Abstract
Systemic lupus erythematosus (SLE) is a disease of unknown cause that may involve one or many organ or systems. Skin involvement is a major feature in this disease, and a wide variety of skin conditions may be present. Lupus erythematosus panniculitis (LEP) constitutes a rare form of cutaneous lupus characterized by recurrent nodular or plaque lesions that can vary from a benign and mild course to a more disfiguring disease. Initial therapy includes corticosteroids, antimalarials, and azathioprine and, in refractory cases, two antimalarials in association, mycophenolate mofetil, or other immunomodulators. Intravenous immuglobulin (IVIG) is used in many autoimmune disorders, like in SLE, although clinical trials have not yet taken place. In this report, we review skin manifestations of SLE and their treatment, IVIG, and finally a case of LEP successfully treated with IVIG when other therapy modalities failed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skin manifestations of SLE
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that can affect almost any organ or system. Immunologic abnormalities, especially the production of antinuclear antibodies, are a prominent feature of this disease. Its presentation and course is highly variable, ranging from indolent to fulminant. Although the specific cause of SLE is unknown, multiple factors are associated with the development of the disease. These include genetic, racial, hormonal, and environmental factors.
Skin involvement is present in 90% of patients with SLE. A wide variety of skin conditions may be present, which can be divided into three main groups:
-
1.
Lupus erythematosus (LE)-specific skin disease: cutaneous forms of LE that occur in isolation or with SLE. These include acute cutaneous LE (ACLE or the “butterfly rash”), subacute cutaneous LE (SCLE), and chronic cutaneous LE (discoid LE (DLE), LE panniculitis, and LE tumidus).
-
2.
LE nonspecific skin disease: nonspecific cutaneous manifestations of SLE, including vasculitis, urticaria, and livedo.
-
3.
Cutaneous complications of drug therapy for LE.
As mentioned previously, skin involvement is a major feature in SLE. Three of the 11 criteria of the American College of Rheumatology (ACR) for the diagnosis of SLE involve cutaneous manifestations (malar rash, photosentivity, and discoid lesions) [1]. Also, multiple other nonspecific cutaneous manifestations are clues to a potential diagnosis of lupus. Photosensitivity refers to an abnormal cutaneous response to ultraviolet radiation (UVR) and can manifest in LE in several ways:
-
Cutaneous forms of LE (ACLE, SCLE, and DLE) may themselves be specifically induced and exacerbated by UVR.
-
Phototoxic reactions usually present as sunburn in patients with LE who are prescribed photosensitizing medications (thiazide diuretics, neuroleptics, and tetracyclines).
Photosensitive SLE patients sometimes report an exacerbation of their systemic symptoms after sun exposure. It appears that exposing skin cells such as epidermal keratinocytes to low-to-moderate doses of UV radiation causes movement of autoantigens from the nucleus to the surface of the cell, where they become accessible to binding by the antinuclear autoantibodies. This may lead to formation of circulating immune complexes that may be deposited on the skin, joints, lungs, and kidneys, causing cell death [2, 3]. There is recent clinical evidence that consistent sunscreen photoprotection in patients with SLE is associated with significantly better clinical outcomes, less frequent renal involvement, thrombocytopenia, hospitalizations, and requirement of aggressive immunesuppression (cyclophosphamide treatment) [3]. A patient history of photosensitivity has been reported in 57% to 73% of SLE patients (including ACLE) [1, 4]. Prior to photosensitivity, polymorphic light eruption (PLE or “prickly heat”) generally precede LE by several years and is particularly common in patients with LE (both cutaneous and systemic forms).
Cutaneous forms of SLE
Acute cutaneous LE
ACLE generally presents as a photosensitive symmetrical, confluent erythema and edema over the malar eminences (butterfly rash), which is transient, usually lasting days or weeks and healing without scarring. Sun exposure is not always required. It is strongly associated with active SLE and with the presence of antinuclear antibodies (ANA) and antidouble stranded DNA antibodies (dsDNA). Cutaneous photosensitivity in LE is commoner in lighter skin types [1, 5, 6].
Subacute cutaneous LE
This condition is characterized by highly photosensitive papulosquamous and/or annular polycyclic lesions on sun-exposed sites, especially the upper back, shoulders, upper arms, and the upper chest that last for weeks or months and usually heal without scarring. In SCLE, there is a paucity of systemic manifestations. Most patients with SCLE are also anti-Ro antibody positive, which is more likely in the annular subgroup (74%) than the papulosquamous group (54%) [7]. The most photosensitive major subset of cutaneous LE is SCLE, in which 70 ± 90% of patients are abnormally photosensitive by the ACR definition [8, 9].
Chronic cutaneous LE
CCLE includes discoid LE, LE panniculitis, and LE tumidus, which also tend to have few systemic manifestations.
-
DLE consists of chronic well-defined plaques with hyperkeratosis, scaling, telangiectasia, atrophy, scarring, follicular plugging, peripheral hyperpigmentation, and central hypopigmentation. Lesions tend to occur in sun-exposed areas, including the face, particularly the dorsum of the nose and malar eminences as well as the ears and scalp, as well as in sun-protected areas such as the inguinal region, perineum, mucosal surfaces, and external auditory canal. Lesions usually heal with scarring. Photosensitivity is estimated to occur in 50% of patients with DLE [10].
-
LE tumidus is a rare form of CCLE characterized by photosensitive edematous plaques. LE-specific changes at the dermo and/or epidermal junction may not be present, although histology usually reveals a superficial and deep perivascular lymphocytic infiltrate.
-
LE panniculitis is a deep inflammatory condition of the lower dermis and subcutaneous fat, characterized clinically by recurrent nodular or plaque lesions. DLE lesions may occur on the skin overlying LE panniculitis in the majority of cases, combination referred to as LE profundus [11]. The relevance of photosensitivity to LE panniculitis is unknown, although the lesions of DLE that overly the majority of cases also suggest a possible pathogenic role for UVR.
Lupus band test
The term lupus band test (LBT) is employed to describe the deposition of immunoglobulins (Igs) and complement at the dermoepidermal junction in the cutaneous lesions of both DLE and SLE and of the normal-appearing skin of SLE patients.
There is a high degree of specificity in these findings for the normal-appearing skin of SLE patients [12]. The prevalence of positive LBT is much higher in lesional than in nonlesional skin, as well as in clinically normal sun-exposed skin than in clinically normal non-sun-exposed skin [13]. LBT can be helpful in the diagnosis of LE only if performed on sun-protected nonlesional (SPNL) skin and the choice of SPNL skin reduces the wide range of variability in LBT results. In addition, different authors have pointed out that, when a biopsy specimen is obtained from SPNL skin, the immunologic composition of junctional deposits supports the diagnosis of SLE, and it may even be indicative of a decreased long-term survival and of an increased prevalence of renal disease [14].
Lupus panniculitis
Lupus erythematosus panniculitis (LEP), first described by Kaposi in 1883, is the involvement of the deep dermis and the subcutaneous fat in LE [11]. Lupus panniculitis is a chronic condition that often involves persistent lesions that subsequently heal with disfigurement. LEP may occur alone or associated with other lesions of cutaneous LE and SLE; included in the spectrum of CCLE and 70% of patients with lupus panniculitis, there will be either preceding, subsequent, or concomitant lesions of discoid lupus erythematosus [15].
Most patients are adults between 20 and 60 years old, with a female to male ratio of approximately two to one [11]. Although LEP is usually detected in young adulthood, there are various reports of childhood onset [16, 17]. The familiar tendency for systemic LE is well known; however, that for LEP is not clear.
LEP occurs in 2% to 5% of patients with SLE [11, 18], and the reported incidence of SLE among patients with lupus profundus is 3 ± 30% [19–21]. LEP may be the presenting sign of cutaneous or systemic LE or may occur as an isolated phenomenon. There can be overlap of findings between LEP and other lupus subtypes, and some reports have suggested it as a precursor to the onset of SLE [11]. Between 10% and 50% of patients with lupus panniculitis will have or eventually develop systemic lupus erythematosus. The progression to systemic LE is, however, still a reason to follow-up in patients with isolated LEP and ANA positivity seems to be an important factor in this regard [22].
Martens et al. [19] Watanabe et al. [20] and Kundig et al. [21] have concluded that those LEP patients who fulfilled criteria for SLE tend to have mild systemic involvement. When present in combination with SLE, lupus profundus has been noted to be a marker of less severe symptoms in patients with SLE [19, 20].
Initial reports stated that LEP follows a relatively benign course [11]. In contradiction to this past literature and despite the variable clinical course, LEP is considered actually not a benign, chronic disease, but rather one that can follow a more serious recurrent course and is associated with considerable morbidity secondary to painful, active cutaneous lesions, and related SLE complications [23].
Clinical manifestations
Lupus panniculitis is a variant of lupus erythematosus that primarily affects subcutaneous fat. In nearly all cases, there are deep, erythematous plaques and nodules and some ulcers, which usually involve the proximal extremities, trunk, breasts, buttocks, and face. LEP may also develop in unusual zones such as the skin of the periocular area and parotid region [24–27].
Martens et al. have reported that the most frequently involved sites by LEP include the proximal extremities, trunk, face, and scalp in a study, which included 40 patients. A recent study conducted on 12 cases has also shown that the face (50%), upper limbs (33%), and scalp (25%) are mostly involved [22].
Lesions may be tender and painful and frequently heal with atrophy and scars. LEP lesions are frequently persistent, but they can ulcerate and gradually result in scarring [28, 29]. The lipoatrophy is irreversible and a serious cosmetic problem. Occasionally, ulceration, drainage, follicular plugging, epidermal atrophy, and scarring (i.e., DLE lesions) may coexist. LEP lesions often occur contiguous with DLE lesions, a combination referred to as LE profundus.
In addition to the classical presentation of LEP as deep dermal or subcutaneous nodules, linear and morphea-like lesions and Parry Romberg syndrome (periorbital erythema with facial hemiatrophy and endopthalmos) have also been reported [23, 30–32]. Associated with irreversible lipoatrophy and extensive cosmetic disfigurement, major depression can be present.
Pathophysiology
The pathophysiological mechanism responsible for the development of LEP is not fully understood. However, panniculitis with an overlying lesion consistent with LE may be interpreted as the deep component of the inflammation resulting in the involvement of subcutaneous tissue. Deep lesional morphology and associated multiorgan diseases suggests that immune complex disease may be involved. Immune complexes can be found on small lesions of LEP, and no significant correlation of specific autoantibodies with LEP has been proven. The effectiveness of cyclosporine (CsA) might suggest T-cell mediation in the pathogenesis of LEP, considering the molecular mechanism of its action and its limited direct effects on B cells or macrophages. In addition, previous reports showed that the lymphocytes infiltrating in the panniculitis lesions are predominantly T helper cells [33, 34].
Diagnosis
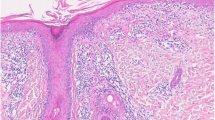
Diagnosis is confirmed primarily by both clinical and histologic findings. Histologic features include epidermal atrophy, hydrophic degeneration of the basal-cell layer of the epidermis, and perivascular/periappendageal lymphocytic inflammation that extends into the subcutaneous fat and that may be accompanied by hyalinized fat necrosis. Mucinous changes and foci of calcification can be seen [15, 20, 28].
Cutaneous lesions of LE may be seen in overlying skin or other areas of the body. Direct immunofluorescence studies frequently reveal a positive lupus band test in overlying skin of the LEP [35].
Often, LEP is associated with a variety of abnormal laboratory findings, including positive ANA, leukopenia, hypocomplementemia, circulating rheumatoid factor, false-positive syphilis serology, and an increased erythrocyte sedimentation rate. Other possible laboratory findings are lymphopenia, anemia, and reduction of C4 levels [15, 20]. These abnormalities are associated with a higher incidence of SLE than that seen in DLE [19]. Association of lymphocytic lobular panniculitis and autoimmune-associated haemophagocytic syndrome in patients with SLE has been described [36].
Treatment
Untreated LE panniculitis/profundus is indolently progressive with ulceration often supervening. Therapy for this disease is discussed below.
Antimalarials
They are indicated in SLE patients in the prevention and treatment of symptoms like erythematous rash, acute and discoid skin lesions, oral ulcers, alopecia, arthritis, athralgias, pleuritis, pericarditis, and asthenia. Increasing evidence suggests that disease-modifying drugs such as hydroxychloroquine can have a steroid-sparing effect. An additional advantage appears to be a photoprotective effect [37].
LEP often responds to treatment with antimalarials, such as hydroxychloroquine (200 mg id or bid) [38, 39]. When monotherapy is ineffective, some cases respond to a combination of antimalarials.
Regarding the use of antimalarials, chloroquine has fallen into disuse as a first line antimalarial in view of its retinal toxicity, binding to corneal tissue more avidly than hydroxychloroquine and causing macular pigmentation that progresses to the typical bull's eye lesion and then to widespread retinal pigment epithelial atrophy resembling retinitis pigmentosa. Ninety-five percent of long-term chloroquine users will develop corneal deposits compared to less than 10% of hydroxychloroquine users [39–41].
The Royal College of Ophthalmologists recommends referral to the patient's usual optician if visual impairment is observed at the baseline check. Also, patients requiring hydroxychloroquine for more than 5 or 10 years should continue annual monitoring of visual acuity and for the detection of new visual impairment, especially loss or distortion of central vision (patient's own optician or by a consultant ophthalmologist) [42].
Other secondary side effects are hematologic (blood dyscrasia) and dermatologic (alopecia, pruritus, eruptions, exacerbation of psoriases, and porfirias).
Corticosteroids
Systemic glucocorticoids like predisolone and deflazacort should be reserved for widespread and resistant lesions [15].
Intralesional corticosteroid therapy is indicated for particularly hyperkeratotic DLE lesions or lesions that are unresponsive to topical corticosteroids. This therapy should be approached with caution since even this minimal form of trauma can cause LE panniculitis lesions to break down and ulcerate and may exacerbate the atrophic healing process. Even a carefully executed diagnostic skin biopsy can at times produce chronic ulceration in these lesions.
Dapsone
Dapsone is a leprostatic agent that can also be used in dermatitis herpetiform and acnes vulgaris. The usual dosage is 100 mg/day PO. The more severe adverse effects are agranulocytosis and methehemoglobinemia. Success in some cases has been described with this agent [43].
Azathioprine
Azathioprine is a purine analog antimetabolite that inhibits DNA synthesis and, in minor proportions, the synthesis of RNA and proteins. The recommended dosage is 1–2 mg/kg/day PO and maximum dosage of 200 mg/day PO. The more frequent adverse reactions are gastrointestinal intolerance, medular depression (leukopenia), hypersensibility, hepatotoxicity, cancer, and susceptibility to infections
Thalidomide
Thalidomide is an immunosuppressant agent indicated in the treatment and prevention of recurrence of erythema nososum leprosum. The initial dosage is 100–300 mg/day PO. The more frequent side effects are skin rash and periferal neuropathy. Success has been described in isolated case reports.
Cyclosporin
Cyclosporin is an immunomodulatory drug, without citotoxicity. It can be given during pregnancy. CsA has been successfully used to treat LEP resistant to the conventional treatments [34].
The initial dosage is 2 mg/kg/day with a maintenance dosage of 5 mg/kg/day. The more frequent adverse reactions are nephrotoxicity, hypertension, neurotoxicity, and long-term adverse effects, especially skin cancer.
If LEP is resistant to conventional treatments, CsA should be considered to maintain remission.
Metotrexate
Metotrexate is an acid folic antimetabolit that stops cellular proliferation. It is mainly used in the articular manifestations resistant to antimalarials, AINEs, and corticosteroids or in situations in which other cytostatics are contraindicated. The initial dosage is 7.5–15 mg weekly (three administrations, 12 h apart). Folic acid supplement is needed. The more frequent side effects are digestive (including mucositis), hematologic, intersticial pneumonitis, hepatotoxicity, medular toxicity, teratogenic effects, vaginal, and other types of cancers. Due to their therapeutic efficacy in other lupus subtypes, these agents should be considered early in the course of recalcitrant or rapidly progressive lupus profundus.
Cyclophosphamide
Cyclophosphamide is an alkylating drug that inhibits DNA replication. It can be administrated PO (1–2 mg/kg/day) or EV (750 mg/m2 of body surface) monthly during 3 months and later every 3 months (during 1–2 years). The more frequent adverse reactions are ovarian toxicity, hemorrhagic cystitis, and bladder cancer,
Due to their therapeutic efficacy in other lupus subtypes, these agents should be considered early in the course of recalcitrant or rapidly progressive lupus profundus [23].
Mycophenolate mofetil
Mycophenolate mofetil (MMF) inhibits DNA synthesis by reducing guanin production. The main use of MMF is in renal transplantations and together with steroids in SLE patients with lupus nephritis whose disease was relapsing or was resistant to cyclophosphamide [44]. The most commonly used dose of MMF was 2 g in adverse events of vomiting and diarrhea. Other side effects are leuko and lymphonenia, susceptibility to infections, and liver enzyme increase.
Reports on MMF treatment of skin manifestations of LE are still rare. The few studies included patients with SLE-associated erythema (Gaubitz et al., a 10-patient study [45]), subacute cutaneus LE (Schanz, two patients [46]), chronic discoid lesions (Goyal, two patients [47]), and Chilblain lupus erythematosus (Boehm et al., one patient [48]). The conclusion of these reports is that SLE skin manifestations have a good response to MMF therapy, requiring the recurrence of symptoms with therapy discontinuation, with a maintenance dose of 1 g of MMF. In summary, LE-associated skin lesions respond well to MMF therapy mainly in a dose of 2 g.
Surgery
Surgical debridement or resection of individual lesions may be attempted when all other modalities have failed and there is appreciable debilitation.
LEP is often recurrent and resistant to these conventional treatments in some cases. This unusual variant of lupus erythematosus should be respected as a potential harbinger of more serious systemic disease and should be treated aggressively to prevent the associated disfigurement and consequential physical and psychological morbidity. Untreated LE panniculitis/profundus is indolently progressive with ulceration often supervening.
Intravenous immunoglobulin
Intravenous immunoglobulin (IVIG) is currently the most widely used plasma component in the world [49] and a standard therapeutic modality for a few autoimmune diseases such as immune thrombocytopenic purpura, Kawasaki disease, Guillain–Barre syndrome, and polymyositis [50]. Moreover, IVIGs are currently an empirical treatment for many other autoimmune diseases like SLE, antiphospholipid syndrome, myasthenia gravis, heparin-induced thrombocytopenia, and vasculitides.
IVIG was first licensed in 1981 to treat primary and secondary immune deficiencies. In 1981, Imbach et al. observed a raise in platelet count while administering IVIG to patients with congenital agammaglobulinemia who were also thrombocytopenic (i.e., Wiskott–Aldrich syndrome). In the same year, he reported the successful treatment of immune thrombocytopenic purpura with IVIG [51].
SLE is a systemic autoimmune disease with various clinical manifestations. Severe cases of SLE are usually managed with high-dose steroids and cytotoxic agents. These can result in an immunodeficient state with the consequent risk of severe infections. IVIG is an immunomodulatory agent capable of not only modulating SLE in animal models and in humans [52] but also providing defense against infection, rather than to be a contributing factor.
The USA Food and Drug Administration approved several conditions for the use of IVIG. These indications for IVIG are [53]:
-
Idiopathic thrombocytopenic purpura
-
Primary immunodeficiency states
-
Secondary immunodeficiency in chronic lymphocytic leukemia
-
Pediatric HIV infection
-
Kawasaki syndrome
-
Prevention of graft versus host disease and infection in adult with hematopoietic cell transplantation
In recent years, several groups have reported on the beneficial effects of IVIG in other diseases such as SLE, although clinical trials have not yet taken place, and there are only a few case series of SLE patients who received IVIG.
The dose of IVIG to be used is also of question. Onouchi et al. [54] reported on a better therapeutic effect in Kawasaki disease with a high-dose (2 g/kg) IVIG than with a low-dose (1 g/kg) IVIG. Nonetheless, it cannot be concluded that this is also true in SLE, as comparative clinical trials have not yet taken place as mentioned previously.
Composition
IVIG is a highly purified IgG fraction extracted from pooled human plasma collected from thousands of healthy donors. Intravenous therapy was initially plagued by frequent severe adverse reactions due perhaps to the tendency of the separated immunoglobulins to form aggregates and activate complement and because of the presence of prekallikrein activator, which could cause circulatory collapse. In its composition, IVIG contains, in addition to IgG, traces of IgA, IgM, F(ab0)2 fragments of IgG, soluble CD4, CD8, TGFb, and an extremely broad spectrum of antigen specificity, including bacteria, viruses, as well as idiotypes.
Products now manufactured using this or related treatments do not pose any significant risk for hepatitis C, hepatitis B, or HIV transmission.
Pharmacokinetics
In the treatment of primary and secondary immunodeficiency disorders characterized by hypogammaglobulinemia, knowledge of the pharmacokinetics of IGIV may be helpful to predict optimal administration regimens to maintain a predefined serum IgG level. In patients with autoimmune disorders, the mechanism of action of IVIG is not clear, and the therapeutic regimens should not be dictated by a target serum IgG concentration.
Bioavailability is 100% with intravenous administration. The pharmacokinetics of IgG following intravenous administration seems to fit with a one-compartment model of degradation, with fairly rapid equilibration into a small volume of distribution. The rate of metabolism of IGIV may vary with the clinical state: The half-life of the infused immunoglobulin is 3 weeks, except for IgG3, which has a half-life of 1 week.
Mechanism of action
The mechanism by which IGIV might act in autoimmune disorders is not completely understood, but the following factors may be important:
-
Downregulation of autoreactive B cells (Fc γ RII) [55]
-
Inhibition of antibody-dependent cell-mediated cytotoxicity
-
Increasing the effect of regulatory T cells (increase in IL-10 and TGF-B) [56]
-
IVIG may alter the number of T cells and T-cell subsets, including an increased number of T suppressor cells and selective reduction of T but not B or interleukin 2 receptor positive cells [57]
-
Antiidiotypic antibodies directed against antineutrophil cytoplasmic antibodies (ANCA) and HLA-specific antibodies may be responsible for the possible benefit in Wegener's granulomatosis and highly sensitized patients awaiting transplantation, respectively [58–60]
-
Solubilization and clearance of immune deposits [61]
-
Neutralizing antibodies: diarrhea-induced hemolytic–uremic syndrome [62], staphylococcal toxic shock syndrome, severe group A streptococcal infections, and Kawasaki disease
-
Accelerating the fractional rate of catabolism of IgG [63]
-
Neutralizing the inflammatory actions of the complement fragments, C3a and C5a, by a physical association between these anaphylatoxins and the constant region of F(ab)'2 [64]
Adverse reactions
IVIG therapy is usually safer than immunosuppressive agents: Adverse effects appear in 10% to 30% of patients and are usually mild and transient. The most frequent adverse effects are headache, low-grade fever, and chills [65]. More severe adverse reactions like acute renal failure and thrombotic events are less frequent.
The adverse reactions related with IVIG therapy can be divided according to their frequency in the following:
-
Frequent (disappear reducing the rate of infusion): fever, headache, liquid overload, hypo- and hypertension, rash, nauseas, vomiting, pruritus
-
Infrequent: aseptic meningitis, thoracic pain, dyspnea, renal failure, thrombotic events
-
Rare: anaphylaxy, alopecia, arthritis, hemolysis, intravascular hemolysis from Rho(D) immune globulin, descamation, hypothermia, susceptibility to infections, neutropenia, respiratory insufficiency, vasculitis
The more severe adverse reactions, acute renal failure [66, 67], and thromboembolic complications (TEC) have been reported especially in patients prone to these complications due to advanced age, atherosclerotic/thrombotic diseases, or other vascular risk factors.
Renal failure
Significant numbers of cases of acute kidney failure have been reported in close temporal association with IVIG infusions. The decline in renal function is attributed to glomerular damage from newly formed immune complexes and to tubular damage secondary to increased blood viscosity [68]. There have been reports in patients with SLE of vasculitis and flare-up or appearance of immune-mediated diseases after IVIG treatment [69, 70]. So, IVIG can cause paradoxical exacerbations of an autoimmune disease, instead of inducing a remission of it.
Thrombotic events
Three basic mechanisms have been cited as contributors to the potential generation of TEC after IVIG administration:
-
1.
Increase in plasma viscosity as a result of the high protein load, especially IgG [71–73] and from protein polymerization
-
2.
Platelet activation [74]
- 3.
The increase in plasma viscosity is the principal contributor to TEC, and in persons with risk factors for thromboembolic events, milder increments may be dangerous [77]. It may happen due to a local/regional increase in viscosity leading to local TEC (ipsilateral arm vein thrombosis [78, 79]) and due to a systemic increase in viscosity after the steady state is reached.
When IVIG therapy is instituted, slow infusion rates as stated are important to decrease the likelihood of TEC and to reduce side effects. Both dose and rate of administration should be given in accordance to 2 g/kg of body weight over 5 days (400 mg/kg per day administered at not more than 50 mg/kg per hour), and each daily dose should be administered during not less than 8 h to minimize risks [80].
IVIG administration should be carefully weighted in each patient taking into account his comorbidity, relative risks, and the potential benefits.
Effects on laboratory studies
IGIV has a variety of transient effects on laboratory and serological tests. If administrated in a 10% maltose solution, it may cause dilutional hyponatremia. IVIG may also cause pseudonatremia due to the protein load and promote a decline in the anion gap, and by increasing serum viscosity, it may reduce the erythrocyte sedimentation rate.
More important, serologic tests (e.g., antiviral titers, ANA, ANCA, and rheumatoid factor) cannot be relied upon shortly after an infusion since it is difficult (unless the patient has agammaglobulinemia) to determine if the patient or the donor is the source of the antibodies. The fact that IVIG preparations contain autoantibodies (e.g., antiphospholipid autoantibodies) meant that it could possibly be a source of passive induction/exacerbation of APS by direct transfer of autoantibodies. Recent evidence support the safety of IVIG use in APS, as IVIG preparations do not contain elevated levels of antiphospholipid autoantibodies (the same elevated levels used for routine diagnosis of APS) [81].
IVIG preparations also contain anti-DNA antibodies, and since these antibodies correlate with disease activity, their levels should be modified following an IVIG course [82].
Lupus panniculitis case reports
As mentioned previously, LEP constitutes a rare form of cutaneous lupus characterized by recurrent nodular or plaque lesions and occurs in 2% to 5% of patients with SLE, and between 10% and 50% of patients with LEP will have or eventually develop SLE.
In over 200 patients with the diagnosis of SLE that we follow up in our outpatient clinic, we have only two patients with LEP.
Case 1
The first patient is a 40-year-old Caucasian woman with past history of hypertension, dislipidemia, mitro-aortic insufficiency, chronic gastritis, and transient ischemic attack with SLE diagnosis in 1997. At the time, she presented with butterfly rash, photosensitivity, proximal interfalangic and wrist arthritis, oral ulcers, pericardial effusion, nonnefrotic proteinuria, elevated autoantibody titles (ANA, anti-dsDNA, and anti-Ro+), and complement consumption. Antiphospholipid autoantibodies were negative at the time of the diagnosis (transient ischemic attack 2 years before). The patient had no history of abortions or fetal loss. Shortly after, she also developed a small number of nodular lesions in the lower and upper limbs that were compatible with LEP. Besides these clinical and laboratory findings, the patient also presented with sicca symptoms/signs, and secondary Sjögren syndrome was diagnosed. She was medicated with deflazacort and later with prednisolone, hydroxychloroquine, and azathioprine with good response.
Since the time of the diagnosis, the nodular lesions recurred only once associated with systemic symptoms, alopecia, elevated autoantibody titles (ANA 1/160, anti-dsDNA 23 nU/mL, anti-Ro+), complement consumption, and deterioration of renal function with nephritic proteinuria in February 2008. The cutaneous lesions responded well to an increase in corticosteroid dosage. Renal biopsy disclosed lupus nephritis classes IIIc and V, after which the patient initiated cyclophosphamide according to the Euro- Lupus Nephritis Trial with marked renal function and systemic symptoms improvement. There was no recurrence of LEP.
In this case, the systemic involvement is more severe than the cutaneous with serositis and nephritic syndrome. The LEP lesions are few in number and responded well to corticosteroids, antimalarials, and azathioprine.
The chronological association between the systemic symptoms, deterioration of renal function, and LEP may be explained by an immune complex disease. This is a case o SLE with important multiorgan involvement, and the presence of LEP lesions was associated with flare-up of the disease. The LEP lesions were easily controlled and marked disfiguration was absent.
Case 2
This patient has a long history of recurrent nodular lesions with 15 years of duration, and LEP follows a more aggressive course with important disfiguration.
The patient is a 51 year-old Caucasian woman, with past history of multinodular goiter, dyslipidemia, and smoking habits (20 “pack-year”). A familiar history of SLE was also present (first degree cousin).
We first have contact with this patient in 2005. Fifteen years before, in 1990, she developed several abdominal and limb nodular lesions that histologically were compatible with lupus panniculitis, associated with positive lupus band test. Shortly after the appearance of the cutaneous lesions, raised autoantibody titers (ANA and anti-DNAds positive), complement consumption, thrombocytopenia (108 × 109/μL), and elevated ESR (69 mm) were noticed. Diagnoses of SLE and LEP was done at that time with follow-up in the Dermatology Outpatient Clinic, and the patient was medicated with hydroxychloroquine 400 mg id and posteriorly with deflazacort 6 mg id, azathioprine 50 mg id with good response and regression of cutaneous lesions.
After 3 years of antimalarial therapy, the patient presented with slight elevation of liver enzymes. Hepatic biopsy revealed unspecific histological findings. The diagnosis of hydroxychloroquine toxic hepatitis was considered and this drug was suspended. From 1990 to 2005, she had periodic exacerbations of subcutaneous nodules, and other lines of therapy were tried: thalidomide (300 mg id) with periferal neuropathy and diarrhea and dapsone (100 mg id) with new rise of liver enzymes (hepatic biopsy with histological findings compatible with toxic and lupus hepatitis). Considering the lines of therapy already tried, hydroxychloroquine was again tried in 1996 and suspended in 1998 due to important visual impairment.
In 2005, the patient was referred to our Auto-Immune Outpatient Clinic with marked systemic symptoms, arthralgias, rapid progression in number of the abdominal, and limb subcutaneous nodules with important disfigurement associated with raised autoantibodies titters (ANA 1/1,280 and anti-dsDNA 45 UI/mL), ESR 117 mm, discrete elevation of liver enzymes (lupus hepatitis?), and hypergammaglobulinemia with increased serum IgG. In an attempt to optimize therapy, deflazacort and azathioprine doses were increased to 30 and 100 mg id, respectively, without response. Patient refused to take prednisolone (standard doses of deflazacort were used), as well as a further increase in the doses of deflazacort and azathioprine.
Considering the lines of therapy already tried and failed or the patient was intolerant, IVIG was tried in February 2005, once monthly for 6 months with complete regression of the subcutaneous nodules after which IVIG pulses were administrated every 3 months until May 2006. Regarding the hypergammaglobulinemia with increased serum IgG, serum and urinary immunofixation and urinary immunoglobulins were unremarkable. With a serological profile with ASMA, anti-LKM, HBV, and HCV negative, the elevation of the liver enzymes in this patient is probably related to disease activity.
Five months after the last cycle, we observed a recurrence of symptoms together with raised autoantibody titers, with the necessity of IVIG until July 2007. Because she became asymptomatic, she discontinued IVIG at this time. In January 2008, we see a new exacerbation of cutaneous manifestations with exuberant lesions in upper limbs and dorsal region (Figs. 1, 2, 4, and 5) associated with raised autoantibody titles, with clinical regression after two therapeutic cycles.
In these 3 years, the patient was medicated with IVIG, and no severe adverse reactions to this therapy were recorded. Only two episodes of headache took place: the first responded by reducing the rate of infusion and the second to the substitution of the IVIG sample.
The patient is currently medicated with deflazacort 6 mg id and azathioprine 50 mg id plus IVIG cycles every 3 months with clinical control of the LEP lesions. Due to 18 years of evolution, the patient has marked disfigurement of the upper limbs and in a smaller degree, abdominal wall (Figs. 1, 2, 3, 4, 5, 6, and 7) but no depressive symptoms have been described. The diagnosis of LEP and SLE was made at the same time. In this case, skin is the organ more severely affected by the disease, and the flare-ups of LEP appear to be related to systemic symptoms. This case is demonstrative of IVIG clinical benefit in SLE when other treatments fail and urges for the need of clinical trials. Besides more aggressive immunosuppresion in the treatment of this chronic and disfiguring condition, IVIG should also be considered a therapeutic option.
Discussion
SLE includes a disease that may involve one or many organ or systems with skin involvement being a major feature. LEP is a rare condition inserted in the group of CCLE. Therapy options include corticosteroids, antimalarials, azathioprine, mycophenolate mofetil, thalidomide, cyclosporin, metotrexate, cyclophosphamide, and dapsone. The course of LEP can be a chronic and benign condition controlled with corticosteroids and antimalarials, as well as a more aggressive disease with important disfiguration that may not respond to conventional therapy and needs aggressive immunosuppression.
The two cases presented in this report are clearly demonstrative of these clinical differences.
In the first case, a patient with SLE and primary systemic involvement presents with small number of LEP lesions related to a flare of the disease. The LEP lesions resolved with corticosteroids and control of the disease with cyclophosphamide.
In the second case, although the disease follows a chronic course during approximately 15 years, the disease is more aggressive, is associated with important disfigurement, and has the necessity of more aggressive immunosuppression. When antimalarials, thalidomide and dapsone were contraindicated, and the therapeutic options were reduced. Despite corticosteroids and azathioprine therapy (although not in optimal doses), the disease was not controlled. Few options were available. The response of the case 2 patient to IVIG was remarkable with complete control of the disease. The indolent and chronic characteristics of LEP are patented in the clinical relapse that occurred in this patient after IVIG periodicity increased, despite corticosteroid and azathioprine maintenance therapy. In an attempt to control the disease, IVIG is actually being administrated every 3 months.
In conclusion, IVIG has obviously an important role in the control of this chronic and disfiguring disease, capable of maintaining a remission state and without the adverse reactions of more aggressive immunosuppresion.
References
Tan EM et al (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25:1271–1277
Ullrich SE (2000) Sun exposure and the lupus patient. Lupus Awareness 22:6–7
Vila LM, Mayor AM, Valentin AH et al (1999) Association of sunlight exposure and photoprotection measures with clinical outcome in systemic lupus erythematosus. Puerto Rico Health Sci J 18:89–94
Doria A et al (1996) Photosensitivity in systemic lupus erythematosus: laboratory testing of ARA/ACR definition. Lupus 5:263–268
Hochberg MC et al (1985) Systemic lupus erythematosus: a review of clinico-laboratory features and immunogenetic markers in 150 patients with emphasis on demographic subsets. Medicine 64:285–295
Mikle MF, Barton EN, Morgan OS, Deceulaer K (1996) Photosensitivity and antinuclear antibodies in black patients with systemic lupus erythematosus. J Assoc Acad Minor Phys 7:53–55
Sontheimer RD et al (1982) Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med 97:664–671
Callen JP, Klein J (1988) Subacute cutaneous lupus erythematosus. Clinical, serologic, immunogenetic, and therapeutic considerations in seventy two patients. Arthritis Rheum 31:1007–1013
Herrero C et al (1988) Subacute cutaneous lupus erythematosus: clinicopathologic findings in thirteen cases. J Am Acad Dermatol 19:1057–1062
Sontheimer RD, Provost TT (1996) Lupus erythematosus. In: Sontheimer RD, Provost TT (eds) Cutaneous manifestations of rheumatic diseases. Williams and Wilkins, Baltimore, pp 1–71
Tuffanelli DL (1971) Lupus erythematosus panniculitis (profundus). Arch Dermatol 103:231–242
Provost TT (1979) Lupus band test. In: Beutner EH, Chorzelski TP, Bean SF (eds) Immunopathology of the skin, vol 22. Wiley, New York, pp 399–410
Burnham TK, Fine G (1971) The immunofluorescent “band” test for lupus erythematosus. III. Employing clinically normal skin. Arch Derm 103:24–32
Levy Y, Sherer Y, Ahmed A, Langevitz P, George J, Fabbrizzi F, Terryberry J, Meissner M, Lorber M, Peter JB, Shoenfeld Y (1999) A study of 20 SLE patients with intravenous immunoglobulin clinical and serologic response. Lupus 8:705
Strober BE (2001) Lupus panniculitis (lupus profundus). Dermatology Online Journal 7(2):20 New York University, Department of Dermatology
Wimmershoff MB, Hohenleutner U, Landthaler M (2003) Discoid lupus erythematosus and lupus profundus in childhood: a report of two cases. Pediatr Dermatol 20:140–145
Bacanli A, Uzun S, Ciftcioglu MA, Alpsoy E (2005) A case of lupus erythematosus profundus with unusual manifestations. Lupus 14:403
Chen MT, Chen KS, Chen MJ et al (1999) Lupus profundus (panniculitis) in a chronic haemodialysis patient. Nephrol Dial Transplant 14:966–968
Martens PB, Moder KG, Ahmed I (1999) Lupus panniculitis: clinical perspectives from a case series. J Rheumatol 26:68–72
Kundig TM, Trueb RM, Krasovec M (1997) Lupus profundus=panniculitis. Dermatology 195:99–101
Watanabe T, Tsuchida T (1996) Lupus erythematosus profundus: a cutaneous marker for a distinct clinical subset? Br J Dermatol 134:123–125
Ng PP, Tan SH, Tan T (2002) Lupus erythematosus panniculitis: a clinicopathologic study. Int J Dermatol 41:488–490
Grossberg E, Scherschun L, Fiven DP (2001) Lupus profundus: not a benign disease. Lupus 10:514
Cernea SS, Kihara SM, Sotto MN et al (1993) Lupus mastitis. J Am Acad Dermatol 29:343–346
Holland NW, McKnight K, Challa VR et al (1995) Lupus panniculitis (profundus) involving the breast: report of 2 cases and review of the literature. J Rheumatol 22:344–346
White WL, Sherertz EF, Berg D et al (1993) Periparotid lupus erythematosus panniculitis: clinicopathologic correlation of two cases presenting as primary parotid disease. Arch Pathol Lab Med 117:535–539
Ogura N, Fujisaku A, Jodo S et al (1997) Lupus erythematosus profundus around the salivary glands: a case resembling submandibular gland disease. Lupus 6:477–479
Peters MS, Su WP (1989) Lupus erythematosus panniculitis. Med Clin North Am 73:1113–1126
Laman SD, Provost TT (1994) Cutaneous manifestations of lupus erythematosus. Rheum Dis Clin North Am 13:641–644
Tada J, Arata J, Katayama H (1991) Linear lupus erythematosus profundus in a child. J Am Acad Dermatol 24:871–874
Heid E (1998) A 17-year old Italian boy with a linear lupus erythematosus profundus. Eur J Dermatol 8:69
Stork J, Vosmik F (1994) Lupus erythematosus panniculitis with morphea-like lesions. Clin Exp Dermatol 19:79–82
Hytiroglou P, Phelps RG, Wattenberg DJ, Strauchen JA (1992) Cytophagic histiocytic panniculitis; molecular evidence for a clonal T cell disorder. J Am Acad Dermatol 27:333–336
Saeki Y, Ohshima S, Kurimoto I, Miura H, Suemura M (2000) Maintaining remission of lupus erythematosus profundus (LEP) with cyclosporin A. Lupus 9:390
Sanchez NP, Peters MS, Winkelmann RK (1981) The histopathology of lupus erythematosus panniculitis. J Am Acad Dermatol 5:673–680
Lonceint J, Sassolas B, Lefur JM, Guillet G, Leroy JP (2001) Panniculitis and macrophage activation syndrome in a child with lupus erythematosus. Ann Dermatol Venereol 128:1339–1342 in French
D'Cruz D (2001) Antimalarial therapy: a panacea for mild lupus? Lupus 10:148
Petri M (1996) Hydroxychloroquine use in the Baltimore Lupus Cohort: effects on lipids, glucose and thrombosis. Lupus 5(Suppl 1):S16–S22
Khamashta MA, Wallace DJ (eds) (1996) Antimalarials in rheumatology. Lupus 5(Suppl 1):S1–S73.
Easterbrook M (1990) Is corneal deposition of antimalarial any indication of retinal toxicity? Can J Ophthalmol 25:249–251
Mavrikakis M, Papazoglou S, Sifkakis PP, Vaiopoulos G, Rougas K (1996) Retinal toxicity in long term hydroxychloroquine treatment. Ann Rheum Dis 55:187–189
Bienfang D, Coblyn JS, Liang MH, Corzillius M (2000) Hydroxychloroquine retinopathy despite regular ophthalmologic evaluation: a consecutive series. J Rheumatol 27:2703–2706
Yamada Y, Dekio S, Jidoi J, Ozasa S (1989) Lupus erythematosus profundus: report of a case treated with dapsone. J Dermatol 16:379–382
Adu D, Cross J, Jayne DR (2001) Treatment of systemic lupus erythematosus with mycophenolate mofetil. Lupus 10:203–208
Gaubitz M, Schorat A, Schotte H, Kern P, Domschke W (1999) Mycophenolate mofetil for the treatment of systemic lupus erythematosus: an open pilot trial. Lupus 8:731–736
Schanz S, Ulmer A, Rassner G, Fierlbeck G (2002) Successful treatment of subacute cutaneous lupus erythematosus with mycophenolate mofetil. Br J Dermatol 147:174–178
Goyal S, Nousari HC (2001) Treatment of resistant discoid lupus erythematosus of the palms and soles with mycophenolate mofetil. J Am Acad Dermatol 45:142–144
Boehm I, Bieber T (2001) Chilblain lupus erythematosus Hutchinson: successful treatment with mycophenolate mofetil. Arch Dermatol 137:235–236
Knezevic-Maramica I, Kruskall MS (2003) Intravenous immune globulins: an update for clinicians. Transfusion 43:1460–1480
Kazatchkine MD, Morell A (eds) (1996) Intravenous immunoglobulin research and therapy. Parthenon, New York
Imbach P, Barandun S, d’Apuzzo V et al (1981) High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet 1:1228–1231
Shoenfeld Y, Krause I (1996) Immunosuppression and immunomodulation of experimental models of systemic lupus erythematosus and antiphospholipid syndrome. Transplant Proc 28:3096–3098
Darabi K, Abdel-Wahab O, Dzik WH (2006) Current usage of intravenous immune globulin and the rationale behind it: the Massachusetts General Hospital data and a review of the literature. Transfusion 46:741
Onouchi Z, Yanagisawa M, Hirayama T et al (1995) Optimal dosage and differences in therapeutic efficacy of IVIg in Kawasaki disease. Acta Paediatr Jpn 37:40–46
Samuelsson A, Towers TL, Ravetch JV (2001) Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 291:484
Fehr J, Hoffman V, Kappeler U (1982) Transient reversal of thrombocytopenia in idiopathic thrombocytopenic purpura by high-dose intravenous gamma globulin. N Engl J Med 306:1254
Tsubakio T, Kurata Y, Katagiri S et al (1983) Alteration of T cell subsets and immunoglobulin synthesis in vitro during high dose gamma globulin therapy in patients with idiopathic thrombocytopenic purpura. Clin Exp Immunol 53:697
Dietrich G, Kaveri SV, Kazatchkine MD (1992) Modulation of autoimmunity by intravenous immune globulin through interaction with the function of the immune/idiotypic network. Clin Immunol Immunopathol 62:S73
Kaveri SV, Dietrich G, Hurez V, Kazatchkine MD (1991) Intravenous immunoglobulins (IVIG) in the treatment of autoimmune diseases. Clin Exp Immunol 86:192
Jayne DR, Davies MJ, Fox CJ et al (1991) Treatment of systemic vasculitis with pooled intravenous immunoglobulin. Lancet 337:1137
Palla R, Cirami C, Panichi V et al (1991) Intravenous immunoglobulin therapy of membranous nephropathy: Efficacy and safety. Clin Nephrol 35:98
Ashkenazi S, Cleary TG, Lopez E, Pickering LK (1988) Anticytotoxin neutralizing antibodies in immune globulin preparations: potential use in hemolytic–uremic syndrome. J Pediatr 113:1008
Bleeker WK, Teeling JL, Hack CE (2001) Accelerated autoantibody clearance by intravenous immunoglobulin therapy: studies in experimental models to determine the magnitude and time course of the effect. Blood 98:3136
Basta M, Van Goor F, Luccioli S et al (2003) F(ab)(2)-mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nat Med 9:431
Sherer Y, Levy Y, Langevitz P, Rauova L, Fabrizzi F, Shoenfeld Y (2001) Adverse effects of intravenous immunoglobulin therapy in 56 patients with autoimmune diseases. Pharmacolgy 62:133–137
Rauova L, Lukac J, Levy Y, Rovensky J, Shoenfeld Y (2001) High-dose intravenous immunoglobulins for lupus nephritis—a salvage immunomodulation. Lupus 10:209–213
Orbach H, Tishler M, Shoenfeld Y (2004) Intravenous immunoglobulin and the kidney—a two-edged sword. Semin Arthritis Rheum 34:593–601
Pasatiempo AMG, Kroser JA, Rudnick M, Hoffmnann BI (1994) Acute renal failure after intravenous immunoglobulin therapy. J Rheumatol 21:347–349
Jordan SC (1989) Intravenous y-globulin therapy in systemic lupus erythematosus and immune complex disease. Ctin Immunol Immunopathol 53:164–169
Hashkes PJ, Lovell DJ (1996) Vasculitis in systemic lupus erythematosus following intravenous immunoglobulin therapy. Clin Exp Rheumatol 14:673–675
Reinhart WH, Berchtold PE (1992) Effect of high-dose intravenous immunoglobulin therapy on blood rheology. Lancet 339:662–644
Dalakas MC (1994) High-dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic events. Neurology 44:223–226
Nishikawa M, Ichiyama T, Hasegawa M, Kawasaki K, Matsubara T, Furukawa S (2003) Safety from thromboembolism using intravenous immunoglobulin therapy in Kawasaki disease: study of whole-blood viscosity. Pediatr Int 45:156–158
Woodruff RK, Grigg AP, Firkin FC, Smith IL (1986) Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet 2:217–218
Voltz R, Rosen FV, Yousry T, Beck J, Hohlfeld R (1996) Reversible encephalopathy with cerebral vasospasm in a Guillain–Barre syndrome patient treated with intravenous immunoglobulin. Neurology 46:250–251
Sztajzel R, Le Floch-Rohr J, Eggimann P (1999) High-dose intravenous immunoglobulin treatment and cerebral vasospasm: a possible mechanism of ischemic encephalopathy? Eur Neurol 41:153–158
Katz U, Shoenfeld Y (2005) Review: intravenous immunoglobulin therapy and thromboembolic complications. Lupus 14:802
Go RS, Call TG (2000) Deep venous thrombosis of the arm after intravenous immunoglobulin infusion: case report and literature review of intravenous immunoglobulin-related thrombotic complications. Mayo Clin Proc 75:83–85
Turner B, Wills AJ (2000) Cerebral infarction complicating intravenous immunoglobulin therapy in a patient with Miller Fisher syndrome. J Neurol Neurosurg Psychiatry 68:790–791
Shoenfeld Y, Krause I (2004) IVIG for autoimmune, fibrosis, and malignant conditions: our experience with 200 patients. J Clin Immunol 24:107–114
Sherer Y, Wu R, Krause I, Peter JB, Shoenfeld Y (2001) Antiphospholipid antibody levels in intravenous immunoglobulin (IVIg) preparations. Lupus 10:568. doi:10.1191/096120301701549705
Krause I, Blank M, Shoenfeld Y (1998) Anti-DNA and antiphospholipid antibodies in IVIg preparations: in vivo study in naive mice. J Clin Immunol 18:52–60
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Espírito Santo, J., Gomes, M.F., Gomes, M.J. et al. Intravenous Immunoglobulin in Lupus Panniculitis. Clinic Rev Allerg Immunol 38, 307–318 (2010). https://doi.org/10.1007/s12016-009-8162-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-009-8162-x