with increasing fish intake (p trend = 0.0349). Although the univariate analysis of Huang et al. [44] showed that higher oily fish intake was associated with higher prevalence of doctor diagnosed asthma, in the multivariate analysis this association was no longer significant. Also, no associations were found for total fish, seafood, and shellfish intake and allergic rhinitis or asthma.
Of the three studies that did not identify any statistically significant associations between fish intake and atopic or allergic outcomes in infants/children, two were prospective cohort studies [47, 48] and one was case–control [46]. All studies were conducted in children older than 1 year of age. Hijazi et al. [46] collected fish intake data at age 12 years, Farchi et al. [47] at age 6–7 years and Wijga et al. [48] at age 2 years. All three studies used a parental/maternal FFQ. The study of Farchi et al. [47] was the only one including oily fish and ‘pasta with oily fish’ as categories. The other two studies collected information only on consumption frequencies of total fish. Also, frequency categories (often, sometimes, rarely, never) in the study of Hijazi et al. [46] were not clearly defined in the questionnaire. What is more, the two prospective cohort studies [47, 48] did not include monthly fish consumption frequencies in their analysis. In contrast, they only compared weekly consumption frequencies which may not have allowed for significant associations to be identified. As far as the outcome measures are concerned, the two prospective cohort studies [47, 48] assessed atopic outcome based on the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaires completed by parents. The ISAAC questions were also used in the study of Hijazi et al. [46], but were answered by the children themselves. Although the two prospective cohort studies had a large sample size, their follow-up lasted only for 1 year which may not have been long enough to allow for atopy/allergy outcomes to be revealed. The cross-sectional data recorded in 1980 during the case-control study, nested in the follow-up study of Dunder et al. [37], identified a null association between fish consumption and any atopic outcome (in 1980). However, as mentioned above, the retrospective follow-up showed a protective effect of fish. In the same study, the cross-sectional data of 1986 were not presented.
Summary and discussion of the findings of these studies
Overall, the evidence from epidemiological studies investigating the effects of fish intake during infancy and childhood on atopic outcomes in those infants or children is inconsistent. However, the majority of the studies (nine of 14) showed a protective effect of fish intake during infancy or childhood on atopic outcomes in those infants/children. The reduction in atopy/allergy risk amongst these studies ranged between 22% and 80%. This variation could be attributed to the fact that studies differed in study design, control of confounding factors, exposure, and outcome measure assessment. Three studies did not observe any associations, and two studies observed increased risk of atopy with higher fish consumption (12% increase of risk for current asthma [45]), although the study of Huang et al. [44] did not show any significant associations in the multivariate analysis. Therefore, based on the evidence available from epidemiological studies, it cannot be clearly concluded with absolute certainty whether fish consumption during infancy or childhood can be protective towards atopic disease development in those infants/children, although a number of studies would support that conclusion.
Intervention studies investigating the effect of early fish oil exposure on atopic outcomes in infancy or childhood
In general, randomized controlled trials (RCTs) or experimental/intervention studies are designs that provide the highest level of testing of cause and effect. This section reviews the literature published to present on RCTs of fish oil supplementation in pregnancy, lactation, or infancy/childhood and atopy/allergy outcomes in infants/children of those pregnancies or those infants/children (Tables 4 and 5). The studies were identified through Ovid Medline (1950–2009) and Pubmed (1950–2009) databases by searching with the following keywords: fish oil, fish supplements, fish-oil capsules, EPA, DHA, trial, maternal, pregnancy, lactation, atopy, allergy, asthma, eczema, dermatitis, hay fever, pre-school children, childhood, infancy.
Randomized controlled trials investigating the effects of fish oil supplementation during pregnancy or lactation on immune or atopic outcomes in the offspring of those pregnancies
Table 4 summarizes RCTs of maternal fish oil supplementation during pregnancy and/or lactation and immune or atopic outcomes in the offspring during infancy or childhood; five such studies were identified. One study investigating the effects of fish oil supplementation during pregnancy has generated a number of publications [50–57], of which six [50–52, 55–57] refer to atopy/allergy outcomes in the offspring or immune markers that may modulate these outcomes (Table 4). A second study, also investigating the effects of fish oil supplementation during pregnancy on the immune system of the mother and the offspring, has published two papers [58, 59] of which one [58] is included in Table 4. A third study was conducted on lactating women undergoing fish oil supplementation and investigating immune markers, but not clinical outcomes, in the children [60]. The fourth study, conducted by Olsen et al. [61] investigated the effects of fish oil intake in the last trimester of pregnancy and followed up the children to assess asthma at 16 years of age. The fifth study investigated the effects of fish oil supplementation both during pregnancy and lactation, on infant allergy risk. This trial has published two relevant papers, one reporting the effects of the supplementation on the offspring during the first year of life [62] and the other the effects of the supplementation during pregnancy on the immune system of the mother [63].
Description of the five studies
The study of Dunstan and colleagues [50–57] was conducted in Australia and was a double-blinded RCT starting at week 20 of pregnancy. The study of Krauss-Etschmann et al. [58, 59] was a European multicenter (Germany, Spain, Hungary) two-factorial double-blinded RCT starting at week 22 of gestation. The study of Lauritzen et al. [60] was conducted in Denmark and was a double-blinded parallel group RCT including women from The Danish National Birth Cohort. In this study, the women were supplemented during lactation and their children were followed up to 2.5 years of age. The study of Furuhjelm et al. [62] was conducted in Sweden and was a RCT starting at week 25 of gestation up to 3–4 months of breastfeeding. The study of Olsen et al. [61], conducted in Denmark, was a double-blinded RCT with stratification by maternal fish oil intake at baseline (low/medium/high), conducted from week 30 gestation to delivery, and children were followed up at 16 years of age. Furuhjelm et al. [62] compared fish oil with soybean oil as placebo. Dunstan and colleagues [50–57], Lauritzen et al. [60], and Olsen et al. [61] compared fish oil with olive oil as placebo. However, in the study of Lauritzen et al. [60] there was a third group of women which was a high-fish-intake reference group and these women did not receive any supplement. In the study of Olsen et al. [61], there was also a third group which was a control group and which received no oil capsules. Krauss-Etschmann et al. [58] included four groups: DHA-rich fish oil, 5-methyl-tetra-hydrofolic acid (400 μg/day), both, or placebo. All were provided in a milk-based drink. The placebo was a plain milk-based supplement of minerals and vitamins recommended for pregnancy. For the intervention groups, fish oil and/or 5- methyl-tetra-hydrofolic acid were added into the placebo supplement. The main inclusion criterion for the women who participated in the study by Dunstan and colleagues [50–57] was the presence of atopy: all women had a history of physician-diagnosed allergic rhinitis and/or asthma and a positive SPT to one or more of six common allergens. The subjects' habitual dietary intake did not exceed two fish meals per week as assessed using a semi-quantitative FFQ prior to the study. Subjects in the study of Krauss-Etschmann et al. [58, 59] were healthy pregnant women (including both atopic and non-atopic subjects). Only women who did not use fish oil, folate, and vitamin B12 supplementation after week 16 gestation were included in the study. Mothers that took part in the Danish study [60] were healthy and non-atopic. Estimation of their habitual long-chain n−3 PUFA intake was conducted using a semi-quantitative 300 item FFQ and only women with an intake below the population median (0.4 g/day) were randomized. Women with an intake in the upper quartile (> 0.8 g/day) were used as a reference group. In the study of Olsen et al. [61] the women were healthy at study entry (atopic and non-atopic), but those with fish allergy were excluded. Food intake was assessed at baseline by a simple FFQ that categorized women in low, medium, and high habitual intake of fish. In the study of Furuhjelm et al. [62], both atopic and non-atopic women were included (family history of allergy assessed by interview and doctor diagnosis and IgE positive test) and those with fish or soy allergy were excluded. Only women that planned to breastfeed their offspring were included. At baseline (25 weeks of gestation) 3-day dietary diaries were conducted. In both groups, EPA and DHA intakes were 0.2 g/day and 0.1 g/day, respectively [62]. Although the window of early life that the intervention took place differed between the five studies (pregnancy and/or lactation), the duration of intervention period prenatally was similar for three studies: between week 20 of gestation and delivery [50–57], between week 22 of gestation and delivery [58, 59], and between week 30 of gestation and delivery [61]. For one study, supplementation occurred only postnatally, during the first 4 months of lactation [60] and for the study conducted by Furuhjelm et al. [62] supplementation occurred perinatally, starting in pregnancy (week 25) and finishing in lactation (average 3–4 months of breastfeeding). The supplementation dosages of long-chain n−3 PUFAs differed between the studies. Women in the fish oil group in the study of Dunstan and colleagues [50–57] received 3.7 g/day of n−3 PUFAs with 56% as DHA and 27.7% as EPA. The control group received 4 g/day of olive oil. In the study of Krauss-Etschmann et al. [58, 59], women in the fish oil groups received 0.5 g/day DHA and 0.15 g/day EPA. Women in the study of Olsen et al. [61] received 2.7 g/day EPA plus DHA, while the placebo group (one of the two control groups) received 4 g/day olive oil. In the trial of Furuhjelm et al. [62], the women assigned to the intervention group consumed 1.6 g/d EPA and 1.1 g/d DHA while the placebo group consumed 4.5 g/day soybean oil containing mainly LA. Mothers in the study of Lauritzen et al. [60] were supplemented with 4.5 g/day of fish oil which provided 1.5 g/day of EPA plus DHA or with 4.5 g/day of olive oil. The supplementation in the studies of Dunstan and colleagues [50–57], Furuhjelm et al. [62], and Olsen et al. [61] was in the form of capsules, in the study of Krauss-Etschmann et al. [58, 59], in the form of powder stirred into a milk-based drink, whereas in the study of Lauritzen et al. [60], the fish oil was incorporated into muesli bars, home-made cookies, and capsules. In the study of Dunstan and colleagues, 85% of the women who started the study completed it. Compliance was monitored by measuring the incorporation of EPA and DHA into the cell membranes of erythrocytes [53]. In the European multicenter study [58], there is no reference to subject compliance rates. However, this trial has published results on EPA and DHA incorporation in the mother and offspring [59]. In this paper, it is mentioned that left over sachets of the supplement were asked to be returned. Also, compliance was assessed in standardized questionnaires at 30 weeks gestation, and at delivery by asking each subject how many days of dosing she had missed. The drop-out rate was 13.18% (270 of the 311 recruited pregnant women completed the study) [59]. In the study conducted by Furuhjelm et al. [62], the overall dropout from gestation week 25 till delivery was 17%, however the dropout was higher in the fish oil group (23%) than in the control group (12%). After birth, 52 infants were followed up in the fish oil group and 65 in the control group. This means that the dropout in the fish oil group was 25% and in the control group 13%, while overall, the attrition rate was 19%. Authors did not comment on compliance, apart from the fact that the research nurses contacted the mothers twice during the last part of pregnancy to remind them of the supplementation/placebo [62]. In the study of Olsen et al. [61], children were followed up and assessed in terms of asthma and other related allergic symptoms at 16 years of life. The follow up rate was extremely high, as 522 were included in analyses 16 years after the intervention. According to the authors compliance was optimized by returning and weighing the empty boxes of capsules at three times, so that the researchers estimated amounts of capsules consumed [61]. Lauritzen et al. [60] stated that the overall self-reported compliance with exclusive breastfeeding in both groups was, on average, 91% (range 67–100%, n = 64). The follow-up rates at 2.5 years of age in the randomized groups and in the high-fish-intake reference group were 72% and 58%, respectively, but in total, the follow-up rate at 2.5 years of infants' age in comparison to the baseline subjects' recruitment was 48% (101 infants out of 211 pregnant women). However, the follow-up women had significantly better compliance with exclusive breastfeeding in the intervention groups compared to the follow-up women in the reference group (89 versus 85%, p = 0.020) [60]. In the study of Dunstan and colleagues, so as to minimize potential confounding factors at randomization, the groups were stratified by parity (no previous-term-birth child versus one or more), pre-pregnancy BMI, age, and maternal allergy (allergic rhinitis or asthma). Results were adjusted for gender, parity, and method of delivery. Dunstan et al. [50] reported that background maternal dietary intake of fatty acids assessed by FFQ was not different between the two groups at study entry or at 30 weeks gestation. In the study of Krauss-Etschmann et al. [58], after randomization, the women in different groups did not differ significantly in parity, height, weight at study entry, smoking habits, or social demographic characteristics. Also, data on dietary habits were obtained at study entry [59]. However, data on their dietary intake were not presented nor controlled for. The neonates did not differ significantly in sex, birth weight, length, Apgar score, and parental history of allergy. For the purposes of analysis, only 158 mother–child pairs were available. Their characteristics did not differ from those of the main trial (n = 311). The analysis of this study was adjusted for study center (reference Hungary) and maternal DHA status at week 20 of gestation (baseline). Confounding factors controlled for were gravity, parity, delivery mode, and maternal smoking at 20 and 30 weeks gestation. Olsen et al. [61] did not control for any factors but randomization was stratified by maternal habitual fish intake (low, medium, and high). Furuhjelm et al. [62] made adjustments in their analysis for allergic symptoms in children for the following factors: LA and AA levels in maternal phospholipids at inclusion, breastfeeding fully until 6 months, number of siblings, exposure, to tobacco smoke, maternal allergic symptoms and eczema in family [62]. Lauritzen et al. [60] did not control for confounding factors in their analysis. However, they assessed subjects' compliance with exclusive breastfeeding, and they found no differences in characteristics of children (2.5 years) between the groups (sex, parity, birth weight, duration and degree of breastfeeding, age, height, weight, family history of atopy, eczema, wheezing, food allergy, plasma IgE).
Findings of the Australian fish oil supplementation study [50–57]
Maternal fish oil supplementation resulted in higher EPA and DHA status and lower AA status in cord blood erythrocytes [53] and in breast milk [52, 57]. Cord blood plasma and urinary F2-isoprostane concentrations, considered to be markers of lipid peroxidation, were lower in the fish oil group [54], suggesting that maternal fish oil supplementation during pregnancy might be protective against oxidative stress in the infants of atopic mothers soon after birth. A number of immunologic effects of maternal fish oil supplementation during pregnancy were reported. These include significantly lower cord plasma IL-13 concentrations [51], a tendency towards lower cord blood mononuclear cell cytokine responses to allergens which was significant for IL-10 in response to cat allergen [50], and lower LTB4 production and higher LTB5 production by cord blood neutrophils [56]. Cord plasma IL-13 and cord neutrophil LTB4 production were inversely related to n−3 PUFA status. Although breast milk IgA, soluble CD14 and cytokines were not different between fish oil and control groups, IgA concentration was positively correlated with breast milk DHA [52]. Denburg et al. [55] showed an altered cord blood hemopoietic progenitor phenotype in the fish oil group: there was an increased number of cord blood CD34+ progenitors and an increased number of IL-5 responsive cord blood eosinophil/basophil colony forming units. The number of CD34+ cells in cord blood significantly increased the risk of atopic eczema in infants at 1 year of age, and cord blood progenitor IL-5 responsiveness increased the risk of atopic eczema and recurrent wheeze significantly. Paradoxically, these findings suggest that fish oil supplementation during pregnancy may actually favor the development of atopic disease in the offspring. In contrast to this conclusion, infants in the fish oil group were significantly less likely (OR 0.09) to have severe atopic dermatitis at 1 year of age and were three times less likely to have a positive SPT to egg at 1 year of age (OR 0.34) [50].
Findings of the European multicenter pregnancy supplementation study [58]
Fish oil supplementation with or without 5-methyl-tetra-hydrofolic acid resulted in an increased proportion of EPA and DHA in maternal plasma at 30 weeks of gestation and at delivery and of DHA in cord blood plasma compared with placebo [59]. Fish oil supplementation also increased the percentage of DHA in placental phospholipids, although placental AA was not significantly different between the groups [64]. Fish oil supplementation during pregnancy was associated with lower mRNA expression of IL-4, IL-13, and CCR4 and decreased frequencies of natural killer cells and CCR3+ CD8+ T cells in cord blood [58]. Fish oil during pregnancy also resulted in lower mRNA levels of IL-1 and interferon (IFN)-γ in maternal blood at delivery [58]. In contrast, mRNA levels of the regulatory cytokine transforming growth factor-β were higher in both maternal blood at delivery and in cord blood following maternal fish oil supplementation [58]. Thus, it appears that fish oil supplementation during pregnancy downregulates Th1 responses in the mother and Th2 responses in the fetus. These results are in line with the observations made by Dunstan et al. [51] of lower cord blood IL-13 concentrations after maternal fish oil supplementation. In addition, Krauss-Etschmann et al. [58] showed that the decrease in cord blood IL-13 mRNA levels was more pronounced in non-allergic mothers. No clinical assessments of the infants from this study have been reported.
Findings of the Danish follow-up study [61]
Olsen et al. [61] conducted the fish oil supplementation in 1992 and related late pregnancy fish oil supplementation to prolonged gestation. After 16 years, they followed up the offspring of those mothers that participated in the study and assessed the prevalence of asthma, atopic dermatitis or allergic rhinitis in those children, obtaining the information from the Danish patient registry. The odds ratios for asthma (all types) and allergic asthma were both significantly lower (by 63% and 87% respectively) in the fish oil group in comparison to the olive oil group (control). There was a lower prevalence of asthma (all types), atopic dermatitis or allergic rhinitis (by 57%) and of allergic asthma, atopic dermatitis or allergic rhinitis (by 69%) in the fish oil group compared to control group. Stratification by maternal fish intake at baseline (low/medium/high) did not have any significant effect on the results.
Findings of the Swedish pregnancy and lactation study [62, 63]
Warstedt et al. [63] showed that plasma phospholipid EPA and DHA increased in the fish-oil-treated women during pregnancy with similar changes seen in both atopic and non-atopic women. Lipopolysaccharide-induced PGE2 secretion from whole blood cultures was decreased in the majority of women in the fish oil group [63]. However, the mean decrease in the fish oil group was not significant, in contrast to the PGE2 production in the control group which increased significantly. The non-significant decrease of PGE2 secretion in the intervention group was probably due to the observation of higher secretion at baseline from the mothers that were randomized to the fish oil group compared to those allocated in the placebo group. The change in PGE2 production differed significantly between the two groups and the decrease in PGE2 secretion in the fish oil group was more pronounced among non-atopic women (but not significant). No differences in secretion of LTB4, chemokines, and cytokines were observed with fish oil supplementation.
Furuhjelm et al. [62] associated the fish oil supplementation during pregnancy and lactation (week 25 of gestation to 3-4 months lactation) with atopic outcomes in the offspring at 6 months and 1 year of age. The prevalence of any positive SPT and of a positive SPT to egg in the infants at 1 year of age was significantly lower in the fish oil group compared to the placebo group. Eczema in the presence of detectable IgE antibodies or positive SPT towards egg, milk, or wheat and food allergy (reaction to egg or milk) during the first 12 months of life were significantly lower in the fish oil group. In a regression analysis, after controlling for confounding factors, it was found that the risk of developing any positive SPT, a positive SPT to egg, or IgE-associated eczema was three to four times less in the fish oil group compared to the placebo. The risk of developing food allergy was reduced ten times in the fish oil group in comparison to the control. These significant effects were seen in the offspring of non-allergic mothers but not of allergic mothers.
Findings of the ‘Danish National Birth Cohort’ study [60]
Erythrocyte n−3 PUFAs at 4 months were higher in infants whose mothers received fish oil during lactation compared to controls. Differences in the erythrocyte fatty acid composition between the groups were no longer evident at 2.5 years of age. The study was not powered to look at clinical outcomes, such as atopic sensitization, and no differences in atopic outcomes or in plasma IgE were observed between the groups. No significant association was found between in vitro cytokine production and plasma IgE levels and there was also no significant association between plasma IgE and eczema, wheezing, or food allergy, although both trends tended to be positive.
Summary and discussion of the main findings of these studies
Dunstan et al. [53] showed that maternal fish oil supplementation resulted in higher n−3 PUFA status (higher EPA and DHA in cord blood erythrocytes) and lower n−6 PUFA status in the neonates. Krauss-Etschmann et al. [59] demonstrated that fish oil supplementation during pregnancy results in higher levels of DHA in both maternal and cord blood. These studies reported effects of maternal fish oil supplementation during pregnancy on cord blood immune markers (blood cytokine mRNA, plasma cytokines, LTB4 production from neutrophils, cytokine production by mononuclear cells) and an altered cord blood hemopoietic progenitor phenotype. These immunologic effects might be expected to impact on allergic sensitization and on the development of atopic disease. Indeed, Dunstan et al. [50] reported beneficial effects on atopic outcomes as a result of maternal fish oil supplementation during pregnancy (less-severe atopic dermatitis, lower risk of positive SPT to egg). The Danish study of Olsen et al. [61] identified that fish oil supplementation in late pregnancy is associated with a marked reduction in atopic manifestations in the offspring at age 16 years, suggesting a long-term effect of any immunologic changes that occurred in pregnancy and early life of those children. The study conducted in Sweden [62, 63] involved fish oil supplementation during both pregnancy and lactation. Again, expected effects on n−3 PUFA status were observed and these were associated with differences in PGE2 production. The latter might be expected to influence Th2 polarization. Indeed, infants from mothers in the fish oil group had reduced risk of developing allergic sensitization to egg, IgE-associated eczema and food allergy during the first year of life. The Danish study of maternal fish oil supplementation during lactation [60] is the only one of these studies investigating immune outcomes in the offspring beyond birth. Infants of lactating mothers who received fish oil supplementation had a higher n−3 PUFA status at 4 months of age and IFN-γ production at 2.5 years of age was higher in the fish oil group, an observation which may reflect faster maturation of the immune system. This study did not assess clinical outcomes.
Thus, it is clear that fish oil supplementation during pregnancy and lactation results in higher provision of n−3 PUFAs to the offspring and so in a higher n−3 PUFA status in the offspring. Early fish oil provision is associated with immunologic changes in cord blood and such changes may persist. These studies suggest clinical effects of early fish oil provision including reduced sensitization to common food allergens and reduced prevalence and severity of atopic dermatitis in the first year of life, again with a possible persistence until adolescence with a reduction in eczema, hay fever, and asthma. The observations of these studies need to be confirmed by future trials powered adequately to examine clinical outcomes in the offspring later on in life in order to be able to draw more definite conclusions and to inform recommendations.
Randomized controlled trials investigating the effects of fish oil supplementation during infancy/childhood on allergic outcomes in those infants/children
Table 5 summarizes RCTs of fish oil supplementation during infancy/childhood on allergic outcomes in those infants/children; five studies were identified. Six papers have been published on the Childhood Asthma Prevention Study (CAPS), of which one describes the study protocol [65]. Table 5 summarizes the results of CAPS at 18 months [66, 67], 3 years [68], and 5 years of age [69, 70]. Damsgaard et al. [71] also studied the impact of fish oil supplementation in infants. Studies done by Hodge et al. [72], Nagakura et al. [73] and Vaisman et al. [74] investigated the effect of fish oil in older children.
Description of the five studies
Both the CAPS [65] and the study of Hodge et al. [72] were conducted in Australia, the study of Nagakura et al. [72] in Japan, the study of Vaisman et al. [74] in Israel, and the study of Damsgaard et al. [71] in Denmark. Infants (aged 6 months) who participated in the CAPS [65] were at high risk of developing asthma, whereas infants (aged 9 months) who took part in the Danish study [71] were not selected according to atopy risk. Children (aged 8 to 12 years) who participated in the study of Vaisman et al. [74] were healthy, but those in the studies of Hodge et al. [72] and Nagakura et al. [73] (aged 8 to 12 years and 4 to 17 years, respectively) were asthmatic. All five trials were controlled, comparing fish oil with placebo. The placebos used in the studies varied. The CAPS [65] used capsules containing sunola oil, a monounsaturated fatty acid rich oil which also contains 7% n−6 PUFAs. Damsgaard et al. [71] used milk or formula without added oils. Hodge et al. [72] used capsules containing a mixture of palm, olive and safflower oils and also replaced the usual dietary fat sources by sunflower oil and a sunflower oil based margarine. Nagakura et al. [73] used capsules containing olive oil and Vaisman et al. [74] used canola oil blended in chocolate spread. According to these strategies, the five studies provided different sources and amounts of n−6 PUFAs to the control group. The mode of provision of fish oil and the dose of long-chain n−3 PUFAs given differed among the five studies. In the CAPS tuna oil was given in capsules in an amount that was related to the age of the infant/child: the capsules were added into milk formula if infants had started bottle feeding before 6 months of age, or into formula and weaning foods after 6 months of age. At the highest possible dose infants received 3.6 mg DHA and 0.8 mg EPA per kilogram of body weight [65]. Also canola oil and canola oil-based margarine were used to lower the n−6 PUFA intake in the fish oil group, with the aim of achieving a dietary ratio of n−6 to n−3 fatty acids of 5. In the Danish study [71] liquid fish oil was used providing a mean daily intake of 571 mg EPA plus 381 mg DHA, although the range of intakes was wide, and it was advised to mix the oil into milk or formula. Hodge et al. [72] used fish oil capsules providing 1.2 g EPA + DHA/day and replaced the usual dietary fat sources with canola oil and canola oil-based margarine. Nagakura et al. [73] used fish oil capsules with the number consumed adjusted according to body weight: thus daily intakes were in the range of 17 to 26.8 mg EPA and 7.3 to 11.5 mg DHA per kg body weight; the heaviest children consumed about 1.4 g EPA plus DHA/day. Finally, Vaisman et al. [74] provided fish oil in chocolate spread delivering 300 mg EPA + DHA/day and in addition provided some canola oil. Three of the studies were double-blinded [72–74], while the CAPS [65] was single-blinded, and the Danish study [71] was not blinded. The CAPS also included a house dust mite exposure modification as a separate arm. The duration of supplementation varied between the studies: 3 months [71, 74], 6 months [72], 10 months [73], and 5 years [65]. Finally, infants and children taking part in these five studies were supplemented during different periods of life. The CAPS conducted the intervention from onset of bottle feeding (or 6 months of age) to 5 years of age [65]. The study of Damsgaard et al. [71] supplemented infants between months 9 and 12 of life. The studies of Hodge et al. [72], Nagakura et al. [73], and Vaisman et al. [74] conducted the intervention on children of a wide age range (8–12, 4–17, and 8–12 years of age, respectively). The differences in duration and dose of fish oil supplementation and in the ages of study subjects among the studies makes direct comparison difficult and may be a source of heterogeneity of findings.
In the CAPS, 68% of the children remaining in the study (excluding those lost to follow-up) were available for assessment and had their blood taken at 18 months of age. At 5 years of age, 84% of the children that participated in the randomized cohort were available for assessment. In the study of Damsgaard et al. [71], the attrition rate was 32% (30 subjects out of 94 dropped out), and the completion rate was 88% (and it did not differ significantly between the groups). In the study of Hodge et al. [72] six children out of 45 dropped out at baseline (13% withdrawal). All children completed the study of Vaisman et al. [74]. In the study of Nagakura et al. [73] one child out of 30 dropped out. In the CAPS, compliance was assessed by counting the number of capsules used. The proportion of parents who reported to have remembered to use the study spreads and oils all or most of the time of the study was 88%, and this proportion was not different between groups [69]. However, when the weight change of the used capsule containers was measured, the median adherence to oil capsules during the period after age 2.5 years was only 56% and was higher in the control versus the fish oil group (62% vs. 51%, p = 0.004). Damsgaard et al. [71] asked volunteers to return remaining bottles and report any waste: they reported that mean fish oil consumption was 3.4 ml/day (range 0.8 ml to 5 ml/day), which was according to the advice given to the subjects (One to two teaspoonful/day of the oil supplement into milk or formula). Hodge et al. [72] assessed compliance by counting the number of unused capsules by the participants and by food diary records repeated at three time points after dietary modification and supplementation. Mean number of capsules taken per day was three instead of four (for both groups), and there were no children with an average of less than two capsules per day. Moreover, subject compliance was confirmed by the observed changes in plasma EPA levels over the whole period of the trial. Vaisman et al. [74] ensured compliance of the participants by recording the empty containers of chocolate spread in both groups on a weekly basis. However, the compliance rate was not reported. In the study of Nagakura et al. [73], compliance was controlled within a hospital setting.
The CAPS assessed clinical outcomes of atopic diseases in children at three different time points (18 months, 3 years, and 5 years of age). Primary outcomes were asthma and cough at 3 years of age, and probable current asthma at 5 years of age. Wheeze was a secondary outcome measure at 5 years of age. Lung function, atopy, and asthma severity were assessed in the study of Hodge et al. [72]. Asthma severity and lung function were assessed in the study of Nagakura et al. [73]. Blood immune markers, but not clinical outcomes, were determined in the trials conducted by Vaisman et al. [74] and Damsgaard et al. [71]. Blood immune markers were also determined in the CAPS and in the study of Hodge et al. [72].
Findings of the CAPS [66–70]
Fish oil supplementation increased plasma n−3 PUFA status and decreased n−6 PUFA status at 18 months, 3 years, and 5 years of age. At 18 months of age there was decreased prevalence of wheeze in the fish oil group and higher plasma n−3 PUFA levels were associated with lower bronchodilator use, irrespective of the supplementation group [66, 67]. Follow-up at 3 years of age suggested that fish oil supplementation from infancy to childhood could reduce allergic sensitization and airway disease at this early age, as the fish oil group had reduced cough, but not wheeze [68]. However, no effect of fish oil was seen on the other end-points measured such as eczema, serum IgE concentration, or doctor diagnosis of asthma. At 5 years of age, there was no significant effect of fish oil on any of the clinical outcomes relating to lung function [69], allergy [69], or asthma [70]. Possible reasons for the lack of beneficial effects of long-chain n−3 PUFAs at 5 years of age may be related to suboptimal adherence to and/or implementation of the intervention (50% and 56% compliance in the intervention and control group, respectively), as well as to the dose of fish oil used, loss to follow-up and lack of power.
Findings of Damsgaard et al. [71]
Fish oil increased erythrocyte n−3 PUFA status. There was a borderline significant effect of fish oil on IFN-γ production by whole blood cultures, which increased, but there were no other significant effects on markers of innate immunity or inflammation, although there was a non-significant trend for reduced IL-10 production.
Findings of Hodge et al. [72]
Fish oil increased plasma phospholipid n−3 PUFA status. TNF-α production by isolated mononuclear cells decreased significantly in the fish oil group but was not different from that in the control group at the end of the intervention. There was no effect of fish oil on lung function or asthma severity.
Findings of Nagakura et al. [73]
Fish oil increased plasma EPA status and significantly reduced asthma severity score (by about 70% by month 10) and improved lung function (the provocative concentration of acetylcholine causing a 20% fall in forced expiratory volume in 1 s was increased from an average of 980 μg/ml at baseline to an average of 1,850 μg/ml at month 10).
Findings of Vaisman et al. [74]
All cytokines measured (TNF-α, IL-1β, IL-6, IL-10, IL-1ra) were increased in both unstimulated and lipopolysaccharide-stimulated mononuclear cell cultures following fish oil provision.
Summary and discussion of the main findings of these studies
Provision of long-chain n−3 PUFAs in the form of fish oil to infants or children increases the status of those fatty acids in plasma [66, 68–70, 72] and blood cells [71]. Fish oil consumption may induce effects on the immune system in infants [71] and older children [72, 74]. In children with asthma fish, oil did not affect lung function or asthma severity in the study conducted in Australia [72], but significantly improved lung function and asthma severity in the study conducted in Japan [73]. These latter two studies had similar sample sizes. The study of Nagakura et al. [73] used a lower dose of n−3 PUFAs than that of Hodge et al. [72], and so this does not explain the differences in outcome observed between these two studies. The study of Nagakura et al. [73] was of longer duration than that of Hodge et al. [72] and did not identify a significant effect of fish oil on asthma score or lung function until month 6 of the intervention; the study of Hodge et al. [72] was of 6 months duration. The studies of Damsgaard et al. [71] in healthy infants and of Vaisman et al. [74] in healthy children did not report clinical outcomes. The CAPS, conducted in infants at risk of allergic disease, is the largest of these studies and involved the longest period of supplementation. This study reported some protective effects of fish oil in these infants at 18 months and 3 years of age [66–68], but these effects did not persist until 5 years at age [69, 70]. Lack of persistence may be due to reduced compliance over time, loss to follow-up, lack of power, or the presence of confounding factors.
Thus, it is clear that fish oil supplementation during infancy or childhood results in higher n−3 PUFA status in those infants or children. Such early fish oil provision may be associated with immunologic changes in the blood but it is not clear if these are of clinical significance and whether they persist. Fish oil supplementation in infancy may decrease the risk of developing some manifestations of allergic disease, but this benefit may not persist as other factors come into play. It is not clear whether fish oil can be used to treat children with asthma as the two studies conducted to date [72, 73] give divergent results. Further studies are needed to identify immunologic and clinical effects of fish oil in infants and in children and to identify protective and therapeutic effects and their persistence.
Conclusions
There are two main families of PUFAs, the n−6 and the n−3 families. Intake of the n−6 PUFA LA increased over the second half of the twentieth century, this increase coinciding with increased prevalence of atopy and its clinical manifestations. It has been suggested that there is a causal relationship between n−6 PUFA intake and allergic disease and there are biologically plausible mechanisms, involving eicosanoid mediators of the n−6 PUFA AA that could explain this. Fish and fish oils are sources of long-chain n−3 PUFAs and these fatty acids act to oppose the actions of n−6 PUFAs. Thus, it is considered that n−3 PUFAs will protect against atopic sensitization and against the clinical manifestations of atopy. Evidence to examine this has been acquired from epidemiologic studies investigating associations between fish intake in pregnancy, lactation, infancy, and childhood and atopic outcomes in infants and children and from intervention studies with fish oil supplements in pregnancy, lactation, infancy, and childhood and atopic outcomes in infants and children All five epidemiological studies investigating the effect of maternal fish intake during pregnancy on atopic or allergic outcomes in infants/children of those pregnancies concluded protective associations. One study investigating the effects of maternal fish intake during lactation did not observe any significant associations. The evidence from epidemiological studies investigating the effects of fish intake during infancy and childhood on atopic outcomes in those infants or children is inconsistent, although the majority of the studies (nine of 14) showed a protective effect of fish intake during infancy or childhood on atopic outcomes in those infants/children. Fish oil supplementation during pregnancy and lactation or during infancy of childhood results in a higher n−3 PUFA status in the infants or children. Fish oil provision to pregnant women is associated with immunologic changes in cord blood and such changes may persist. Studies performed to date indicate that provision of fish oil during pregnancy may reduce sensitization to common food allergens and reduce prevalence and severity of atopic dermatitis in the first year of life, with a possible persistence until adolescence with a reduction in eczema, hay fever, and asthma. Fish oil provision to infants or children may be associated with immunologic changes in the blood but it is not clear whether these are of clinical significance and whether they persist. Fish oil supplementation in infancy may decrease the risk of developing some manifestations of allergic disease, but this benefit may not persist as other factors come into play. It is not clear whether fish oil can be used to treat children with asthma as the two studies conducted to date give divergent results. Further studies of increased long-chain n−3 PUFA provision in during pregnancy, lactation, and infancy are needed to more clearly identify the immunologic and clinical effects in infants and children and to identify protective and therapeutic effects and their persistence.
Abbreviations
- AA:
-
Arachidonic acid
- ALA:
-
α-Linolenic acid
- CAPS:
-
Childhood Asthma Prevention Study
- COX:
-
Cyclooxygenase
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FFQ:
-
Food Frequency Questionnaire
- IFN:
-
Interferon
- Ig:
-
Immunoglobulin
- IL:
-
Interleukin
- ISAAC:
-
International Study of Asthma and Allergies in Childhood
- LA:
-
Linoleic acid
- LOX:
-
Lipoxygenase
- LT:
-
Leukotriene
- PG:
-
Prostaglandin
- PUFA:
-
Polyunsaturated fatty acid
- RCT:
-
Randomized controlled trial
- SPT:
-
Skin prick test
- TNF:
-
Tumor necrosis factor
- TX:
-
Thromboxane
References
Calder PC, Burdge GC (2004) Fatty acids. In: Nicolaou A, Kafatos G (eds) Bioactive lipids. The Oily Press, Bridgewater, pp 1–36
Calder PC, Yaqoob P (2009) Omega-3 polyunsaturated fatty acids and human health outcomes. Biofactors 35:266–272
Black PN, Sharp S (1997) Dietary fat and asthma: is there a connection? Eur Resp J 10:6–12
Hodge L, Peat J, Salome C (1994) Increased consumption of polyunsaturated oils may be a cause of increased prevalence of childhood asthma. Aust N Z J Med 24:727
Calder PC, Miles EA (2000) Fatty acids and atopic disease. Pediat Allergy Immunol Suppl. 13:29–36
Calder PC (2006) Abnormal fatty acid profiles occur in atopic dermatitis but what do they mean? Clin Exp Allergy 36:138–141
Burdge GC, Calder PC (2006) Dietary α-linolenic acid and health-related outcomes: a metabolic perspective. Nutr Res Rev 19:26–52
British Nutrition Foundation. Report of the Task Force on Unsaturated Fatty Acids. Nutritional and Physiological Significance. Chapman & Hall, London, 1992
British Nutrition Foundation. Briefing Paper: N-3 Fatty Acids and Health. British Nutrition Foundation, London, 1999.
Calder PC (2007) Immunomodulation by omega-3 fatty acids. Prostagland Leukotr Essent Fatty Acids 77:327–335
Calder PC (2008) The relationship between the fatty acid composition of immune cells and their function. Prostagland Leukotr Essent Fatty Acids 79:101–108
Nicolaou A (2004) Prostanoids. In: Nicolaou A, Kafatos G (eds) Bioactive lipids. The Oily Press, Bridgewater, pp 197–222
Fiore S (2004) Leukotrienes and lipoxins. In: Nicolaou A, Kafatos G (eds) Bioactive lipids. The Oily Press, Bridgewater, pp 223–243
Calder PC (2006) N−3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 83:1505S–1519S
Lewis RA, Austen KF, Soberman RJ (1990) Leukotrienes and other products of the 5-lipoxygenase pathway: biochemistry and relation to pathobiology in human diseases. N Eng J Med 323:645–655
Tilley SL, Coffman TM, Koller BH (2001) Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest 108:15–23
Moore Ml, Peebles RS (2006) Update on the role of prostaglandins in allergic lung inflammation: separating friends from foes, harder than you might think. J Allergy Clin Immunol 117:1036–1039
Park GY, Christman JW (2006) Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol 290:L797–L805
Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN (2001) Lipid mediator class switching during acute inflammation: signals in resolution. Nature Immunol 2:612–619
Vachier I, Chanez P, Bonnans C, Godard P, Bousquet J, Chavis C (2002) Endogenous anti-inflammatory mediators from arachidonate in human neutrophils. Biochem Biophys Res Commun 290:219–224
Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, Chan L, Van Dyke TE (2003) Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol 171:6856–6865
Peterson LD, Jeffery NM, Thies F, Sanderson P, Newsholme EA, Calder PC (1998) Eicosapentaenoic and docosahexaenoic acids alter rat spleen leukocyte fatty acid composition and prostaglandin E2 production but have different effects on lymphocyte functions and cell-mediated immunity. Lipids 33:171–180
Rees D, Miles EA, Banerjee T, Wells SJ, Roynette CE, Wahle KWJW et al (2006) Dose-related effects of eicosapoentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am J Clin Nutr 83:331–342
Healy DA, Wallace FA, Miles EA, Calder PC, Newsholme P (2000) The effect of low to moderate amounts of dietary fish oil on neutrophil lipid composition and function. Lipids 35:763–768
Serhan CN, Arita M, Hong S, Gotlinger K (2004) Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids 39:1125–1132
Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, Okajima F, Dobashi K, Mori M (2008) Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun 367:509–515
Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD (2008) Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol 9:873–879
Sausenthaler S, Koletzko S, Schaaf B, Lehmann I, Borte M, Herbarth O, von Berg A, Wichmann HE, Heinrich J (2007) Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr 85:530–537
Romieu I, Torrent M, Garcia-Esteban R, Ferrer C, Ribas-Fito N, Anto JM, Sunyer J (2007) Maternal fish intake during pregnancy and atopy and asthma in infancy. Clin Exp Allergy 37:518–525
Willers S, Devereux G, Craig L, McNeill G, Wijga A, Bou El-Magd W, Turner S, Helms P, Seaton A (2007) Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax 62:746–748
Salam MT, Li YF, Langholz B, Gilliland FD (2005) Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma 42:513–518
Calvani M, Alessandri C, Sopo SM, Panetta V, Pingitore G, Tripodi S, Zappala D, Zicari AM (2006) Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol 17:94–102
Hoppu U, Rinne M, Lampi AM, Isolauri E (2005) Breast milk fatty acid composition is associated with development of atopic dermatitis in the infant. J Pediatr Gastroenterol Nutr 41:335–338
Martin JC, Bougnoux P, Fignon A, Theret V, Antoine JM, Lamisse F, Couet C (1993) Dependence of human milk essential fatty acids on adipose stores during lactation. Am J Clin Nutr 58:653–659
Demmelmair H, Baumheuer M, Koletzko B, Dokoupil K, Kratl G (1998) Metabolism of U13C-labelled linoleic acid in lactating women. J Lipid Res 39:1389–1396
Hodge L, Salome CM, Peat JK, Haby MM, Xuan W, Woolcock AJ (1996) Consumption of oily fish and childhood asthma risk. Med J Aust 164:137–140
Dunder T, Kuikka L, Turtinen J, Rasanen L, Uhari M (2001) Diet, serum fatty acids, and atopic diseases in childhood. Allergy 56:425–428
Nafstad P, Nystad W, Magnus P, Jaakkola JJ (2003) Asthma and allergic rhinitis at 4 years of age in relation to fish consumption in infancy. J Asthma 40:343–348
Andreasyan K, Ponsonby AL, Dwyer T, Kemp A, Dear K, Cochrane J, Carmichael A (2005) A differing pattern of association between dietary fish and allergen-specific subgroups of atopy. Allergy 60:671–677
Kim JL, Elfman L, Mi Y, Johansson M, Smedje G, Norback D (2005) Current asthma and respiratory symptoms among pupils in relation to dietary factors and allergens in the school environment. Indoor Air 15:170–182
Kull I, Bergstrom A, Lilja G, Pershagen G, Wickman M (2006) Fish consumption during the first year of life and development of allergic diseases during childhood. Allergy 61:1009–1015
Chatzi L, Torrent M, Romieu I, Garcia-Esteban R, Ferrer C, Vioque J, Kogevinas M, Sunyer J (2007) Diet, wheeze, and atopy in school children in Menorca, Spain. Pediatric Allergy Immunol 18:480–485
Antova T, Pattenden S, Nikiforov B, Leonardi GS, Boeva B, Fletcher T, Rudnai P, Slachtova H, Tabak C, Zlofkowska R, Houthvijs D, Brunekreef B, Holikova J (2003) Nutrition and respiratory health in children in six Central and Eastern European countries. Thorax 58:231–236
Huang SL, Lin KC, Pan WH (2001) Dietary factors associated with physician-diagnosed asthma and allergic rhinitis in teenagers: analyses of the first nutrition and health survey in Taiwan. Clin Exp Allergy 31:259–264
Takemura Y, Sakurai Y, Honjo S, Tokimatsu A, Gibo M, Hara T, Kusakari A, Kugai N (2002) The relationship between fish intake and the prevalence of asthma: the Tokorozawa childhood asthma and pollinosis study. Prev Med 34:221–225
Hijazi N, Abalkhail B, Seaton A (2000) Diet and childhood asthma in a society in transition: a study in urban and rural Saudi Arabia. Thorax 55:775–779
Farchi S, Forastiere F, Agabiti N, Corbo G, Pistelli R, Fortes C, Dell'Orco V, Perucci CA (2003) Dietary factors associated with wheezing and allergic rhinitis in children. Eur Respir J 22:772–780
Wijga AH, Smit HA, Kerkhof M, de Jongste JC, Gerritsen J, Neijens HJ, Boshuizen HC, Brunekreef B (2003) Association of consumption of products containing milk fat with reduced asthma risk in pre-school children: the PIAMA birth cohort study. Thorax 58:567–572
Alm B, Aberg N, Erdes L, Mollborg P, Pettersson R, Norvenius SG, Goksor E, Wennergren G (2009) Early introduction of fish decreases the risk of eczema in infants. Arch Dis Child 94:11–15
Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, Prescott SL (2003) Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol 112:1178–1184
Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, Prescott SL (2003) Maternal fish oil supplementation in pregnancy reduces interleukin-13 levels in cord blood of infants at high risk of atopy. Clin Exp Allergy 33:442–448
Dunstan JA, Roper J, Mitoulas L, Hartmann PE, Simmer K, Prescott SL (2004) The effect of supplementation with fish oil during pregnancy on breast milk immunoglobulin A, soluble CD14, cytokine levels and fatty acid composition. Clin Exp Allergy 34:1237–1242
Dunstan JA, Mori TA, Barden A, Beilin LJ, Holt PG, Calder PC, Taylor AL, Prescott SL (2004) Effects of omega-3 polyunsaturated fatty acid supplementation in pregnancy on maternal and fetal erythrocyte fatty composition. Eur J Clin Nutr 58:429–437
Barden AE, Mori TA, Dunstan JA, Taylor AL, Thornton CA, Croft KD, Beilin LJ, Prescott SL (2004) Fish oil supplementation in pregnancy lowers F2-isoprostanes in neonates at high risk of atopy. Free Radical Res 38:233–239
Denburg JA, Hatfield HM, Cyr MM, Hayes L, Holt PG, Sehmi R, Dunstan JA, Prescott SL (2005) Fish oil supplementation in pregnancy modifies neonatal progenitors at birth in infants at risk of atopy. Pediatric Res 57:276–281
Prescott SL, Barden AE, Mori TA, Dunstan JA (2006) Maternal fish oil supplementation in pregnancy modifies neonatal leukotriene production by cord-blood-derived neutrophils. Clin Sci 113:409–416
Dunstan JA, Mitoulas LR, Dixon G, Doherty DA, Hartmann PE, Simmer K, Prescott SL (2007) The effects of fish oil supplementation in pregnancy on breast milk fatty acid composition over the course of lactation: a randomized controlled trial. Pediatr Res 62:689–694
Krauss-Etschmann S, Hartl D, Rzehak P, Heinrich J, Shadid R, Ramirez-Tortosa MC, Campoy C, Pardillo S, Schendel DJ, Decsi T, Demmelmair H, Koletzko BV (2008) Decreased cord blood IL-4, IL-13, and CCR4 and increased TGF-beta levels after fish oil supplementation of pregnant women. J Allergy Clin Immunol 121:464–470
Krauss-Etschmann S, Shadid R, Campoy C, Hoster E, Demmelmair H, Jimenez M, Gil A, Rivero M, Veszpremi B, Decsi T, Koletzko BV (2007) Effects of fish-oil and folate supplementation of pregnant women on maternal and fetal plasma concentrations of docosahexaenoic acid and eicosapentaenoic acid: a European randomized multicenter trial. Am J Clin Nutr 85:1392–1400
Lauritzen L, Kjaer TMR, Fruekilde M-B, Michaelsen KF, Frokiaer H (2005) Fish oil supplementation of lactating mothers affects cytokine production in 2 1/2-year-old children. Lipids 40:669–676
Olsen SF, Osterdal ML, Salvig JD, Mortensen LM, Rytter D, Secher NJ, Henriksen TB (2008) Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr 88:167–175
Furuhjelm C, Warstedt K, Larsson J, Fredriksson M, Fageras Bottcher M, Falth-Magnusson K, Duchen K (2009) Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr 98:1461–1467
Warstedt K, Furuhjelm C, Duchen K, Falth-Magnusson K, Fageras M (2009) The effects of omega-3 fatty acid supplementation in pregnancy on maternal eicosanoid, cytokine, and chemokine secretion. Pediatr Res 66:212–217
Larque E, Krauss-Etschmann S, Campoy C, Hartl D, Linde J, Klingler M, Demmelmair H, Cano A, Gil A, Bondy B, Koletzko BV (2006) Docosahexaenoic acid supply in pregnancy affects placental expression of fatty acid transport proteins. Am J Clin Nutr 84:853–861
Mihrshahi S, Peat JK, Webb K, Tovey ER, Marks GB, Mellis CM, Leeder SR (2001) The childhood asthma prevention study (CAPS): design and research protocol of a randomized trial for the primary prevention of asthma. Controlled Clin Trials 22:333–354
Mihrshahi S, Peat JK, Marks GB, Mellis CM, Tovey ER, Webb K, Britton WJ, Leeder SR (2003) Eighteen-month outcomes of house dust mite avoidance and dietary fatty acid modification in the childhood asthma prevention study (CAPS). J Allergy Clin Immunol 111:162–168
Mihrshahi S, Peat JK, Webb K, Oddy W, Marks GB, Mellis CM (2004) Effect of omega-3 fatty acid concentrations in plasma on symptoms of asthma at 18 months of age. Pediatr Allergy Immunol 15:517–522
Peat JK, Mihrshahi S, Kemp AS, Marks GB, Tovey ER, Webb K, Mellis CM, Leeder SR (2004) Three-year outcomes of dietary fatty acid modification and house dust mite reduction in the Childhood Asthma Prevention Study. J Allergy Clin Immunol 114:807–813
Marks GB, Mihrshahi S, Kemp AS, Tovey ER, Webb K, Almqvist C, Ampon RD, Crisafulli D, Belousova EG, Mellis CM, Peat JK, Leeder SR (2006) Prevention of asthma during the first 5 years of life: a randomized controlled trial. J Allergy Clin Immunol 118:53–61
Almqvist C, Garden F, Xuan W, Mihrshahi S, Leeder SR, Oddy W, Webb K, Marks GB (2007) Omega-3 and omega-6 fatty acid exposure from early life does not affect atopy and asthma at age 5 years. J Allergy Clin Immunol 119:1438–1444
Damsgaard CT, Lauritzen L, Kjaer TMR, Holm PMI, Fruekilde M-B, Michaelsen KF, Frokiaer H (2007) Fish oil supplementation modulates immune function in healthy infants. J Nutr 137:1031–1036
Hodge L, Salome CM, Hughes JM, Liu-Brennan D, Rimmer J, Allman M, Pang D, Armour C, Woolcock AJ (1998) Effect of dietary intake of omega-3 and omega-6 fatty acids on severity of asthma in children. Eur Resp J 11:361–365
Nagakura T, Matsuda S, Shichijyo K, Sugimoto H, Hata K (2000) Dietary supplementation with fish oil rich in omega-3 polyunsaturated fatty acids in children with bronchial asthma. Eur Resp J 16:861–865
Vaisman N, Zaruk Y, Shirazi I, Kaysar N, Barak V (2005) The effect of fish oil supplementation on cytokine production in children. Eur Cytokine Network 16:194–198
Acknowledgment
L-SK, MV, PSN, NDD, and EAM are supported by funding from the European Commission under Framework 6 (FOOD-CT-2006-16249).
Author information
Authors and Affiliations
Corresponding author
Additional information
Lefkothea-Stella Kremmyda and Maria Vlachava made an equal contribution to this article
Rights and permissions
About this article
Cite this article
Kremmyda, LS., Vlachava, M., Noakes, P.S. et al. Atopy Risk in Infants and Children in Relation to Early Exposure to Fish, Oily Fish, or Long-Chain Omega-3 Fatty Acids: A Systematic Review. Clinic Rev Allerg Immunol 41, 36–66 (2011). https://doi.org/10.1007/s12016-009-8186-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-009-8186-2