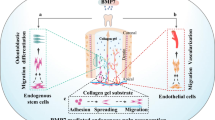
Abstract
Deep caries, trauma, and severe periodontitis result in pulpitis, pulp necrosis, and eventually pulp loss. However, no clinical therapy can regenerate lost pulp. A novel pulp regeneration strategy for clinical application is urgently needed. Signaling transduction plays an essential role in regulating the regenerative potentials of dental stem cells. Cytokines or growth factors, such as stromal cell-derived factor (SDF), fibroblast growth factor (FGF), bone morphogenetic protein (BMP), vascular endothelial growth factor (VEGF), WNT, can promote the migration, proliferation, odontogenic differentiation, pro-angiogenesis, and pro-neurogenesis potentials of dental stem cells respectively. Using the methods of signaling modulation including growth factors delivery, genetic modification, and physical stimulation has been applied in multiple preclinical studies of pulp regeneration based on cell transplantation or cell homing. Transplanting dental stem cells and growth factors encapsulated into scaffold regenerated vascularized pulp-like tissue in the root canal. Also, injecting a flowable scaffold only with chemokines recruited endogenous stem/progenitor cells for pulp regeneration. Notably, dental pulp regeneration has gradually developed into the clinical phase. These findings enlightened us on a novel strategy for structural and functional pulp regeneration through elaborate modulation of signaling transduction spatially and temporally via clinically applicable growth factors delivery. But challenges, such as the adverse effects of unphysiological signaling activation, the controlled drug release system, and the safety of gene modulation, are necessary to be tested in future works for promoting the clinical translation of pulp regeneration.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The dental pulp is an indispensable part of the tooth. It locates in the root canal space and communicates with periapical tissue through the apical foramen [1]. Histologically, it is rich in mature pulp cells or progenitors/stem cells and extracellular matrix with abundant collagenous fibers. Functionally, it is responsible for nutrients supply, dentin formation, sensory function, and defensive reaction [2]. However, during deep caries, tooth trauma, and severe periodontitis, the dental pulp usually suffers pulpitis, then traditionally removed under root canal therapy (RCT). But tooth without pulp may exhibit discoloration and increased fragility. Two decades ago, researchers isolated a clonogenic, highly proliferative population of stem cells from human dental pulp. These postnatal cells, defined as dental pulp stem cells (DPSCs), can regenerate dentin/pulp-like tissue [3]. Moreover, stem cells from human exfoliated deciduous teeth (SHED), stem cells from apical papilla (SCAPs), were also identified to share the robust self-renewal and multi-potent characteristics. Further researches on the developmental and regenerative mechanism of the dental pulp have gradually uncovered the specific signaling transductions that regulating the regenerative capabilities in dental stem cells, suggesting the possibility of dental pulp regeneration by mediating signaling pathways. Based on these findings, two burgeoning strategies for pulp regeneration have been developed. The one is the tissue engineering approach based on exogenous cell transplantation with scaffold and growth factors [4]. The other is cell homing approach based on endogenous cell migration controlled by chemokines [5]. At present, dental pulp regeneration has been a front topic in current oral medicine and striking developments have been achieved. Therefore, this review aims to pay attention to the researches about the reactivation of signaling transduction contributing to regenerative potentials in dental stem cells, then comprehensively discuss the challenges and prospects of signaling modulation in clinical translation of pulp regeneration.
Dental Stem Cells for Dental Pulp Repair and Regeneration
Several dental stem cell populations, such as DPSCs, SHED, SCAPs, have been isolated from dental pulp and apical papilla [6,7,8,9]. These dental stem cells share an important prospect for dental pulp repair and regeneration. During deep caries, DPSCs migrated toward the damaged site and differentiated into odontoblasts, which secreted reparative dentin to protect the dental pulp tissue from bacteria [10, 11]. Moreover, transplantation of DPSCs regenerated dentin-pulp like tissue with blood vessels remodeling in preclinical experiments, including ectopic regeneration in nude mice and orthotopic regeneration in dogs or minipigs, even clinical studies [12,13,14,15,16,17,18]. Compared to DPSCs, SHED presents a higher proliferation rate, but lower ALP activity level and odontogenic differentiation markers expression [19]. When in vivo transplanting hydroxyapatite/tricalcium phosphate (HA/TCP) or tooth slices with SHED in the nude mouse, dentin-pulp-like tissues were also observed [20, 21]. Likewise, SHED with puramatrix (peptide hydrogel) or recombinant human Collagen type I in the premolar roots were implanted into immunodeficient mice, which regenerated pulp-like tissues with new tubular dentin [22]. Apical papilla from immature teeth retains a population of SCAPs that presenting the regenerative characteristics of high proliferation, and odontoblastic phenotype differentiation [23, 24]. SCAPs-derived pellet transplantation or co-transplanting with 3D-printed hydroxyapatite scaffold regenerate dental pulp-like tissue with vascularity and dentine-like deposition in ectopic nude mouse models [15, 25]. Moreover, SCAPs can still be recruited into the root canal space as an endogenous cell source for pulp-dentin regeneration [26]. These stem cells colonize and proliferate in the root canal while secreting vasotrophic factors and neurotrophic factors to induce the sprouts of blood vessels and nerves. The stem cells adjacent to the dentin further differentiate into the specific odontoblasts integrating with the native dentin. Collectively, the discovery of dental stem cells makes it possible to postnatally regenerate dental pulp tissue based on their latent regenerative capabilities, including migration, proliferation, odontogenic differentiation, pro-angiogenesis, and pro-neurogenesis, which is largely controlled by the specific signaling pathways (Fig. 1).
Schematic diagram of dental stem cells-based dental pulp regeneration. Activation of the specific signaling pathways drives the migration, proliferation, odontogenic differentiation, pro-angiogenesis, and pro-neurogenesis capabilities of dental stem cells. These regenerative potency of dental stem cells ensures structural and functional pulp regeneration
Signaling Transduction Regulates the Regenerative Potency of Dental Stem Cells
Epithelial-mesenchymal interaction plays a vital role in the early stage of tooth development. Sonic hedgehog (SHH) signaling controls the tooth morphogenesis by regulating cell proliferation [27]. FGF signaling ensures the odontogenic fate of dental mesenchymal cells [28]. BMP signaling participates in tissue homeostasis by regulating odontogenic differentiation and mesenchymal stem cell (MSC) lineage commitment [29]. Moreover, when exposed to the low-grade or short-termed inflammation, the reparative capacities of DPSCs in adhesion, migration, and proliferation were triggered by the SDF-1/CXCR4 axis, NF-kB pathways, and MAPK pathway [30, 31]. In theory, dental pulp regeneration can be achieved by simulating the signaling pattern that resembled what dental epithelial cells delivered to mesenchyme during pulp formation [32]. It is necessary to understand the mechanism of reactivation of specific signaling pathways contributing to migration, proliferation, odontogenic differentiation potentials of dental stem cells, and angiogenesis, neurogenesis of dental pulp (Fig. 2).
The pivotal signaling pathways and downstream transductions contributing to the regenerative capabilities of dental stem cells. Growth factors such as the WNT, the FGF, the TGF, the VEGF, et al., activate the corresponding pathways and controlling different cellular events. Intracellular downstream PI3K/AKT, MEK/ERK, and MAPK are responsible for multiple upstream signals and perform different functions in regulating regenerative potentials in dental stem cells
Cell Migration‐related Signaling
Various chemokines share a positive function in promoting the migration of dental stem cells, suggesting the possibility of recruiting endogenous stem cells into the root canal. SDF-1 and CXC chemokine ligand 14 (CXCL14), members of the CXC cytokine family, can potently induce the migration of both DPSCs and SCAPs via activating the phosphorylation of FAK (focal adhesion kinases), PI3K (phosphoinositide 3-kinase), and AKT, while enhancing the expression of CXCR4 and the translocation of cytoplasmic CXCR4 to the cell membrane through the SDF-1(CXCL)-CXCR4 axis [26, 33,34,35,36,37]. Moreover, SDF-1α activates autophagy, which is positively correlated with the enhanced migration of DPSCs [34]. Stem cell factor (SCF)/the tyrosine kinase proto-oncogene Kit (c-Kit) system enhanced migration potential and cytoskeleton rearrangement in dental pulp progenitors through both MEK/ERK and PI3K signaling transduction [38]. Similarly, glutamine (Gln) dose-dependently promoted human dental pulp cells (HDPCs) migration accompanied by the up-regulation of chemokines through MAPKs (ERK, p38, JNK) and PI3K/AKT signaling transduction [39].
The above signaling pathways (CXCL/CXCR4-PI3K/AKT pathways, SCF/c-Kit- MEK/ERK and PI3K/AKT pathways, Gln-MAPK and PI3K/AKT pathways) are promising targets for promoting dental stem cells migration via modulating the adhesion, autophagy, cytoskeleton rearrangement, and upregulating the expression of chemokines and their receptors.
Cell Proliferation‐related Signaling
Cell proliferation is an indispensable factor for the regeneration of dental pulp tissue. Researches have shown that FGF2 enhanced the colony-forming efficiency and proliferation rate of DPSCs in vitro [40, 41]. The FGF/FGFR signaling transduction strongly stimulated the proliferative potential of HDPCs (containing a mixed population of stem and progenitor cells) via initiating cell cycle progression and mitosis and upregulating the expression of cdc2 and cyclin B1. The MEK/ERK signaling transduction was activating in FGF-FGFR mediated proliferation [42]. Also, the canonical WNT proteins enhanced the proliferation of HDPCs by promoting their self-renewing abilities. Overexpression of WNT10a in HDPCs can promote cell proliferation by increasing G2/M and S phases cells via the canonical WNT/β-catenin signaling transduction [43]. Conversely, knockdown of WNT10a inhibited proliferation [44]. Besides, silencing stathmin(soluble tubulin) in DPSCs down-regulated WNT5a and β-catenin, which caused a decreased proliferation rate and cells in S phase due to the inhibition of the WNT/β-catenin signaling. But treatment with lithium (LiCl, an activator of the WNT/β-catenin pathway) rescued these inhibitory effects [45]. Additionally, 0.4-T static magnetic fields (SMFs) surprisingly promoted DPSCs proliferation associated with cytoskeleton reorganization via activating the p38/MAPK signaling pathway [46]. SHH also enhanced HDPCs proliferation via SHH/Gli 1 signaling transduction [47].
To sum up, the FGF/FGFR-MEK/ERK, WNT/β-catenin, SMF-p38/MAPK, and SHH/Gli 1 signaling pathways can effectively improve the proliferation potential of dental stem cells via controlling cell cycle, cytoskeleton reorganization, and self-renewing ability.
Odontogenic Differentiation‐related Signaling
The odontogenic differentiation potential of dental stem cells is the key point of pulp regeneration, which ensures the regenerated pulp tissue to acquire the capability of dentin formation. DPSCs under transforming growth factor-β1 (TGF-β1) treatment exhibited the polarized odontoblasts-like appearance, the ultrastructural changes, and the expressions of specific odontogenic proteins and genes such as dentin matrix protein 1 (DMP-1) and dentin sialophosphoprotein (DSPP) [41]. BMP2, a member of the TGF-β superfamily, promoted the ALP activity, mineralized nodules formation, and odontoblastic differentiation of DPSCs via the phosphorylation and nuclear translocation of Smad 1/5 [48, 49]. BMP7 delivery increased the odontoblastic differentiation and the formation of mineralized nodules in DPSCs as well [33]. Glutamine, mineral trioxide aggregates (MTA), extracellular Ca2+, and Mg2+ also promoted odontogenic differentiation of DPSCs with increased gene expressions of DMP-1 and DSPP by enhancing BMP2 or BMP4 expressions and its downstream Smad1/5/8 phosphorylation [39, 50,51,52].
Besides, Both LiCl and MTA extracts (containing Ca2+) stimulated odontogenic differentiation with nuclear translocation of β-catenin and the expression of WNT3a, β-catenin, and Axin2 in SCAP and HDPCs respectively via the canonical WNT/β-catenin signaling transduction [53, 54]. Besides, glutamine stimulated the phosphorylation of GSK-3β, which indicated that the WNT/β-catenin pathway was involved in Gln-induced odontoblastic differentiation [39]. The high stiffness of scaffolds indirectly activated the canonical WNT/β- catenin pathway, which induced odontogenic differentiation in DPSCs [55]. An electrospun polystyrene nanofibrous (PSF) matrix promoted the expression of WNT3a and β-catenin that leading to remarkable DSPP expression in DPSCs, concomitant with increased alkaline phosphatase activity and alizarin red staining, indicating that the PSF matrix promoted odontoblastic differentiation via the canonical WNT/β-catenin signaling transduction [56]. As for, there is no evidence indicating the function of the non-canonical WNT pathway on odontogenic differentiation of dental stem cells.
FGF2 promoted odontoblastic differentiation of DPSCs with the enhancement of ALP activity, mineralization, and odontogenic markers expression via the MAPK (p38, ERK) and PI3K/AKT signaling transduction [57]. Lipopolysaccharide(LPS), epiregulin(EREG) and parathyroid hormone(PTH), or materials like demineralized dentin matrix (DDM) and ceramic bovine bone (CBB) enhanced DSPP and DMP-1 expressions of DPSCs through activating the MAPK (p38 and ERK) signaling pathways [58,59,60,61]. DPSCs-derived exosomes under odontogenic differentiation conditional medium triggered the MAPK (p38) transduction, which promoted the DSPP expression and odontogenic differentiation of DPSCs [62].
Collectively, the TGF-β, the BMP, the canonical WNT/β-catenin, the MAPK (p38 and ERK) signaling pathways play a critical role in the odontogenic differentiation of dental stem cells.
Angiogenesis Related Signaling
Vasculogenesis vitally ensures dental pulp regeneration. This process involves multiple cellular signals, which regulate endothelial cell migration, proliferation, branching, and tube formation [63]. Pericytes and endothelial cells interact to regulate new vessel formation. A study found that DPSCs can act as pericyte-like cells to secrete proangiogenic factors such as VEGF for vascularization in pulp regeneration [64]. Hinokitiol, Iloprost, MTA, TNF-α, and LPS upregulated the expression of proangiogenic factors (VEGF, hypoxia-inducible factor-1alpha (HIF-1α), Ang-1, vWF) in HDPCs by activating the MAPK (p38 and ERK) pathway [65,66,67,68]. EphrinB2/ EphB4 signaling promoted VEGF secretion in DPSCs via the MEK/ERK1/2 and MAPK (p38) signaling transduction, which induced sprouting angiogenesis of endothelial cells [69]. Under the hypoxia condition, SCAP presented an upregulated expression of VEGF via activation of the HIF-1α signaling, which accelerated the formation of vessel-like structures by human umbilical vein endothelial cells (HUVECs) [70].
Besides, dental stem cells can directly differentiate into the endothelial cells to promote angiogenesis [71]. A study indicated that VEGF induced SHED to differentiate into angiogenic endothelial cells through binding to VEGFR1 and activating downstream ERK and AKT signaling [72]. The Sema4D/PlexinB1 signaling up-regulated the VEGF secretion via the phosphorylation of AKT and ERK1/2, which is contributing to the endothelial differentiation of DPSCs [73]. Moreover, VEGF or Wnt1 treatment upregulated the expressions of endothelial cell differentiation markers (VEGFR2, VE-Cadherin, Tie-2, and CD31) respectively in DPSC and SHED via the canonical WNT/β-catenin pathway, along with the increased expressions of LRP-6 and Fzd-6 (the WNT receptors) and β-catenin [74].
Altogether, dental stem cells can not only act as pericytes secreting pro-angiogenic factors to induce endothelial cells for vascularization, but also directly differentiate into endothelial cells via the VEGF, the HIF-1α, the MAPK (p38 and ERK), the canonical WNT/β-catenin, and the PI3K/AKT signaling transduction.
Neurogenesis Related Signaling
The innervation is crucial for DPSCs proliferation and apoptosis, and tooth homeostasis [75]. Research has revealed that dental stem cells shared the neurotrophic function for neurogenesis [76]. Nerve growth factor (NGF) was upregulated in the odontoblasts adjacent to caries and injury sites. NGF signaling induced axonal migration and growth of Schwann cells during dental pulp repair [77]. Brain-derived neurotrophic factor (BDNF) signaling triggered the neurotrophic effects of dental mesenchymal stem cells (including SCAP, DPSCs) by enhancing neurotrophic factors expressions, such as BDNF, NGF, neurotrophin 3 (NT-3) [78]. Besides, FGF2 signaling transduction also induced HDPCs to release neurotrophic factors to support axonal regeneration [79].
Dental stem cells can differentiate into neural cells for nerve sprouting. Basic-FGF increased neurosphere size of DPSCs and expressions of neurogenic markers via FGF-FGFR and PLCγ intracellular transduction, suggesting neural differentiation of DPSCs [80]. NGF and Basic-FGF together stimulated the expressions of neuronal markers (Nestin, βIII-tubulin), and promoted the neural differentiation of DPSCs via the ERK and AKT signaling transduction [81]. Otherwise, the chitosan scaffold induced neural differentiation of DPSCs attributing to the activation of the WNT/β-catenin signaling pathway with upregulating the levels of BDNF, NGF, and NT-3 expressions [82].
Neurotrophic factors like BDNF, NGF secreted by dental stem cells can promote neurogenesis. Moreover, the FGF-FGFR signaling and the WNT/β-catenin signaling may contribute to both neurotrophic effects and neural differentiation of dental stem cells.
Methods in Regulating Signaling Transduction
The reactivation of signaling transduction can be modulated by multiple methods, which are summarized below (Fig. 3).
Several means in modulating cellular signaling transduction. a The drug delivery method is based on topically delivering some bioactive growth factors, small molecules, or metal ions to modulates cellular activities; b Genetic modification include inputting or deleting target gene fragments from the genome through gene editing or gene transfection to modulate signaling transduction in the host cell; c Physical stimulation such as stiffness of scaffolds, local treatment of low-frequency ultrasound and magnetic field are also possibly applied for specific signaling activation
Drug Delivery
Growth factors share the role in signaling regulation by binding to their receptors. The activators of the WNT pathway (WNT1, WNT3a), the BMP pathway (BMP2, BMP7), and the FGF pathway (FGF2), et al., can activate the related signaling transduction [42, 57, 83,84,85]. On the contrary, some inhibitory factors, rhDKK1 and noggin, negatively regulate the WNT pathway and the BMP pathway respectively [85, 86]. Besides, amounts of chemically synthesized small molecules as inhibitors blocked the transduction of the BMP signaling (LDN193189), and the TGF-β signaling (SB431542) [85, 87]. CHIR99021 (CHIR), an adenosine triphosphate (ATP)–competitive glycogen synthase kinase-3 (GSK-3) inhibitor, can stimulate the WNT/β-catenin signaling activity [88]. LiCl can also inhibit GSK-3β to indirectly activate the WNT signaling transduction [53, 89]. Moreover, metal ions such as Ca2+ and Mg2+ can activate the BMP pathway [51, 52]. Together, the specific drug delivery by pre-encapsulated into scaffolds is a clinically feasible way to modulate the signaling pathway. But an effective sustained-release system should be established in further studies.
Genetic Modification
Genetic modification refers to the use of biochemical methods to modify DNA sequences including inputting target gene fragments into host cells or deleting specific gene fragments from the genome to change the host cell genotype or strengthen the original genotype [90]. A study showed that overexpressing the platelet-derived growth factor-BB (PDGF-BB) by lentivirus significantly facilitated the migration of DPSCs in vitro and promoted the dentin-pulp regeneration in vivo via activating the PI3K/AKT signaling transduction [91]. When transfected with VEGF and SDF-1 overexpressed lentiviral particles, DPSCs acquired stronger potentials for the vascularized pulp regeneration [4]. Moreover, transfecting the Delta-1 (the Notch pathway ligands) genes by retrovirus enhanced the proliferation and odontogenic differentiation of DPSCs [92]. Compared to drug delivery, gene modification possesses the superiority of stable and persistent effects on activating the signaling. However, the usage of viral vectors has latent adverse effects such as immunoreaction. Genome editing techniques like CRISPR/Cas system may have higher security than viral transfection, but it is also necessary to consider how to terminate the persistent activation of specific signaling pathways.
Physical Stimulation
Physical stimulation influences signaling transduction. Elastic polydimethylsiloxane substrates raised the proliferation and odontogenic differentiation in DPSCs along with the stiffness increasing, which is accompanied by the activation of the WNT/β-catenin pathway [55]. Static Magnetic Fields (SMFs) arouses higher proliferative rate and intracellular calcium ions change of DPSCs via the p38 signaling [46]. Magnetic nanofiber scaffold can also improve odontogenic differentiation and angiogenesis of HDPCs by activating the WNT/MAPK/NF-kB pathways [93]. Furthermore, low-frequency ultrasound treatment promoted VEGF expression in odontoblast-like cells [94]. Therefore, the physical properties of scaffolds (stiffness, magnetic materials) and local treatment of low-frequency ultrasound are possibly applied to improve the regenerative potentials of dental stem cells.
Modulation of Regeneration‐related Signaling in Pulp Regeneration Strategies
Cell Homing Approach
The cell homing approach load chemokines into bioactive scaffolds to recruit periapical stem/progenitor cells, to regenerate pulp tissue in the root canal endogenously (Fig. 4a) [95]. Reinstating regenerative related signals in the scaffold is conducive to pulp regeneration.
Schematic diagram of growth factors in pulp regeneration strategies. a The cell homing approach is based on transplanting bioactive scaffolds loading the chemokines into the root canal to recruit endogenous stem/progenitor cells to regenerate pulp tissue; b The cell transplantation approach is based on in vitro cell culture and in vivo transplantation of exogenous stem cells with growth factors and scaffolds for dental pulp tissue regeneration
Chemotaxis of cytokines strongly recruited endogenous stem cells for dental pulp regeneration. Implanting collagen gel with Basic-FGF into the endodontically prepared root canals successfully yielded pulp-like tissue with abundant cells [33]. Stem cell factor (SCF) promoted pulp regeneration by increasing cell number and capillaries, as well as collagen sponge remodeling and collagen fiber neogenesis. PI3K/AKT and MEK/ERK signaling were involved in that process [38]. SDF-1 potently enhanced the migration of CXCR4 positive DPSCs and promoted pulp regeneration with dentin, nerves, vasculature formation [96]. Moreover, SDF-1α-loaded silk fibroin scaffolds improved the de novo formation of pulp-like tissues in pulpectomized dog teeth [34]. These findings seemly indicated that CXCL/CXCR signaling transduction is a promising target for the cell homing approach of pulp regeneration.
In a preclinical orthotopic minipig model, researchers infused recombinant human WNT3a with collagen gel into root canals, which successfully recruited endogenous stem cells to regenerate neurovascular stroma and parenchymal odontoblast-like cells embedded into newly formed dentin. Besides, BMP7 or WNT3a + BMP7 delivery both lead to excessive mineralization in the regenerated pulp-like tissue [84]. WNT and BMP pathways may play a critical role in admitting postnatal endogenous stem cells with an innate regenerative capacity to regenerate pulp tissues.
Platelet-rich plasma (PRP) contains many growth factors including PDGF-BB, TGF-β1, IGF-1, VEGF, and Basic-FGF [97]. Transplantation of the PRP into beagle dog teeth regenerated newly formed tissue (cementum-like and periodontal ligament-like), without significant difference compared to combined transplantation of DPSCs and PRP [98]. Besides, canine teeth with preexisting necrotic pulps and periapical lesions were filled with the PRP, which significantly promoted apical development and hard tissue deposition than not using the PRP [99]. A clinical case reported that after injecting the PRP into the root canal of maxillary premolar promoted root development, apical foramen closure with normal response to cold and electric pulp tests [100].
Tissue Engineering Approach
The procedure of the tissue engineering approach is transplanting exogenous stem cells and growth factors with scaffolds into the endodontically prepared root canal to regenerate dental pulp tissue (Fig. 4b) [101]. Currently, exogenous stem cells including DPSCs, SHED, SCAP, et al., have been commonly utilized in vitro and in vivo studies of pulp regeneration [102]. Besides, the scaffold should be biocompatible and biodegradable, which resembles the extracellular matrix. Growth factors exert essential biological functions in regulating cellular events and have been applied to pulp regeneration.
Ectopically transplanting DPSCs with SDF-1α into nude mice recruited host-derived cells. Two types of cells together regenerated pulp-like tissue with vascularity, well-organized fibrous matrix, and dentin deposition [34]. Exosomes (containing multiple growth factors) derived from DPSCs under odontogenic differentiation conditions triggered the p38/MAPK signaling activation. When co-transplanting the exosomes and DPSCs into tooth root slices and implanting subcutaneously in the back of nude mice regenerated dental pulp-like tissue [62]. Besides, downregulation of the RA signaling enhanced the odontogenic differentiation of DPSCs and increased bone-like tissue formation when subcutaneously transplanting with HA/TCP ceramic particles with DPSCs into nude mice [103]. Combined delivery of different growth factors potently enhanced pulp regeneration due to the modulation of different regenerative capabilities of dental stem cells. Transplanting DPSCs with a group of growth factors, TGF-β1, Basic-FGF, and VEGF into self-assembling peptide hydrogel into the back of immunocompromised mice successfully regenerated a vascularized dental pulp-like tissue with odontoblast-like cells embedded into the dentinal tubules [104].
Angiogenesis is essential for the transplanted constructs. PDGF-BB overexpression in DPSCs regenerated dentin-pulp like tissue infiltrated with blood vessels by subcutaneously transplanted with porous calcium phosphate cement (CPC) scaffold in nude mice model [91]. Transplanting DPSCs with Granulocyte Colony Stimulating Factor (G-CSF) into the root canal of the dog yielded a large amount of dentin-pulp like complex with neovascularization [14]. The root fragments seeded with two types of DPSCs (overexpressed SDF-1α and overexpressed VEGF) were co-implanted into nude mice, which produced an increased vessel area density in the regenerated pulp-like tissue [4].
Based on current evidence, the cell homing approach is seemly a more convenient strategy than the cell transplantation approach. it does not require the isolation, culture, and transplantation of stem cells. But this strategy still encounters some hardships about the shortage of endogenous stem cells in defect sites and the effective delivery of chemotactic factors. The tissue engineering approach has achieved considerable pulp regeneration in animal models. Notably, bioactive molecules play an essential role in pulp regeneration via directly or indirectly modulating signaling transduction. However, stem cells and growth factors modulating the key signaling pathways still needs a long run to validate for clinical application.
Challenges of Signaling Modulation in the Clinical Application of Pulp Regeneration
The researches of pulp regeneration are gradually transferring into the clinical phase. Some preclinical studies have demonstrated the safety and efficacy of stem cell transplantation [12, 17]. As mentioned above, pulp regeneration by modulating specific signaling transduction can regulate the regenerative capabilities of dental stem cells, which shares a promising prospect in the clinical application of pulp regeneration strategies. But several issues should be resolved before clinical application.
Controlled Release of Growth Factors
Clinical pulp regeneration will be a long-term procedure. Growth factors can be delivered only once, while gene modification is difficult for clinical use because of its security issues. Therefore, growth factors sustained-release system becomes extremely critical for the long-term biological effects on signaling modulation. Currently, growth factors encapsulated into hydrogels and microspheres, and crosslinked with scaffolds surface are available for sustained-release delivery [105,106,107]. But the sustained-release systems are not suitable for drugs with very short or very long biological half-lives, high effective dose, or low solubility. A novel scaffold with injectable property, biocompatibility, maintenance of growth factors activity, and sustained-release of growth factors is very appropriate for the clinical translation of pulp regeneration.
Single or Combined Deliveries of Growth Factors
Pulp regeneration involves multiple regenerative potentials of dental stem cells. Modulating a single signaling pathway fail to meet the requirements of structural and functional pulp regeneration. The combinations of different growth factors targeting to different signaling pathways may be the more feasible approach for pulp regeneration. However, different growth factors may cause intricate crosstalk. Some pathways promote each other and some antagonize each other. Balancing the effects of growth factors to control the complicated signaling crosstalk is a tough but meaningful job.
Safety of Modulating Signaling Transduction
Safe and effective strategies are necessary for the clinical application of pulp regeneration. Generally, drug delivery can be applied by encapsulating growth factors into scaffolds and then injected into the root canal [108]. However, it is noteworthy that these signaling molecules may regulate a wide range of cell types and cellular events, which probably results in side effects during clinical use. Genetic modification maybe not clinically suitable due to its latent safety problems of viral vectors and gene editing. Perhaps, physical stimulation may unite the drug delivery to enhance the therapeutic effect via synergistically regulating the related signaling. Further researches should target to maintain the safe, stable, and controllable effects of signaling modulation when clinically applied.
The Barriers of Complex Clinical Conditions
Endogenous stem cell migration is the key to cell homing strategy. Treatments of apical foramen and the elimination of local inflammation are necessary during the procedure of pretreating the root canals. Moreover, the senescence of dental stem cells limits their regenerative potentials [109]. It is difficult to obtain vigorous autologous stem cells from aged patients. The stem cells from the elders may also exhibit limited reactivity to signals.
Control the Abnormal Calcification in Regenerated Pulp Tissue
The level of dentinogenesis in regenerated pulp should be similar to that of the normal pulp. But odontogenic differentiation-related signaling may increase the risk of pulp calcification [110]. Extensive calcification is harmful to long-term outcomes. For young permanent teeth with an open apex, the potential of dentinogenesis should be enhanced for the continued apical development. However, in mature permanent teeth, the dentinogenesis should be maintained or moderately inhibited to prevent excessive calcification.
The Future Perspective of the Stem Cell-based Dental Pulp Regeneration
The finding of dental stem cells suggests a possibility of dental pulp regeneration through activation of autogenous stem cells or transplantation of exogenous stem cells. The good manufacturing practices (GMP)-compliant facilities and stem cells provide the safety and quality for cell-transplanted pulp regeneration strategy, which have been confirmed by series of preclinical/clinical trials. Since the functionality largely depends on the signals, understanding the mechanisms of signals (such as the WNT pathway and the BMP pathway) determining the function of dental stem cells is essential to dental pulp regeneration. In the future, transplanting autologous/allogeneic stem cells or recruiting the endogenous stem cells in periapical niches are expected to be applied in dental clinical practice as an ideal therapy instead of root canal therapy (RCT) for pulpitis, periapical inflammation, and other pulp diseases. Meanwhile, precisely activating and controlling the postnatally pivotal signaling pathways will make pulp regeneration encounter big prospects and future.
Conclusions
Signaling transduction controls a series of cellular events. Dental stem cells such as DPSCs, SCAPs, SHED, play an essential role in pulp regeneration. Multiple evidence has shown that the reactivation of specific signaling transduction is vitally critical for the regenerative potency of dental stem cells. Both cell transplantation and cell homing approaches are dependent on the regenerative capacities of dental stem cells. These findings inspire us to consider a novel strategy that synergistically or antagonistically integrating different signaling pathways especially contributing to the migration, proliferation, odontogenic differentiation, pro-angiogenesis, and pro-neurogenesis potentials of dental stem cells via orchestrating gene expression precisely. In the meantime, the methods of modulating signaling must be clinically feasible and effective. Growth factor delivery and physical stimuli approaches are more appropriate for clinical use. Collectively, further researches should focus on the comprehensive mechanism of signaling modulation during pulp regeneration. Postnatally reactivation of pivotal signaling pathways in dental stem cells will share an essential prospect in pulp regeneration strategies and especially clinical translation of strategies based on the cell homing approach.
Data Availability
Not applicable.
References
Itoh, I., et al. (1987). [Relationships among the tooth crown, the root structure and the pulp cavity in human molars]. Shikwa Gakuho, 87(3), 529–538.
Kleinert, A., et al. (2018). Endodontium - together or separately? Folia Morphologica (Warsz), 77(3), 409–415. https://doi.org/10.5603/FM.a2018.0008.
Gronthos, S., et al. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America, 97(25), 13625–13630. https://doi.org/10.1073/pnas.240309797.
Zhu, L., et al. (2019). Dental pulp stem cells overexpressing stromal-derived factor-1alpha and vascular endothelial growth factor in dental pulp regeneration. Clinical Oral Investigations, 23(5), 2497–2509. https://doi.org/10.1007/s00784-018-2699-0.
Kim, J. Y., et al. (2010). Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Engineering. Part A, 16(10), 3023–3031. https://doi.org/10.1089/ten.TEA.2010.0181.
Gronthos, S., et al. (2002). Stem cell properties of human dental pulp stem cells. Journal of Dental Research, 81(8), 531–535. https://doi.org/10.1177/154405910208100806.
Miura, M., et al. (2003). SHED: stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences of the United States of America, 100(10), 5807–5812. https://doi.org/10.1073/pnas.0937635100.
Guo, L., et al. (2013). Comparison of odontogenic differentiation of human dental follicle cells and human dental papilla cells. PLoS One, 8(4), e62332. doi:https://doi.org/10.1371/journal.pone.0062332.
Lei, M., et al. (2014). Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after in vivo transplantation. Biomaterials, 35(24), 6332–6343. doi:https://doi.org/10.1016/j.biomaterials.2014.04.071.
Nowicka, A., et al. (2013). Response of human dental pulp capped with biodentine and mineral trioxide aggregate. Journal of Endodontia, 39(6), 743–747. https://doi.org/10.1016/j.joen.2013.01.005.
Yoshida, S., et al. (2016). Semaphorin 3A induces odontoblastic phenotype in dental pulp stem cells. Journal of Dental Research, 95(11), 1282–1290. https://doi.org/10.1177/0022034516653085.
Iohara, K., et al. (2013). A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem Cells Translational Medicine, 2(7), 521–533. https://doi.org/10.5966/sctm.2012-0132.
Zhu, X., et al. (2018). A miniature swine model for stem cell-based de novo regeneration of dental pulp and dentin-like tissue. Tissue Engineering. Part C, Methods, 24(2), 108–120. https://doi.org/10.1089/ten.tec.2017.0342.
Iohara, K., et al. (2018). Allogeneic transplantation of mobilized dental pulp stem cells with the mismatched dog leukocyte antigen type is safe and efficacious for total pulp regeneration. Stem Cell Research & Therapy, 9(1), 116. https://doi.org/10.1186/s13287-018-0855-8.
Hilkens, P., et al. (2017). The angiogenic potential of DPSCs and SCAPs in an in vivo model of dental pulp regeneration. Stem Cells International, 2017, 2582080. https://doi.org/10.1155/2017/2582080.
Itoh, Y., et al. (2018). Pulp regeneration by 3-dimensional dental pulp stem cell constructs. Journal of Dental Research, 97(10), 1137–1143. https://doi.org/10.1177/0022034518772260.
Nakashima, M., et al. (2017). Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Research & Therapy, 8(1), 61. https://doi.org/10.1186/s13287-017-0506-5.
Xuan, K., et al. (2018). Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Science Translational Medicine, 10(455). https://doi.org/10.1126/scitranslmed.aaf3227.
Sabbagh, J., et al. (2020). Differences in osteogenic and odontogenic differentiation potential of DPSCs and SHED. Journal of Dentistry, 101, 103413. https://doi.org/10.1016/j.jdent.2020.103413.
Shi, S., et al. (2005). The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthodontics & Craniofacial Research, 8(3), 191–199. https://doi.org/10.1111/j.1601-6343.2005.00331.x.
Cordeiro, M. M., et al. (2008). Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. Journal of Endodontia, 34(8), 962–969. https://doi.org/10.1016/j.joen.2008.04.009.
Rosa, V., et al. (2013). Dental pulp tissue engineering in full-length human root canals. Journal of Dental Research, 92(11), 970–975. https://doi.org/10.1177/0022034513505772.
Yoo, Y. J., et al. (2016). Regenerative characteristics of apical papilla-derived cells from immature teeth with pulpal and periapical pathosis. Journal of Endodontia, 42(11), 1626–1632. https://doi.org/10.1016/j.joen.2016.08.004.
Zhang, H., et al. (2015). Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs). Biomaterials, 39, 145–154. doi:https://doi.org/10.1016/j.biomaterials.2014.11.007.
Na, S., et al. (2016). Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. Journal of Tissue Engineering and Regenerative Medicine, 10(3), 261–270. https://doi.org/10.1002/term.1686.
Liu, J. Y., et al. (2015). CXC Chemokine Receptor 4 Is Expressed Paravascularly in Apical Papilla and Coordinates with Stromal Cell-derived Factor-1alpha during Transmigration of Stem Cells from Apical Papilla. J Endod, 41(9), 1430–1436. doi:https://doi.org/10.1016/j.joen.2015.04.006.
Yu, J. C., et al. (2015). Hedgehog signaling regulates dental papilla formation and tooth size during zebrafish odontogenesis. Developmental Dynamics, 244(4), 577–590. https://doi.org/10.1002/dvdy.24258.
Liu, C., et al. (2013). FGF signaling sustains the odontogenic fate of dental mesenchyme by suppressing beta-catenin signaling. Development, 140(21), 4375–4385. doi:https://doi.org/10.1242/dev.097733.
Shi, C., et al. (2019). BMP signaling in regulating mesenchymal stem cells in incisor homeostasis. Journal of Dental Research, 98(8), 904–911. https://doi.org/10.1177/0022034519850812.
Li, D., et al. (2014). The effects of LPS on adhesion and migration of human dental pulp stem cells in vitro. Journal of Dentistry, 42(10), 1327–1334. https://doi.org/10.1016/j.jdent.2014.07.007.
Simon, S., et al. (2010). The MAP kinase pathway is involved in odontoblast stimulation via p38 phosphorylation. Journal of Endodontia, 36(2), 256–259. https://doi.org/10.1016/j.joen.2009.09.019.
Smith, A. J., et al. (2001). Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Critical Reviews in Oral Biology and Medicine, 12(5), 425–437. https://doi.org/10.1177/10454411010120050501.
Suzuki, T., et al. (2011). Induced migration of dental pulp stem cells for in vivo pulp regeneration. Journal of Dental Research, 90(8), 1013–1018. https://doi.org/10.1177/0022034511408426.
Yang, J. W., et al. (2015). Autophagy in SDF-1alpha-mediated DPSC migration and pulp regeneration. Biomaterials, 44, 11–23. doi:https://doi.org/10.1016/j.biomaterials.2014.12.006.
Xiao, M., et al. (2019). Stromal-derived Factor-1alpha signaling is involved in bone morphogenetic protein-2-induced odontogenic differentiation of stem cells from apical papilla via the Smad and Erk signaling pathways. Experimental Cell Research, 381(1), 39–49. https://doi.org/10.1016/j.yexcr.2019.04.036.
Hayashi, Y., et al. (2015). CXCL14 and MCP1 are potent trophic factors associated with cell migration and angiogenesis leading to higher regenerative potential of dental pulp side population cells. Current Stem Cell Research & Therapy, 6, 111. https://doi.org/10.1186/s13287-015-0088-z.
Li, M., et al. (2017). SDF-1/CXCR4 axis induces human dental pulp stem cell migration through FAK/PI3K/Akt and GSK3beta/beta-catenin pathways. Scientific Reports, 7, 40161. https://doi.org/10.1038/srep40161.
Pan, S., et al. (2013). SCF promotes dental pulp progenitor migration, neovascularization, and collagen remodeling - potential applications as a homing factor in dental pulp regeneration. Stem Cell Reviews and Reports, 9(5), 655–667. https://doi.org/10.1007/s12015-013-9442-7.
Kim, D. S., et al. (2014). Effects of glutamine on proliferation, migration, and differentiation of human dental pulp cells. Journal of Endodontia, 40(8), 1087–1094. https://doi.org/10.1016/j.joen.2013.11.023.
Kim, J., et al. (2014). Treatment of FGF-2 on stem cells from inflamed dental pulp tissue from human deciduous teeth. Oral Diseases, 20(2), 191–204. https://doi.org/10.1111/odi.12089.
He, H., et al. (2008). Effects of FGF2 and TGFbeta1 on the differentiation of human dental pulp stem cells in vitro. Cell Biology International, 32(7), 827–834. https://doi.org/10.1016/j.cellbi.2008.03.013.
Chang, Y. C., et al. (2017). Basic fibroblast growth factor regulates gene and protein expression related to proliferation, differentiation, and matrix production of human dental pulp cells. Journal of Endodontia, 43(6), 936–942. https://doi.org/10.1016/j.joen.2017.01.024.
Zhang, Z., et al. (2014). Effects of WNT10A on proliferation and differentiation of human dental pulp cells. Journal of Endodontia, 40(10), 1593–1599. https://doi.org/10.1016/j.joen.2014.07.009.
Liu, Y., et al. (2013). Down-regulation of Wnt10a affects odontogenesis and proliferation in mesenchymal cells. Biochemical and Biophysical Research Communications, 434(4), 717–721. https://doi.org/10.1016/j.bbrc.2013.03.088.
Zhang, X., et al. (2019). Stathmin regulates the proliferation and odontoblastic/osteogenic differentiation of human dental pulp stem cells through Wnt/beta-catenin signaling pathway. Journal of Proteomics, 202, 103364. https://doi.org/10.1016/j.jprot.2019.04.014.
Lew, W. Z., et al. (2018). Static magnetic fields enhance dental pulp stem cell proliferation by activating the p38 mitogen-activated protein kinase pathway as its putative mechanism. Journal of Tissue Engineering and Regenerative Medicine, 12(1), 19–29. https://doi.org/10.1002/term.2333.
Xia, L., et al. (2013). Enhanced dentin-like mineralized tissue formation by AdShh-transfected human dental pulp cells and porous calcium phosphate cement. PLoS One, 8(5), e62645. doi:https://doi.org/10.1371/journal.pone.0062645.
Qin, W., et al. (2012). Smad 1/5 is involved in bone morphogenetic protein-2-induced odontoblastic differentiation in human dental pulp cells. Journal of Endodontia, 38(1), 66–71. https://doi.org/10.1016/j.joen.2011.09.025.
Yuan, X., et al. (2019). Ciliary IFT80 regulates dental pulp stem cells differentiation by FGF/FGFR1 and Hh/BMP2 signaling. International Journal of Biological Sciences, 15(10), 2087–2099. https://doi.org/10.7150/ijbs.27231.
Woo, S. M., et al. (2016). Combination of mineral trioxide aggregate and platelet-rich fibrin promotes the odontoblastic differentiation and mineralization of human dental pulp cells via BMP/Smad signaling pathway. Journal of Endodontia, 42(1), 82–88. https://doi.org/10.1016/j.joen.2015.06.019.
Li, S., et al. (2015). Extracellular Ca2 + promotes odontoblastic differentiation of dental pulp stem cells via BMP2-mediated Smad1/5/8 and Erk1/2 pathways. Journal of Cellular Physiology, 230(9), 2164–2173. https://doi.org/10.1002/jcp.24945.
Kong, Y., et al. (2019). Magnesium-enriched microenvironment promotes odontogenic differentiation in human dental pulp stem cells by activating ERK/BMP2/Smads signaling. Stem Cell Research & Therapy, 10(1), 378. https://doi.org/10.1186/s13287-019-1493-5.
Wang, J., et al. (2012). Effects of Wnt/beta-catenin signalling on proliferation and differentiation of apical papilla stem cells. Cell Proliferation, 45(2), 121–131. https://doi.org/10.1111/j.1365-2184.2012.00806.x.
Chen, Y. W., et al. (2016). The ionic products from mineral trioxide aggregate-induced odontogenic differentiation of dental pulp cells via activation of the Wnt/beta-catenin signaling pathway. Journal of Endodontia, 42(7), 1062–1069. https://doi.org/10.1016/j.joen.2016.04.019.
Liu, N., et al. (2018). Stiffness regulates the proliferation and osteogenic/odontogenic differentiation of human dental pulp stem cells via the WNT signalling pathway. Cell Proliferation, 51(2), e12435. https://doi.org/10.1111/cpr.12435.
Rahman, S. U., et al. (2018). Fibrous topography-potentiated canonical Wnt signaling directs the odontoblastic differentiation of dental pulp-derived stem cells. ACS Applied Materials & Interfaces, 10(21), 17526–17541. https://doi.org/10.1021/acsami.7b19782.
Kim, Y. S., et al. (2010). Effects of fibroblast growth factor-2 on the expression and regulation of chemokines in human dental pulp cells. Journal of Endodontia, 36(11), 1824–1830. https://doi.org/10.1016/j.joen.2010.08.020.
He, W., et al. (2015). LPS promote the odontoblastic differentiation of human dental pulp stem cells via MAPK signaling pathway. Journal of Cellular Physiology, 230(3), 554–561. https://doi.org/10.1002/jcp.24732.
Cui, D., et al. (2019). Epiregulin enhances odontoblastic differentiation of dental pulp stem cells via activating MAPK signalling pathway. Cell Proliferation, 52(6), e12680. https://doi.org/10.1111/cpr.12680.
Ge, X., et al. (2020). Parathyroid hormone enhances the osteo/odontogenic differentiation of dental pulp stem cells via ERK and P38 MAPK pathways. Journal of Cellular Physiology, 235(2), 1209–1221. https://doi.org/10.1002/jcp.29034.
Zhang, H., et al. (2012). Natural mineralized scaffolds promote the dentinogenic potential of dental pulp stem cells via the mitogen-activated protein kinase signaling pathway. Tissue Engineering. Part A, 18(7–8), 677–691. https://doi.org/10.1089/ten.TEA.2011.0269.
Huang, C. C., et al. (2016). Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials, 111, 103–115. doi:https://doi.org/10.1016/j.biomaterials.2016.09.029.
Holderfield, M. T., et al. (2008). Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-β in vascular morphogenesis. Circulation Research, 102(6), 637–652. https://doi.org/10.1161/circresaha.107.167171.
Janebodin, K., et al. (2013). VEGFR2-dependent angiogenic capacity of pericyte-like dental pulp stem cells. Journal of Dental Research, 92(6), 524–531. https://doi.org/10.1177/0022034513485599.
Kim, M. K., et al. (2014). Hinokitiol increases the angiogenic potential of dental pulp cells through ERK and p38MAPK activation and hypoxia-inducible factor-1alpha (HIF-1alpha) upregulation. Archives of Oral Biology, 59(2), 102–110. https://doi.org/10.1016/j.archoralbio.2013.10.009.
Huang, S. C., et al. (2015). Role of the p38 pathway in mineral trioxide aggregate-induced cell viability and angiogenesis-related proteins of dental pulp cell in vitro. International Endodontic Journal, 48(3), 236–245. https://doi.org/10.1111/iej.12305.
Limjeerajarus, C. N., et al. (2014). Iloprost up-regulates vascular endothelial growth factor expression in human dental pulp cells in vitro and enhances pulpal blood flow in vivo. Journal of Endodontia, 40(7), 925–930. https://doi.org/10.1016/j.joen.2013.10.025.
Shin, M. R., et al. (2015). TNF-alpha and LPS activate angiogenesis via VEGF and SIRT1 signalling in human dental pulp cells. International Endodontic Journal, 48(7), 705–716. https://doi.org/10.1111/iej.12396.
Gong, T., et al. (2019). EphrinB2/EphB4 signaling regulates DPSCs to induce sprouting angiogenesis of endothelial cells. Journal of Dental Research, 98(7), 803–812. https://doi.org/10.1177/0022034519843886.
Yuan, C., et al. (2015). Coculture of stem cells from apical papilla and human umbilical vein endothelial cell under hypoxia increases the formation of three-dimensional vessel-like structures in vitro. Tissue Engineering. Part A, 21(5–6), 1163–1172. https://doi.org/10.1089/ten.TEA.2014.0058.
Bento, L. W., et al. (2013). Endothelial differentiation of SHED requires MEK1/ERK signaling. Journal of Dental Research, 92(1), 51–57. https://doi.org/10.1177/0022034512466263.
Sakai, V. T., et al. (2010). SHED differentiate into functional odontoblasts and endothelium. Journal of Dental Research, 89(8), 791–796. https://doi.org/10.1177/0022034510368647.
Zou, T., et al. (2019). Sema4D/PlexinB1 promotes endothelial differentiation of dental pulp stem cells via activation of AKT and ERK1/2 signaling. Journal of Cellular Biochemistry, 120(8), 13614–13624. https://doi.org/10.1002/jcb.28635.
Zhang, Z., et al. (2016). Wnt/beta-catenin signaling determines the vasculogenic fate of postnatal mesenchymal stem cells. Stem Cells, 34(6), 1576–1587. https://doi.org/10.1002/stem.2334.
Liu, A. Q., et al. (2020). Sensory nerve-deficient microenvironment impairs tooth homeostasis by inducing apoptosis of dental pulp stem cells. Cell Proliferation, 53(5), e12803. https://doi.org/10.1111/cpr.12803.
Pisciotta, A., et al. (2020). Neural crest derived stem cells from dental pulp and tooth-associated stem cells for peripheral nerve regeneration. Neural Regeneration Research, 15(3), 373–381. https://doi.org/10.4103/1673-5374.266043.
Mitsiadis, T. A., et al. (2017). Nerve growth factor signalling in pathology and regeneration of human teeth. Scientific Reports, 7(1), 1327. https://doi.org/10.1038/s41598-017-01455-3.
Kolar, M. K., et al. (2017). The neurotrophic effects of different human dental mesenchymal stem cells. Scientific Reports, 7(1), 12605. https://doi.org/10.1038/s41598-017-12969-1.
Nagashima, K., et al. (2017). Priming with FGF2 stimulates human dental pulp cells to promote axonal regeneration and locomotor function recovery after spinal cord injury. Scientific Reports, 7(1), 13500. https://doi.org/10.1038/s41598-017-13373-5.
Osathanon, T., et al. (2011). Basic fibroblast growth factor inhibits mineralization but induces neuronal differentiation by human dental pulp stem cells through a FGFR and PLCgamma signaling pathway. Journal of Cellular Biochemistry, 112(7), 1807–1816. https://doi.org/10.1002/jcb.23097.
Zhang, J., et al. (2017). Effects of nerve growth factor and basic fibroblast growth factor promote human dental pulp stem cells to neural differentiation. Neurochemical Research, 42(4), 1015–1025. https://doi.org/10.1007/s11064-016-2134-3.
Zhang, J., et al. (2016). Chitosan scaffolds induce human dental pulp stem cells to neural differentiation: potential roles for spinal cord injury therapy. Cell and Tissue Research, 366(1), 129–142. https://doi.org/10.1007/s00441-016-2402-1.
Silva, G. O., et al. (2017). Lipoprotein receptor-related protein 6 signaling is necessary for vasculogenic differentiation of human dental pulp stem cells. Journal of Endodontia, 43(9S), S25–S30. https://doi.org/10.1016/j.joen.2017.06.006.
He, L., et al. (2019). Parenchymal and stromal tissue regeneration of tooth organ by pivotal signals reinstated in decellularized matrix. Nature Materials, 18(6), 627–637. https://doi.org/10.1038/s41563-019-0368-6.
Yang, J., et al. (2015). Bone morphogenetic protein 2-induced human dental pulp cell differentiation involves p38 mitogen-activated protein kinase-activated canonical WNT pathway. International Journal of Oral Science, 7(2), 95–102. https://doi.org/10.1038/ijos.2015.7.
Sagomonyants, K., et al. (2015). Enhanced dentinogenesis of pulp progenitors by early exposure to FGF2. Journal of Dental Research, 94(11), 1582–1590. https://doi.org/10.1177/0022034515599768.
Xu, J. G., et al. (2018). Inhibition of TGF-beta Signaling in SHED enhances endothelial differentiation. Journal of Dental Research, 97(2), 218–225. https://doi.org/10.1177/0022034517733741.
Zaugg, L. K., et al. (2020). Translation approach for dentine regeneration using GSK-3 antagonists. Journal of Dental Research, 22034520908593. https://doi.org/10.1177/0022034520908593.
Ali, M., et al. (2019). Lithium-containing surface pre-reacted glass fillers enhance hDPSC functions and induce reparative dentin formation in a rat pulp capping model through activation of Wnt/β-catenin signaling. Acta Biomaterialia, 96, 594–604. doi:https://doi.org/10.1016/j.actbio.2019.06.016.
Cockrell, A. S., et al. (2007). Gene delivery by lentivirus vectors. Molecular Biotechnology, 36(3), 184–204. https://doi.org/10.1007/s12033-007-0010-8.
Zhang, M., et al. (2017). The effects of platelet-derived growth factor-BB on human dental pulp stem cells mediated dentin-pulp complex regeneration. Stem Cells Translational Medicine, 6(12), 2126–2134. https://doi.org/10.1002/sctm.17-0033.
He, F., et al. (2009). Effects of Notch ligand Delta1 on the proliferation and differentiation of human dental pulp stem cells in vitro. Archives of Oral Biology, 54(3), 216–222. https://doi.org/10.1016/j.archoralbio.2008.10.003.
Yun, H. M., et al. (2016). Magnetic nanofiber scaffold-induced stimulation of odontogenesis and pro-angiogenesis of human dental pulp cells through Wnt/MAPK/NF-kappaB pathways. Dental Materials, 32(11), 1301–1311. https://doi.org/10.1016/j.dental.2016.06.016.
Scheven, B. A., et al. (2009). VEGF and odontoblast-like cells: stimulation by low frequency ultrasound. Archives of Oral Biology, 54(2), 185–191. https://doi.org/10.1016/j.archoralbio.2008.09.008.
Eramo, S., et al. (2018). Dental pulp regeneration via cell homing. International Endodontic Journal, 51(4), 405–419. https://doi.org/10.1111/iej.12868.
Nakashima, M., et al. (2011). Regeneration of dental pulp by stem cells. Advances in Dental Research, 23(3), 313–319. https://doi.org/10.1177/0022034511405323.
Qiao, J., et al. (2017). Quantification of growth factors in different platelet concentrates. Platelets, 28(8), 774–778. doi:https://doi.org/10.1080/09537104.2016.1267338.
Zhu, X., et al. (2012). Transplantation of dental pulp stem cells and platelet-rich plasma for pulp regeneration. Journal of Endodontia, 38(12), 1604–1609. https://doi.org/10.1016/j.joen.2012.09.001.
Torabinejad, M., et al. (2015). Histologic examination of teeth with necrotic pulps and periapical lesions treated with 2 scaffolds: an animal investigation. Journal of Endodontia, 41(6), 846–852. https://doi.org/10.1016/j.joen.2015.01.026.
Torabinejad, M., et al. (2011). Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: a case report. Journal of Endodontia, 37(2), 265–268. https://doi.org/10.1016/j.joen.2010.11.004.
Dave, J. R., et al. (2018). Dental tissue-derived mesenchymal stem cells: applications in tissue engineering. Critical Reviews in Biomedical Engineering, 46(5), 429–468. https://doi.org/10.1615/CritRevBiomedEng.2018027342.
Shi, X., et al. (2020). Concise review: Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Translational Medicine. https://doi.org/10.1002/sctm.19-0398.
Wang, J., et al. (2020). Retinoic acid signal negatively regulates osteo/odontogenic differentiation of dental pulp stem cells. Stem Cells International, 2020, 1–12. https://doi.org/10.1155/2020/5891783.
Galler, K. M., et al. (2012). A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Engineering. Part A, 18(1–2), 176–184. https://doi.org/10.1089/ten.TEA.2011.0222.
Abasalizadeh, F., et al. (2020). Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. Journal of Biological Engineering, 14, 8. https://doi.org/10.1186/s13036-020-0227-7.
Zhang, B., et al. (2018). Surface-decorated hydroxyapatite scaffold with on-demand delivery of dexamethasone and stromal cell derived factor-1 for enhanced osteogenesis. Materials Science & Engineering. C, Materials for Biological Applications, 89, 355–370. https://doi.org/10.1016/j.msec.2018.04.008.
Skop, N. B., et al. (2013). Heparin crosslinked chitosan microspheres for the delivery of neural stem cells and growth factors for central nervous system repair. Acta Biomaterialia, 9(6), 6834–6843. https://doi.org/10.1016/j.actbio.2013.02.043.
Fukushima, K. A., et al. (2019). Screening of hydrogel-based scaffolds for dental pulp regeneration-A systematic review. Archives of Oral Biology, 98, 182–194. https://doi.org/10.1016/j.archoralbio.2018.11.023.
Ma, L., et al. (2019). Maintained properties of aged dental pulp stem cells for superior periodontal tissue regeneration. Aging and Disease, 10(4), 793–806. https://doi.org/10.14336/AD.2018.0729.
Wang, X., et al. (2014). Promotion of dentin regeneration via CCN3 modulation on Notch and BMP signaling pathways. Biomaterials, 35(9), 2720–2729. doi:https://doi.org/10.1016/j.biomaterials.2013.12.029.
Funding
This work was supported by grants from the National Key Research and Development Program of China (2017YFA0104800), the Fundamental Research Funds for the Central Universities (YJ201878), Technology Innovation Research and Development Project of Chengdu (2019-YF05-00705-SN), Key Project of Sichuan province (2019YFS0311, 2019YFS0515), and the Nature Science Foundation of China (81600912, 31601113).
Author information
Authors and Affiliations
Contributions
CL contributed to the idea for this article and performed the literature search and original draft preparation. LL contributed to the idea for this article, draft preparation, and revision of the manuscript. WDT critically revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Ethics Approval
Not applicable.
Consent for Publication
Not applicable.
Consent to Participate
Not applicable.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, C., Liao, L. & Tian, W. Stem Cell‐based Dental Pulp Regeneration: Insights From Signaling Pathways. Stem Cell Rev and Rep 17, 1251–1263 (2021). https://doi.org/10.1007/s12015-020-10117-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-10117-3