Abstract
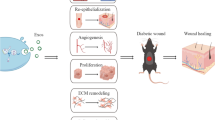
Diabetic foot ischemia and ulcer (DFU) persists as a serious diabetes mellitus complication in spite of increased understanding of the pathophysiology and the cellular and molecular responses. Contributing to this pessimistic situation is the lack of effective treatments that are slow to heal the deep chronic wounds and microvascular obstruction. Mesenchymal stromal cells (MSCs) have been tested as a promising cell-based therapy for diabetes in vitro and in vivo, which is able to accelerate wound closure with increased epithelialization, granulation tissue formation and angiogenesis by differentiation into skin cells and paracrine pathways to repair injured cells. The secretomes of MSCs, including cytokines, growth factors, chemokines, and extracellular vesicles containing mRNA, proteins and microRNAs, have immunomodulatory and regenerative effects. This review will shed new light on the therapeutic potential of MSC-derived extracellular vesicles (MSC-EVs) for the treatment of diabetes-induced lower limb ischemia and ulcers. The identification of underlying mechanisms for MSC-EVs regulation on impaired diabetic wound healing might provide a new direction for MSC-centered treatment for diabetic lower limb ischemia and ulcers.

Immunomodulatory and angiogenic effects of MSC-derived extracellular vesicles on diabetic foot ulcer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the growth of aging populations, dietary changes, and sedentary lifestyles, world-wide rates of diabetes mellitus (DM) have risen steadily. According to epidemiological studies as of the year of 2019, diabetes affects more than 463 million adults worldwide [1]. DM is a group of metabolic disorders and the pathophysiology of DM is underpinned by insulin-resistance and pancreatic β cell dysfunction leading to long-term hyperglycemia [2]. Patients with DM are prone to suffer from multiple complications, such as infections, diabetic nephropathy, diabetic retinopathy, diabetic peripheral neuropathy, and diabetic foot ulcers (DFU) [3]. It is estimated that, annually, between 9.1 million and 26.1 million people develop DFUs worldwide [4]. Moreover, the lifetime incidence of DFU ranges between 15 and 25% among persons with diabetes, but when the patient’s history of foot ulcer is considered, 19% to 34% of persons with diabetes are likely to be affected [4]. DFU, which is mainly caused by ischemic, neuropathic, or combined neuro ischemic abnormalities [5], results in a huge illness burden both to society, and to the patients and their families. The risk of death at 5 years for a patient with a diabetic foot ulcer is 2.5 times higher than as the risk for a patient with diabetes who does not have a foot ulcer [6]. Diabetic ulcers are the most common cause of nontraumatic amputations and approximately 20% of moderate or severe diabetic foot infections lead to different levels of amputation [4, 6]. Decreased vascularization, elevated oxidative stress, and infection contribute to the pathological hallmarks of non-healing chronic diabetic wounds [7].
Therefore, the principles of management of DFU include increasing angiogenesis, removal of oxidative stress, and eradication of infection from the ulcer [8]. However, routine and advanced treatments, such as blood sugar control, debridement, hyperbaric oxygen therapy, electrical stimulation, negative pressure wound therapy and bioengineered skin still present difficulties in the treatment of DFU due to repeated infections, limb ischemia, and nerve damage [3]. Mesenchymal stromal cells (MSCs) obtained from a variety of tissues such as bone marrow, the umbilical cord, fat tissue, the placenta and menstrual blood etc., have been proven that as a promising regenerative therapy for DFU because of their multipotency and paracrine secretions [9,10,11,12,13]. MSCs are capable of secreting cytokines, chemokines, growth factors, and extracellular vesicles containing proteins, mRNA, microRNAs, and mitochondria, contributing to enhanced angiogenesis, fastened re-epithelialization, improved granulation, and contribute to chronic wound closure [14]. The secretome from MSCs has been recognized as the dominant mechanism for ameliorating the symptoms of DFU. In addition, emerging evidence has demonstrated that MSC-EVs are the trophic mediator modulating many biological processes, including anti-inflammation and anti-apoptosis. In this review, we summarize the updated knowledge about the mechanisms of MSC-EVs on DFU and explain the underlying potential for future clinical applications.
Mesenchymal Stromal Cells in DFU
MSCs are being investigated as possessing the peculiar characteristics of differentiating into keratinocytes and endothelial cells, and the secretion of trophic immunoregulatory and reparative factors [15]. The ability of MSCs to home and migrate into damaged sites contributes to the eradication of infections and orchestrates tissue homeostasis. Additionally, the properties of immunomodulation, regeneration in different damaged organs reveal the potential of MSCs as a promising therapeutic alternative that could be translated into clinical work.
The Ability of Homing to Injured Tissue
MSCs possess the ability to migrate and home through the expression of the receptors of cytokines and chemokines, and integrins on the cellular surface, such as C-X-C chemokine receptor ligand 4(CXCR4), C-C cytokine receptor type 2 (CCR2), CCR7, integrin β1 and Integrin α4, which contact with the vascular cell adhesion protein 1 (VCAM-1) on the endothelial cells [16,17,18]. MSCs secrete matrix metalloproteinase 2 (MMP-2) to transmigrate across endothelial monolayers to accelerate the process of homing [19]. In addition, MSCs pretreated with the pro-inflammatory cytokines, such as TGF-β1, IL-1β, and TNF-α could increase MMP-2, MT1-MMP, and/or MMP-9 production in MSCs, resulting in a strong stimulation of chemotactic migration through the extracellular matrix (ECM) [20]. However, the comprehensive mechanisms of MSC homing and how MSCs contact with endothelial cells remains speculative.
The Mechanisms of MSCs in DFU
The paracrine secretions of MSCs are recognized as the main molecular mechanisms in the treatment of DFU to facilitate diabetic wound healing. MSCs are capable of secreting various kinds of angiogenic growth factors, immunomodulatory factors, anti-microbial peptides, reparative molecules and chemokines to enhance granulation tissue formation, wound re-epithelialization, and promote wound angiogenesis and collagen metabolism (Table 1).
In the in vitro diabetic wound model, umbilical cord-derived MSCs (UC-MSCs) were demonstrated to promote diabetic wound repair by secreting VEGF and fibroblast growth factor (FGF), and transforming growth factor-β production (TGF-β), compared to a fibroblast group [24]. Specifically, UC-MSCs can be localized to the injured ulcerated tissue and enhance the tissue recover by upregulating cytokeratin 10 secretion from keratinocytes and accelerating extracellular matrix formation [29]. Additionally, UC-MSC-conditional medium (CM) has been shown to have a greater therapeutic potential than UC-MSCs in contributing to an increase in vascular density and the recovery of sensory function by secreting VEGF, keratinocyte growth factor (KGF), platelet-derived growth factor (PDGF) [23]. More importantly, UC-MSCs have also shown the great therapeutic potential to recover diabetic femoral neural (FN) degeneration by increasing capillaries in FN-innervated gastrocnemius, serum nerve growth factor (NGF) expression, and restoring the slow conduction of FN in the model of diabetic ulceration rats [30]. In a mouse hind limb ischemia model, Wharton’s Jelly MSCs have been also demonstrated that the proangiogenic actions were mediated by secretion of angiogenin, interleukin-8, monocyte chemoattractant protein-1 (MCP-1), and vascular endothelial growth factor (VEGF) [21].
Adipose-derived MSCs (ASCs) have been shown to have a rich secretome and multi-differentiation ability, whereby the critical role of ASCs is manifested in angiogenesis and immunoregulation [31, 32]. ASCs also produce angiogenic growth factors, such as VEGF, KGF, TGF-β1, but ASCs also secrete hepatocyte growth factor (HGF), epidermal growth factor (EGF) and insulin-like growth factor (IGF-1) to promote the wound healing [22, 26]. In a Zucker diabetic fatty rat model, transplantation of ASC sheets was able to decrease the wound area, and increase blood vessel density in full-thickness skin defects [22]. In addition, silk fibroin patches (SFP) cellularized with ASCs and SFP decellularized with ASCs accelerated the rate of wound healing by the significant upregulation of the angiogenic gene (Wnt5α, Wisp1 and TGFβγ3) and the genes involved in ECM deposition and remodeling such as MMP2, Col5α1, Col5α2, Col4α1 etc. [28]
Simultaneously, MSCs, which are identified as having profound immunosuppressive effects, can restore the balance of the immune response in the process of chronic inflammation by modulating the cytokine network and other humoral and cellular effectors. MSC-secreted prostaglandin E2 (PGE2) mediated macrophage polarization from inflammatory macrophages (M1) toward anti-inflammatory macrophages (M2) [33]. Moreover, MSCs also can suppress T/B cell proliferation, and the maturation and differentiation of dendritic cells. However, the comprehensive understanding of MSC effects on the immune system is limited.
Human bone marrow-derived-MSCs, placental derived-MSCs, hair follicle dermal sheath-derived-MSCs and gingiva derived-MSCs were all demonstrated to promote angiogenesis and immunomodulation in similar DFU models [25, 27, 34]. Nevertheless, due to poor cell engraftment and inconsistent stem cell potency, accumulating evidence reported that EVs are one of the most important effectors in the MSC secretome to recapitulate the therapeutic effect of their parent MSCs.
Limitations of MSCs: Reasons to Investigate MSC-EVs
MSCs are not retained and do not survive in the injured tissue for long, normally disappearing gradually within 24 hours [35], and the lack of standardized and optimized criteria contributes to the main challenge of MSC application in DFU. Discrepancies from bench to bedside include donor-based MSC heterogeneity, the conditions of the cell isolation and culture, cryopreservation methods, dose, frequency and route of MSC infusion, timepoint of cell administration, and follow-up period. Moreover, the ambiguous impacts of MSC transplantation include potential tumorigenicity, untargeted tissue differentiation, and undesired immune responses, the most important of which is malignant promotion and transformation. MSCs exhibit tumor tropism [36] and the possibility of tumorigenicity increases if the MSCs are expanded in culture [37]. In addition, despite possessing immune-privileged properties, allogeneic MSCs can be recognized by the host immune system and induce anti-donor immune responses due to the alloantigen on the cell surface [38]. These issues of whole-cell administration initiate the investigation of MSC-EVs’ effect on DFU.
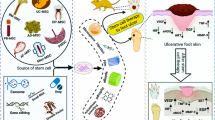
MSC-EVs: Definition, Isolation, Characterization
Extracellular Vesicles (EVs) are small circular structures surrounded by a phospholipid membrane that are released by cells and are vital in intercellular communication as they can transport a variety of substances large distances across the body and influence recipient cell behavior via the delivery of functional bio-molecules [39]. According to the nomenclature recommendation from the Minimal information for studies of extracellular vesicles 2018 (MISEV2018), three different operational terms are used to define EV subtypes: 1) physical characteristics of EVs, such as size with ranges (“small EVs” (sEVs): less than 100 nm or 200 nm [small], and “medium/large EVs” (m/lEVs): more than 200 nm [large and/or medium]) or density (low, middle, high, with each range defined); 2) biochemical composition (CD63+/CD81 + - EVs, Annexin A5-stained EVs, etc.); or 3) descriptions of conditions or cell of origin (podocyte EVs, hypoxic EVs, large oncosomes, apoptotic bodies) [40]. Moreover, EVs transfer specific genetic information via microRNAs, proteins, mitochondria, coding RNAs and non-coding RNAs [41]. These biological and effective molecules have been shown to regulate cell proliferation, anti-inflammation, and angiogenesis in recipient cells, which play a fundamental role in normal physiological processes as well as a pathological process as a result of altered gene regulatory networks and/or epigenetic programming [42].
Ultracentrifugation (differential centrifugation), density gradient centrifugation, and ultrafiltration are the most common isolation approaches towards MSC-EVs, of which ultracentrifugation is the most widely used and easily handled of separating EVs by size and can be handled [43]. In terms of distinct methods, the purity, yield, and the sedimentation efficiency of the isolation differ from each other. In more detail, the steps of ultracentrifugation are typically divided into two stages, including a low-speed spin at 300 g to eliminate apoptotic debris followed by a higher speed spin at 100,000 g to precipitate EVs [44]. However, ultracentrifugation washing procedures lead to the low yield EVs. Density gradient centrifugation is the second most commonly used means to isolate EVs from MSC-CM, which has a higher EV purity and a larger number of EV proteins and RNAs than ultracentrifugation [45]. In addition, Birke et al. have found that the yield and purity of ultrafiltration combined with size exclusion chromatography were superior to ultracentrifugation [46].
There are four distinct methods in checking the characterization of EVs, based on the minimal information for studies of EVs 2018 from the international society of extracellular vesicles 2018 (MISEV2018). First, the source of MSC-EVs and EV preparation are supposed to quantitatively describable. Next, EV general characterization should show at least three positive protein markers of EVs by flow cytometry and western blot, such as CD63, CD81 and CD82, and at least one negative protein marker, such as CD45 [47]. The third approach is to detect the characterization of single vesicles: using an electron or atomic force microscope or single-particle analyzers. The last method recommends that the topology of EV-associated components be assessed [40].
MSC-EVs Effects on DFU in Experimental Models
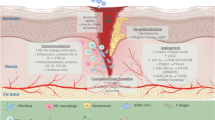
Multiple studies have presented MSC-EVs as being beneficial to the regeneration process of DFU, including the inflammation stage, angiogenesis stage, re-epithelialization, and remodeling stage [48]. miRNAs (“angiomiRs”) and proteins, such as those deleted in malignant brain tumors 1 (DMBT1), OxOband, nuclear factor erythroid 2-related factor 2 (NRF2), miR-21, miR-23a, miR-124a, miR125b, miR126, lncRNA H19, let-7b, mmu_circ_0000250, miR-130a, miR-132 encapsulated in MSC-EVs play a vital role in promoting cell proliferation of connective tissues through activating targeting signaling pathways to modulate the immune response, autophagy, and neovascularization [49, 50] (Table 2and Fig. 1).
MSCs show great therapeutic potential in immunomodulatory and angiogenesis phases of DFU. In immunoregulatory phase, MSCs are capable of secreting miR124a/125b to relieve inflammation response, producing leb-7b, PEG2 to modulate macrophage polarization, and secreting miR21 to regulate dendritic cell differentiation. In angiogenic phase, MSCs own the ability to produce NRF2, mmu_circ_0000250, DMBT1, lncRNA H19, OxOband, miR126, miR23, and miR21 to promote the process of angiogenesis, granulation tissue formation and re-epithelialization. SC: stratum corneum; SG: stratum granulosum; SS: stratum spinosum; SB: stratum basale
DMBT1
Exosomes isolated human urine-derived MSCs (USCs) induced a remarkable proliferation and migration of keratinocytes and fibroblasts, which were detected by CCK8 analysis, and by a scratch wound healing assay and a transwell assay [65]. In addition, USC-Exos stimulated human vascular endothelial cells (HMECs) to generate more capillary-like structures and motility. More importantly, after identifying the components of USC-Exos by bioinformatics analysis, DMBT1, a potent promoter of angiogenesis [63], and VEGFA, a positive mediator of physiological and pathological of angiogenesis, were enriched in the exosomes. In addition, DMBT1 is capable of increasing VEGFA expression through the PI3K-Akt signaling pathway. To confirm the DMBT1 effect on diabetic wound healing, shRNA-DMBT1 was transfected into USC to suppress DMBT1 expression, and the exosomes were isolated from DMBT1-silenced USCs (USCsshDMBT1-Exos). Interestingly, the USCsshDMBT1-Exos lost promising effect on tube formation partially and delayed cutaneous wound healing in a diabetic mice model.
OxOband
ADSC-Exos labeled with Calcein AM dye endocytosed by keratinocytes and fibroblasts promoted the migration of these two kinds of cells, reduced the oxidative stress, and elevated the metabolic activity in neuronal cells in the hyperglycaemic microenvironment. Meanwhile, results of a Live/Dead viability/integrity assay using Calcein AM/PI staining demonstrated that Exos cultured keratinocytes promote their survival rate in an H2O2-induced oxidative stress condition, compared to the non-treated cells [66]. In addition, ADSC-Exos containing metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) can be transferred into keratinocytes and fibroblasts and targeted on miR124 to promote cell proliferation, migration, and inhibit cell apoptosis through stimulating the Wnt/β-catenin pathway [62]. Moreover, ADSC-Exos embedded into the scaffolds of oxygen-releasing antioxidant polymeric cryogel (PUAO-CPO-Exos) revealed a slower release than PUAO -Exo embedded in antioxidant polyurethane (PUAO) scaffolds, which meant PUAO-CPO-Exos had a prolonger effect on damaged tissue in different phases of chronic wound closure. Importantly, exosomes laden oxygen releasing antioxidant scaffolds PUAO-CPO-Exos (OxOband) are capable of reducing ROS release, supply adequate oxygen, generate hair follicles and sebaceous glands to enhance wound healing [66].
NRF2
NRF2 is a nuclear mediator of cellular resistance to oxidants that regulate the physiological and pathophysiological outcomes of oxidant exposure [67]. In the presence of ADSC-Exos, the apoptosis rate of endothelial progenitor cells (EPCs), which were stained with Annexin-V/propidium iodide (PI), was significantly decreased [68]. ADSC-Exos also enhances the tube formation capability of EPCs which were seeded in a Matrigel-coated plate. Moreover, the intracellular ROS levels also decreased, as determined by DCH-DA staining, and the pro-inflammatory cytokine (IL-1β, IL-6, and TNF-α) secretion of EPCs co-cultured with ADSC-Exos was suppressed compared to the exosomes isolated from ADSCs pretreated GW4869, a neutral sphingomyelinase inhibitor that blocks exosome generation, leading to the conclusion that ADSC-Exos were able to attenuate the hyperglycemia-induced endothelial progenitor cells (EPCs) senescence. Additionally, exosomes isolated from NRF2 overexpressed-ADSCs also decrease ROS production and pro-inflammatory cytokine expression, and promote collagen deposition and granulation tissue formation, as measured by Masson Trichrome staining and HE staining [68].
miR-21
miR-21 is one of the most investigated miRNAs in the context of various diabetic complications, including DFU, myocardial ischemia/reperfusion, and diabetic kidney diseases [64, 69]. miR21 is a responsive protective molecule which has been reported to present different mechanisms in diabetic wound healing. For example, miR-21 was positively associated with MMP9 expression and DC differentiation through downregulating phosphatase and tensin homolog (PTEN) and stimulating PI3K/AKT signalling pathway in a full-thickness wound rat model [70]. In what could be another wound healing mechanism, that TGF-β activated NF-κB signaling pathway, and the CHIP assay demonstrated that TGF-β facilitated NF-κB p65 subunit to bind with miR-21 promoter directly in fibroblasts in a high glucose condition, which may promote fibroblast migration [51]. Remarkably, miR-21-3p enriched in UCB-Exos was able to inhibit PTEN and sprout homolog I (SPRY I), contributing to accelerated re-epithelialization, reduced scar widths, and angiogenesis [52].
miR-23
The miR-23 family (miR-23a/b/c) has been reported to enhance angiogenesis by activating angiogenic signaling through targeting Sprouty2 and Sema6A [53]. However, data from the tissue biopsies of DFU in patients reported that only miR23c regulates DFU healing by targeting stromal cell-derived factor-1α (SDF-1α/CXCL12) [71]. In a full-thickness skin defect mouse model, miR-23 carried by UB-MSC-Exos played a critical role in myofibroblast differentiation during scare formation via suppression of TGF-β/SMAD2 stimulation [72].
miR-126
miR-126 is recognized as a master regulator which mediates physiological angiogenesis and inflammation [54, 73, 74]. Jason et al. have shown that miR-126 regulated the response of endothelial cells to VEGF, and directly suppressed the negative mediators of the VEGF and FGF signaling pathway, including the Sprouty-related protein SPRED1 and phosphoinositol-3 kinase regulatory subunit 2 (PIK3R2/p85-β) [56, 75]. Exosomes derived from miR-126 overexpressed synovium MSCs (SMSC-126-Exos) activated the proliferation of human dermal fibroblasts and human dermal microvascular endothelial cells (HMEC-1), and SMSC-126-Exos also stimulated the migration and tube formation of HMEC-1 and collagen maturity [57].
lncRNA H19
Long noncoding RNAs H19 (lncRNAs H19) have been reported to participate in the regulation of hepatic glucose production and insulin resistance in a hyperglycemia microenvironment [58]. Fibroblasts isolated from patients with DFU co-cultured with exosomes derived from lncRNA H19 overexpressed MSCs demonstrated that lncRNA H19 may exert a positive effect on proliferation, migration, and suppression of apoptosis and inflammation of the fibroblasts through miR-152-3p/ PTEN axis [76], contributing to a stimulated wound-healing process in DFU.
Let-7b
LPS preconditioned UC-MSC exosomes showed a stronger effect on modulating macrophage polarization and increasing anti-inflammatory cytokine secretion than untreated UC-MSC exosomes. Moreover, miRNA microarray analysis exhibited that UC-MSC exosomes shuttled let-7b to macrophages and regulated macrophage plasticity through activating TLR4/NF-κB/STAT3/AKT signaling pathway in a high glucose (HG) condition [59].
mmu_circ_0000250
There is emerging evidence that circular RNAs (circ RNAs) exert important roles in mediating the microenvironment of wound healing [60]. In an in vitro tubule formation model of EPCs on Matrigel-coated culture wells, angiogenesis was inhibited in the HG microenvironment, and the inhibition was reversed by mmu_circ_0000250 modified ADSCs-Exos. Moreover, a luciferase reporter assay and qPCR revealed that overexpression of mmu_circ_0000250 suppressed miR-128-3p expression and enhanced autophagic plaque formation in EPCs in a HG microenvironment. In a streptozotocin-induced diabetic mice model, mmu_circ_0000250 was able to accelerate full-thickness cutaneous wound healing in the feet [77].
Recently, accumulating data have shown that human circulating fibrocytes (CD34+ bone marrow-derived progenitor cells) possess the capacity to differentiate into osteoblasts and chondrocytes, which meet the definition of MSCs partially and are able to remodel extracellular matrix components and acquire myofibroblast-like properties in wounds [61, 78]. Geiger et al. have demonstrated fibrocyte-derived exosomes containing various kinds of miRNAs that possess distinct effects in accelerating wound healing, such as regulating collagen deposition (miR-21), proangiogenic (miR-126, miR-130, miR-132) and anti-inflammatory (miR-124a and miR-125b) [55].
Barriers in MSC-EVs for Clinical Application
Unfortunately, until now there are no clinical trials aimed at MSC-EV treatment in DFU. Even though MSC-EVs show therapeutic value in DFU models, the disparities between experimental results and potential actual outcomes of clinical trials still require a more profound view of the role of MSC-based therapy in regenerative medicine. Based on other on-going and completed clinical trials of MSC treatment in DFU, some clinical challenges and underlying limitations need to be addressed before MSC-EVs can be administrated in humans.
The lack of standardized and optimized criteria contributes to the main challenge of MSC-EV application. Protocols from different institutes vary in relation to EV preparation, including EV isolation, characterization, and purification. Hence, the academic societies (ISEV, ISCT, and ISBT) should take on the responsibility of proposing consolidated and well-standardized criteria, and collaborate with biomedical centers worldwide to share cutting-edge improvements.
Finally, developing a safety and efficacy approach to generating large-scale MSC-EVs is the main headwind to deal with. Although some protocols have been established for biomanufacturing exosomes, knowledge about the biomanufacturing of microvesicles remains limited [79, 80].
Conclusions
Today millions of DM patients are still fighting with DFU, and controlling blood glucose level is recognized as the first step to fight this chronic complication. Pro-clinical data support the idea that MSC-EV therapy possesses immunomodulatory and reparative properties which accelerate diabetic wound healing. However, to overcome the barriers to use which exist from laboratory to hospitals, the mechanisms of MSC-EV’s therapeutic potential must be fully understood and good manufacturing practice protocols found.
Despite the challenges that must be overcome, the evidence indicates MSC-EVs deserve further invsitigation due to their promising value in combating DFU.
References
Internation Diabetes Federation. IDF Diabetes Atlas Ninth. Dunia : IDF (2019).
Cerf, M. E. (2013). Beta cell dysfunction and insulin resistance. Front. Endocrinol. (Lausanne)., 4, 1–12.
Yazdanpanah, L. (2015). Literature review on the management of diabetic foot ulcer. World Journal of Diabetes, 6, 37–53.
Armstrong, D. G., Boulton, A. J. M., & Bus, S. A. (2017). Diabetic foot ulcers and their recurrence. The New England Journal of Medicine, 376, 2367–2375.
Andrew, J. (2005). M Boulton, Loretta Vileikyte, Gunnel Ragnarson-Tennvall. J. A. The global burden of diabetic foot diseas. Lancet, 366, 1719–1724.
Walsh, J. W., Hoffstad, O. J., Sullivan, M. O., & Margolis, D. J. (2016). Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabetic Medicine, 33, 1493–1498.
Shiekh, P. A., Singh, A., & Kumar, A. (2020). Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials, 249, 120020.
Hart, T., Milner, R., & Cifu, A. (2017). Management of a diabetic foot. JAMA - J. Am. Med. Assoc., 318, 1387–1388.
Lee, H. C., An, S. G., Lee, H. W., Park, J. S., Cha, K. S., Hong, T. J., Park, J. H., Lee, S. Y., Kim, S. P., Kim, Y. D., Chung, S. W., Bae, Y. C., Shin, Y. B., Kim, J. I., & Jung, J. S. (2012). Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: - a pilot study. Circulation Journal, 76, 1750–1760.
Mathew, S. A., Naik, C., Cahill, P. A., & Bhonde, R. R. (2019). Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cellular and Molecular Life Sciences, 77, 253–265. https://doi.org/10.1007/s00018-019-03268-1.
Gao, W., Chen, D., Liu, G., & Ran, X. (2019). Autologous stem cell therapy for peripheral arterial disease: A systematic review and meta-analysis of randomized controlled trials. Stem Cell Research & Therapy, 10, 1–14.
Gu, J., Huang, L., Zhang, C., Wang, Y., Zhang, R., Tu, Z., Wang, H., Zhou, X., Xiao, Z., Liu, Z., Hu, X., Ke, Z., Wang, D., & Liu, L. (2020). Therapeutic evidence of umbilical cord-derived mesenchymal stem cell transplantation for cerebral palsy: A randomized, controlled trial. Stem Cell Research & Therapy, 11, 43.
Dalirfardouei, R., Jamialahmadi, K., Jafarian, A. H., & Mahdipour, E. (2019). Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. Journal of Tissue Engineering and Regenerative Medicine, 13, 555–568.
Huang, Y.-Z., Gou, M., Da, L.-C., Zhang, W.-Q., & Xie, H.-Q. (2020). Mesenchymal stem cells for chronic wound healing: current status of preclinical and clinical studies. Tissue Eng. Part B Rev., 1–114. https://doi.org/10.1089/ten.teb.2019.0351.
Cao, Y., Gang, X., Sun, C., & Wang, G. (2017). Mesenchymal stem cells improve healing of diabetic foot ulcer. Journal Diabetes Research, 2017, 1–10.
Li, Q., Zhang, A., Tao, C., Li, X., & Jin, P. (2013). The role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose tissue-derived mesenchymal stem cells in vitro. Biochemical and Biophysical Research Communications, 441, 675–680.
Prütz, W. A., & Mönig, H. (1987). Human Adult CD34_ Progenitor Cells Functionally Express the Chemokine Receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but Not CXCR4. Int. J. Radiat. Biol. Relat. Stud. Physics, Chem. Med., 52, 677–682.
Segers, V. F. M., et al. (2006). Mesenchymal stem cell adhesion to cardiac microvascular endothelium: Activators and mechanisms. Am. J. Physiol. - Hear. Circ. Physiol., 290, 1370–1377.
Steingen, C., Brenig, F., Baumgartner, L., Schmidt, J., Schmidt, A., & Bloch, W. (2008). Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. Journal of Molecular and Cellular Cardiology, 44, 1072–1084.
Fukuda, T., & Ohnishi, Y. (1991). MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of. Acta Pathologica Japonica, 41, 466–472.
Kim, H. K. et al. A subset of paracrine factors as efficient biomarkers for predicting vascular regenerative efficacy of Mesenchymal stromal/stem cells. Stem Cells 37, 77–88 (2019).
Kato, Y., Iwata, T., Morikawa, S., Yamato, M., Okano, T., & Uchigata, Y. (2015). Allogeneic transplantation of an adipose-derived stem cell sheet combined with artificial skin accelerates wound healing in a rat wound model of type 2 diabetes and obesity. Diabetes, 64, 2723–2734.
Shrestha, C., Zhao, L., Chen, K., He, H., & Mo, Z. (2013). Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. International Journal of Endocrinology, 2013, 1–10.
You, H. J., Namgoong, S., Han, S. K., Jeong, S. H., Dhong, E. S., & Kim, W. K. (2015). Wound-healing potential of human umbilical cord blood-derived mesenchymal stromal cells in vitro-a pilot study. Cytotherapy, 17, 1506–1513.
Ma, D., Kua, J. E. H., Lim, W. K., Lee, S. T., & Chua, A. W. C. (2015). In vitro characterization of human hair follicle dermal sheath mesenchymal stromal cells and their potential in enhancing diabetic wound healing. Cytotherapy, 17, 1036–1051.
Progenitor, T. (2015) T ISSUE -S PECIFIC P ROGENITOR AND S TEM C ELLS Therapeutic Potential of Adipose-Derived SSEA-3-Positive Muse Cells for Treating Diabetic Skin Ulcers. 146–155.
Cao, Y., Gang, X., Sun, C., & Wang, G. (2017). Mesenchymal stem cells improve healing of diabetic foot ulcer. Journal Diabetes Research, 2017, 1–10.
Navone, S. E., et al. (2014). Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Stem Cell Research & Therapy, 5, 1–15.
Zhao, Q. S., Xia, N., Zhao, N., Li, M., Bi, C. L., Zhu, Q., Qiao, G. F., & Cheng, Z. F. (2013). Localization of human mesenchymal stem cells from umbilical cord blood and their role in repair of diabetic foot ulcers in rats. International Journal of Biological Sciences, 10, 80–89.
Xia, N., Xu, J. M., Zhao, N., Zhao, Q. S., Li, M., & Cheng, Z. F. (2015). Human mesenchymal stem cells improve the neurodegeneration of femoral nerve in a diabetic foot ulceration rats. Neuroscience Letters, 597, 84–89.
An, R., et al. (2020). Adipose stem cells isolated from diabetic mice improve cutaneous wound healing in streptozotocin-induced diabetic mice. Stem Cell Research & Therapy, 11, 1–11.
Marfia, G., Navone, S. E., di Vito, C., Ughi, N., Tabano, S., Miozzo, M., Tremolada, C., Bolla, G., Crotti, C., Ingegnoli, F., Rampini, P., Riboni, L., Gualtierotti, R., & Campanella, R. (2015). Mesenchymal stem cells: Potential for therapy and treatment of chronic non-healing skin wounds. Organogenesis, 11, 183–206.
Zhang, Q. Z., Su, W. R., Shi, S. H., Wilder-Smith, P., Xiang, A. P., Wong, A., Nguyen, A. L., Kwon, C. W., & le, A. D. (2010). Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells, 28, 1856–1868.
Tong, C., Hao, H., Xia, L., Liu, J., Ti, D., Dong, L., Hou, Q., Song, H., Liu, H., Zhao, Y., Fu, X., & Han, W. (2016). Hypoxia pretreatment of bone marrow - derived mesenchymal stem cells seeded in a collagen-chitosan sponge scaffold promotes skin wound healing in diabetic rats with hindlimb ischemia. Wound Repair and Regeneration, 24, 45–56.
Wu, Y., Huang, S., Enhe, J., Ma, K., Yang, S., Sun, T., & Fu, X. (2014). Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. International Wound Journal, 11, 701–710.
Klopp, A. H., Gupta, A., Spaeth, E., Andreeff, M., & Marini, F. (2011). Concise review: Dissecting a discrepancy in the literature: Do mesenchymal stem cells support or suppress tumor growth? Stem Cells, 29, 11–19.
Prockop, D. J., Brenner, M., Fibbe, W. E., Horwitz, E., le Blanc, K., Phinney, D. G., Simmons, P. J., Sensebe, L., & Keating, A. (2010). Defining the risks of mesenchymal stromal cell therapy. Cytotherapy, 12, 576–578.
Ankrum, J. A., Ong, J. F., & Karp, J. M. (2014). Mesenchymal stem cells: Immune evasive, not immune privileged. Nature Biotechnology, 32, 252–260.
Lötvall, J., et al. (2014). Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. Journal of Extracellular Vesicles, 3, 1–6.
Théry, C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., Ayre D.C., Bach J.M., Bachurski D., Baharvand H., Balaj L., Baldacchino S., Bauer N.N., Baxter A.A., Bebawy M., Beckham C., Bedina Zavec A., Benmoussa A., Berardi A.C., Bergese P., Bielska E., Blenkiron C., Bobis-Wozowicz S., Boilard E., Boireau W., Bongiovanni A., Borràs F.E., Bosch S., Boulanger C.M., Breakefield X., Breglio A.M., Brennan M.Á., Brigstock D.R., Brisson A., Broekman M.L.D., Bromberg J.F., Bryl-Górecka P., Buch S., Buck A.H., Burger D., Busatto S., Buschmann D., Bussolati B., Buzás E.I., Byrd J.B., Camussi G., Carter D.R.F., Caruso S., Chamley L.W., Chang Y.T., Chen C., Chen S., Cheng L., Chin A.R., Clayton A., Clerici S.P., Cocks A., Cocucci E., Coffey R.J., Cordeiro-da-Silva A., Couch Y., Coumans F.A.W., Coyle B., Crescitelli R., Criado M.F., D’Souza-Schorey C., Das S., Datta Chaudhuri A., de Candia P., de Santana Junior E.F., de Wever O., del Portillo H.A., Demaret T., Deville S., Devitt A., Dhondt B., di Vizio D., Dieterich L.C., Dolo V., Dominguez Rubio A.P., Dominici M., Dourado M.R., Driedonks T.A.P., Duarte F.V., Duncan H.M., Eichenberger R.M., Ekström K., el Andaloussi S., Elie-Caille C., Erdbrügger U., Falcón-Pérez J.M., Fatima F., Fish J.E., Flores-Bellver M., Försönits A., Frelet-Barrand A., Fricke F., Fuhrmann G., Gabrielsson S., Gámez-Valero A., Gardiner C., Gärtner K., Gaudin R., Gho Y.S., Giebel B., Gilbert C., Gimona M., Giusti I., Goberdhan D.C.I., Görgens A., Gorski S.M., Greening D.W., Gross J.C., Gualerzi A., Gupta G.N., Gustafson D., Handberg A., Haraszti R.A., Harrison P., Hegyesi H., Hendrix A., Hill A.F., Hochberg F.H., Hoffmann K.F., Holder B., Holthofer H., Hosseinkhani B., Hu G., Huang Y., Huber V., Hunt S., Ibrahim A.G.E., Ikezu T., Inal J.M., Isin M., Ivanova A., Jackson H.K., Jacobsen S., Jay S.M., Jayachandran M., Jenster G., Jiang L., Johnson S.M., Jones J.C., Jong A., Jovanovic-Talisman T., Jung S., Kalluri R., Kano S.I., Kaur S., Kawamura Y., Keller E.T., Khamari D., Khomyakova E., Khvorova A., Kierulf P., Kim K.P., Kislinger T., Klingeborn M., Klinke II D.J., Kornek M., Kosanović M.M., Kovács Á.F., Krämer-Albers E.M., Krasemann S., Krause M., Kurochkin I.V., Kusuma G.D., Kuypers S., Laitinen S., Langevin S.M., Languino L.R., Lannigan J., Lässer C., Laurent L.C., Lavieu G., Lázaro-Ibáñez E., le Lay S., Lee M.S., Lee Y.X.F., Lemos D.S., Lenassi M., Leszczynska A., Li I.T.S., Liao K., Libregts S.F., Ligeti E., Lim R., Lim S.K., Linē A., Linnemannstöns K., Llorente A., Lombard C.A., Lorenowicz M.J., Lörincz Á.M., Lötvall J., Lovett J., Lowry M.C., Loyer X., Lu Q., Lukomska B., Lunavat T.R., Maas S.L.N., Malhi H., Marcilla A., Mariani J., Mariscal J., Martens-Uzunova E.S., Martin-Jaular L., Martinez M.C., Martins V.R., Mathieu M., Mathivanan S., Maugeri M., McGinnis L.K., McVey M.J., Meckes Jr D.G., Meehan K.L., Mertens I., Minciacchi V.R., Möller A., Møller Jørgensen M., Morales-Kastresana A., Morhayim J., Mullier F., Muraca M., Musante L., Mussack V., Muth D.C., Myburgh K.H., Najrana T., Nawaz M., Nazarenko I., Nejsum P., Neri C., Neri T., Nieuwland R., Nimrichter L., Nolan J.P., Nolte-’t Hoen E.N.M., Noren Hooten N., O’Driscoll L., O’Grady T., O’Loghlen A., Ochiya T., Olivier M., Ortiz A., Ortiz L.A., Osteikoetxea X., Østergaard O., Ostrowski M., Park J., Pegtel D.M., Peinado H., Perut F., Pfaffl M.W., Phinney D.G., Pieters B.C.H., Pink R.C., Pisetsky D.S., Pogge von Strandmann E., Polakovicova I., Poon I.K.H., Powell B.H., Prada I., Pulliam L., Quesenberry P., Radeghieri A., Raffai R.L., Raimondo S., Rak J., Ramirez M.I., Raposo G., Rayyan M.S., Regev-Rudzki N., Ricklefs F.L., Robbins P.D., Roberts D.D., Rodrigues S.C., Rohde E., Rome S., Rouschop K.M.A., Rughetti A., Russell A.E., Saá P., Sahoo S., Salas-Huenuleo E., Sánchez C., Saugstad J.A., Saul M.J., Schiffelers R.M., Schneider R., Schøyen T.H., Scott A., Shahaj E., Sharma S., Shatnyeva O., Shekari F., Shelke G.V., Shetty A.K., Shiba K., Siljander P.R.M., Silva A.M., Skowronek A., Snyder II O.L., Soares R.P., Sódar B.W., Soekmadji C., Sotillo J., Stahl P.D., Stoorvogel W., Stott S.L., Strasser E.F., Swift S., Tahara H., Tewari M., Timms K., Tiwari S., Tixeira R., Tkach M., Toh W.S., Tomasini R., Torrecilhas A.C., Tosar J.P., Toxavidis V., Urbanelli L., Vader P., van Balkom B.W.M., van der Grein S.G., van Deun J., van Herwijnen M.J.C., van Keuren-Jensen K., van Niel G., van Royen M.E., van Wijnen A.J., Vasconcelos M.H., Vechetti Jr I.J., Veit T.D., Vella L.J., Velot É., Verweij F.J., Vestad B., Viñas J.L., Visnovitz T., Vukman K.V., Wahlgren J., Watson D.C., Wauben M.H.M., Weaver A., Webber J.P., Weber V., Wehman A.M., Weiss D.J., Welsh J.A., Wendt S., Wheelock A.M., Wiener Z., Witte L., Wolfram J., Xagorari A., Xander P., Xu J., Yan X., Yáñez-Mó M., Yin H., Yuana Y., Zappulli V., Zarubova J., Žėkas V., Zhang J.Y., Zhao Z., Zheng L., Zheutlin A.R., Zickler A.M., Zimmermann P., Zivkovic A.M., Zocco D., Zuba-Surma E.K. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7.
Di Liegro, C. M., Schiera, G. & Di Liegro, I. (2017) Extracellular vesicle-associated RNA as a carrier of epigenetic information. Genes (Basel) 8.
Lee, Y., El Andaloussi, S., & Wood, M. J. A. (2012). Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Human Molecular Genetics, 21, 125–134.
Gardiner, C., et al. (2016). Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles, 5, 1–6.
Momen-Heravi, F., Balaj, L., Alian, S., Mantel, P. Y., Halleck, A. E., Trachtenberg, A. J., Soria, C. E., Oquin, S., Bonebreak, C. M., Saracoglu, E., Skog, J., & Kuo, W. P. (2013). Current methods for the isolation of extracellular vesicles. Biological Chemistry, 394, 1253–1262.
Konoshenko, M. Y., Lekchnov, E. A., Vlassov, A. V., & Laktionov, P. P. (2018). Isolation of extracellular vesicles: General methodologies and latest trends. BioMed Research International, 2018, 1–27.
Benedikter, B. J., et al. (2017). Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Scientific Reports, 7, 1–13.
Fafián-Labora, J., Morente-López, M., Sánchez-Dopico, M. J., Arntz, O. J., van de Loo, F. A. J., de Toro, J., & Arufe, M. C. (2020). Influence of mesenchymal stem cell-derived extracellular vesicles in vitro and their role in ageing. Stem Cell Research & Therapy, 11, 13.
Wu, P., Zhang, B., Shi, H., Qian, H., & Xu, W. (2018). MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy, 20, 291–301.
Casado-Díaz, A., Quesada-Gómez, J. M., & Dorado, G. (2020). Extracellular vesicles derived from Mesenchymal stem cells (MSC) in regenerative medicine: Applications in skin wound healing. Frontiers in Bioengineering and Biotechnology, 8, 1–19.
Zhang, D., Xuan, J., Zheng, B. B., Zhou, Y. L., Lin, Y., Wu, Y. S., Zhou, Y. F., Huang, Y. X., Wang, Q., Shen, L. Y., Mao, C., Wu, Y., Wang, X. Y., Tian, N. F., Xu, H. Z., & Zhang, X. L. (2017). Metformin improves functional recovery after spinal cord injury via autophagy flux stimulation. Molecular Neurobiology, 54, 3327–3341.
Madhyastha, R., Madhyastha, H. K., Pengjam, Y., Nakajima, Y., Omura, S., & Maruyama, M. (2014). NFkappaB activation is essential for miR-21 induction by TGFβ1 in high glucose conditions. Biochemical and Biophysical Research Communications, 451, 615–621.
Hu, Y., Rao, S. S., Wang, Z. X., Cao, J., Tan, Y. J., Luo, J., Li, H. M., Zhang, W. S., Chen, C. Y., & Xie, H. (2018). Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics, 8, 169–184.
Zhou, Q., Gallagher, R., Ufret-Vincenty, R., Li, X., Olson, E. N., & Wang, S. (2011). Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23∼27∼24 clusters. Proceedings of the National Academy of Sciences of the United States of America, 108, 8287–8292.
Huang, F., et al. (2013). Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. International Journal of Molecular Medicine, 31, 484–492.
Geiger, A., Walker, A., & Nissen, E. (2015). Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochemical and Biophysical Research Communications, 467, 303–309.
Wang, S., Aurora, A. B., Johnson, B. A., Qi, X., McAnally, J., Hill, J. A., Richardson, J. A., Bassel-Duby, R., & Olson, E. N. (2008). The endothelial-specific MicroRNA miR-126 governs vascular integrity and angiogenesis. Developmental Cell, 15, 261–271.
Tao, S.-C., Guo, S. C., Li, M., Ke, Q. F., Guo, Y. P., & Zhang, C. Q. (2017). Chitosan wound dressings incorporating Exosomes derived from MicroRNA-126-overexpressing Synovium Mesenchymal stem cells provide sustained release of Exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Translational Medicine, 6, 736–747.
Zhang, N., Geng T., Wang Z., Zhang R., Cao T., Camporez J.P., Cai S.Y., Liu Y., Dandolo L., Shulman G.I., Carmichael G.G., Taylor H.S., Huang Y. (2018) Elevated hepatic expression of H19 long noncoding RNA contributes to diabetic hyperglycemia. JCI insight 3.
Ti, D., et al. (2015). LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. Journal of Translational Medicine, 13, 1–14.
Yang, Z. G., Awan, F. M., du, W. W., Zeng, Y., Lyu, J., Wu, D., Gupta, S., Yang, W., & Yang, B. B. (2017). The circular RNA interacts with STAT3, increasing its nuclear translocation and wound repair by modulating Dnmt3a and miR-17 function. Molecular Therapy, 25, 2062–2074.
Bianchetti, L., Barczyk, M., Cardoso, J., Schmidt, M., Bellini, A., & Mattoli, S. (2012). Extracellular matrix remodelling properties of human fibrocytes. Journal of Cellular and Molecular Medicine, 16, 483–495.
He, L. et al. ADSC - Exos containing MALAT1 promotes wound healing by targeting miR - 124 through activating Wnt / β - catenin pathway. Biosci Rep. 2020;40(5)BSR20192549. doihttps://doi.org/10.1042/BSR20192549.
Mollenhauer, J., et al. (2000). DMBT1 encodes a protein involved in the immune defense and in epithelial differentiation and is highly unstable in cancer. Cancer Research, 60, 1704–1710.
Das, A., Ganesh, K., Khanna, S., Sen, C. K., & Roy, S. (2014). Engulfment of apoptotic cells by macrophages: A role of MicroRNA-21 in the resolution of wound inflammation. Journal of Immunology, 192, 1120–1129.
Chen, C. Y., Rao, S. S., Ren, L., Hu, X. K., Tan, Y. J., Hu, Y., Luo, J., Liu, Y. W., Yin, H., Huang, J., Cao, J., Wang, Z. X., Liu, Z. Z., Liu, H. M., Tang, S. Y., Xu, R., & Xie, H. (2018). Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics, 8, 1607–1623.
Shiekh, P. A., Singh, A., & Kumar, A. (2020). Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials, 249, 120020.
Ma, Q. (2015) Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 401–426 doi:https://doi.org/10.1146/annurev-pharmtox-011112-140320.Role.
Li, X., Xie, X., Lian, W., Shi, R., Han, S., Zhang, H., Lu, L., & Li, M. (2018). Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Experimental & Molecular Medicine, 50, 29.
Tsuji, K., Kitamura, S., & Wada, J. (2020). Immunomodulatory and regenerative effects of mesenchymal stem cell-derived extracellular vesicles in renal diseases. International Journal of Molecular Sciences, 21.
Han, Z., Chen, Y., Zhang, Y., Wei, A., Zhou, J., Li, Q., & Guo, L. (2017). MiR-21/PTEN Axis promotes skin wound healing by dendritic cells enhancement. Journal of Cellular Biochemistry, 118, 3511–3519.
Amin, K. N., et al. (2020). miR-23c regulates wound healing by targeting stromal cell-derived factor-1α (SDF-1α/CXCL12) among patients with diabetic foot ulcer. Microvasc. Res, 127, 103924.
Fang, S., Xu, C., Zhang, Y., Xue, C., Yang, C., Bi, H., Qian, X., Wu, M., Ji, K., Zhao, Y., Wang, Y., Liu, H., & Xing, X. (2016). Umbilical cord-derived Mesenchymal stem cell-derived Exosomal MicroRNAs suppress Myofibroblast differentiation by inhibiting the transforming growth factor-beta/SMAD2 pathway during wound healing. Stem Cells Translational Medicine, 5, 1425–1439.
Chen, J. J., & Zhou, S. H. (2011). Mesenchymal stem cells overexpressing MiR-126 enhance ischemic angiogenesis via the AKT/ERK-related pathway. Cardiology Journal, 18, 675–681.
Hu, J., Zeng, L., Huang, J., Wang, G., & Lu, H. (2015). MiR-126 promotes angiogenesis and attenuates inflammation after contusion spinal cord injury in rats. Brain Research, 1608, 191–202.
Fish, J. E., et al. (2008). miR-126 regulates Angiogenic signaling and vascular integrity. Dev Cell, 15, 272–284.
Li, B., Luan, S., Chen, J., Zhou, Y., Wang, T., Li, Z., Fu, Y., Zhai, A., & Bi, C. (2020). The MSC-derived Exosomal lncRNA H19 promotes wound healing in diabetic foot ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol. Ther. - Nucleic Acids, 19, 814–826.
Shi, R., Jin, Y., Hu, W., Lian, W., Cao, C., Han, S., Zhao, S., Yuan, H., Yang, X., Shi, J., & Zhao, H. (2020). Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am. J. Physiol. Physiol., 318, C848–C856.
Choi, Y. H., Burdick, M. D., & Strieter, R. M. (2010). Human circulating fibrocytes have the capacity to differentiate osteoblasts and chondrocytes. The International Journal of Biochemistry & Cell Biology, 42, 662–671.
Pachler, K., Lener, T., Streif, D., Dunai, Z. A., Desgeorges, A., Feichtner, M., Öller, M., Schallmoser, K., Rohde, E., & Gimona, M. (2017). A good manufacturing practice–grade standard protocol for exclusively human mesenchymal stromal cell–derived extracellular vesicles. Cytotherapy, 19, 458–472.
Mendt, M., et al. (2018). Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI insight, 3, 1–22.
Funding
This study was supported by grants from Health Science and Medical technology of Zhejiang Province (2020KY344).
Author information
Authors and Affiliations
Contributions
Tao An: Conceptualization, Tables and diagram, Writing- Original draft preparation.
Yi Chen: Syntax checking. Yingchun Tu: Syntax checking.
Ping Lin: Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Competing Interests
No financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Additional information
Guest Editor: Giovanni Camussi
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Special Issue on Exosomes and Microvesicles: from Stem Cell Biology to Translation in Human Diseases
Rights and permissions
About this article
Cite this article
An, T., Chen, Y., Tu, Y. et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles in the Treatment of Diabetic Foot Ulcers: Application and Challenges. Stem Cell Rev and Rep 17, 369–378 (2021). https://doi.org/10.1007/s12015-020-10014-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-10014-9