Abstract
The ionotropic P2X7 receptor (P2X7R) is involved in bone homeostasis but its role in osteogenesis is controversial. Thus, we investigated the expression of P2X7R and the effects exerted by its modulation in mesenchymal stromal cells from human subcutaneous adipose tissue (S-ASCs), which have potential therapeutic application in bone regenerative medicine. We found that undifferentiated S-ASCs expressed P2X7R and its functional splice variants P2X7AR and P2X7BR. Cell stimulation by P2X7R agonist BzATP (100 μM) neither modified proliferation nor caused membrane pore opening while increasing intracellular Ca2+ levels and migration. The P2X7R antagonist A438079 reversed these effects. However, 25-100 μM BzATP, administered to S-ASCs undergoing osteogenic differentiation, dose-dependently decreased extracellular matrix mineralization and expression of osteogenic transcription factors Runx2, alkaline phosphatase and osteopontin. These effects were not coupled to cell proliferation reduction or to cell death increase, but were associated to decrease in P2X7AR and P2X7BR expression. In contrast, expression of P2X7R, especially P2X7BR isoform, significantly increased during the osteogenic process. Noteworthy, the antagonist A438079, administered alone, at first restrained cell differentiation, enhancing it later. Accordingly, A438079 reversed BzATP effects only in the second phase of S-ASCs osteogenic differentiation. Apyrase, a diphosphohydrolase converting ATP/ADP into AMP, showed a similar behavior. Altogether, findings related to A438079 or apyrase effects suggest an earlier and prevailing pro-osteogenic activity by endogenous ATP and a later one by adenosine derived from endogenous ATP metabolism. Conversely, P2X7R pharmacological stimulation by BzATP, mimicking the effects of high ATP levels occurring during tissue injuries, depressed receptor expression/activity impairing MSC osteogenic differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesenchymal stem cells (MSCs), isolated from embryonic annexes (i.e. umbilical cord or blood, placenta, amniotic fluid) or adult tissues (i.e. bone marrow, teeth) show high self-renewal and multipotent differentiation capacity. These properties, pointed out in a wide number of reports, have created a great expectation on the possible use of these cells in regenerative medicine, especially for bone reconstruction/repair [1]. However, there are several limits in the collection and in vitro expansion of MSCs, since there is a low amount of these cells with variable proliferation potential in embryonic annexes, whereas, in adult tissues, MSC number decreases with patient’s age and sampling procedures result invasive and may cause morbidity [2,3,4]. Human adipose-derived stem cells (ASCs) demonstrate several advantages over MSCs from other sources, including a less invasive harvesting procedure, a higher number of stem cells from an equivalent amount of tissue harvested, increased proliferation and differentiation capacities as well as greater angiogenic and osteogenic properties in vivo. Additionally, donor age does not influence cellular senescence and yield of ASC isolation from subcutaneous adipose tissue [5]. Therefore, ASCs represent a valuable experimental model to investigate the influence of different stimuli on their osteogenic differentiation ability to be exploited in repairing/improving bone damages/reconstruction.
Purines may be included among the several growth factors and signaling molecules which influence the growth and differentiation of ASCs [6]. Purines are ancestral and ubiquitous substances playing a key role in intracellular metabolism, as components of nucleic acids (DNA, RNA), essential molecules for energy storage and supply (ATP or GTP), cofactors in biochemical reactions (i.e. NAD) and intracellular cell signaling (cAMP or cGMP). Remarkably, purines, in particular adenine-based compounds, also act as neurotransmitters/modulators. Indeed, ATP, except from leakage consequent to plasma membrane damages occurring in pathological conditions, is constitutively released from cells in physiological conditions and its extracellular levels are considerably increased by different types of stimulation [7]. Once released, ATP is metabolized to ADP, AMP and adenosine up to xanthine and uric acid by a series of ecto-enzymes similar to those present inside the cells. This complex system is today recognized as “purinome” also in MSCs [8]. At extracellular level, either ATP or adenosine activate specific receptors. The P1 subgroup, recognizing adenosine as an endogenous agonist, includes four G protein-coupled receptors (A1, A2A, A2B, A3) [9], while the P2 family for ATP is more complex, being composed of seven ionotropic P2X and eight metabotropic P2Y receptors [10]. So far, a number of papers have documented the influence exerted by the modulation of different purine receptors on the behavior of MSCs [11,12,13,14], which are likely exposed to adenine nucleotides and adenosine, when these cells migrate in vivo or are transplanted into an injured tissue. Adenosine mostly inhibits adipogenic differentiation, while promoting osteogenesis [15,16,17], whereas ATP regulates both adipogenic and osteogenic differentiation, mainly via different P2Y receptor subtypes [18] the roles of which have been extensively reviewed in bone formation and function [19, 20]. Conversely, the importance of P2X receptors for physiology of MSCs and their differentiation into osteoblasts is yet undetermined. In the last years, a certain interest has been paid to P2X7 receptor (P2X7R), which is unique among the P2X receptor family as it is activated by high concentrations of ATP (> 100 μM) [21, 22] that open Ca2+ permeable channels. In addition, prolonged exposure to this natural agonist leads to formation of large cytolytic pores in the cell membrane [23]. Recent literature reports the expression and involvement of the P2X7R at various differentiation stages of bone cells [19] and a functional P2X7R seems to be essential for differentiation and survival of both osteoclasts and osteoblasts. However, its role in the osteogenic differentiation of MSCs from rodents or humans is still controversial [reviewed in 14, 19].
Attempting to clarify the role of these receptors in osteogenesis, we undertook this study adopting human ASCs as experimental model. We evaluated either the presence and the function of P2X7R in undifferentiated cells or the effect resulting from its stimulation on the osteogenic differentiation process. Additionally, we considered that among the nine splice variants identified so far (P2X7A–J) [24, 25], in humans the only two isoforms showing an ion channel activity, when assembled to form a trimeric receptor, are the variants A and B. The former is a 595 amino acid protein and is also the monomer of the first cloned P2X7R, responsive to high extracellular ATP levels that trigger a sustained pore activity. The latter is a naturally occurring shorter variant, characterized by 18 extra amino acid after the residue 346 in the TM2 domain and lacking the C terminal tail (249 amino acids) due to the insertion of a stop codon. Therefore, the trimeric P2X7B receptor (P2X7BR) lacks the pore formation ability even when stimulated by high ATP concentrations [26]. Assuming that these two splice variants and the related receptors might differently affect ASC osteogenic differentiation, we also investigated their expression in ASCs prior to and along their differentiation towards an osteogenic phenotype.
Materials and Methods
Materials
Disposables for tissue culture were from Falcon (Steroglass, Perugia, Italy). Minimum Essential Medium (MEM) Alpha Medium was purchased from EuroClone S.p.A. (Milan, Italy). L-Glutamine for culture medium, penicillin/streptomycin, amphotericin B, ascorbic acid, dexamethasone, β-glycerophosphate disodium salt, as well as apyrase (VII grade) and all the other chemicals, unless differently indicated, were from Sigma-Aldrich (Milan, Italy). 3-[[5-(2,3-Dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine hydrochloride (A438079), 2′(3′)-O-(4-benzoylbenzoyl)adenosine-5’triphosphate tri(triethylammonium) salt (BzATP) and 8,8′-[carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino)]bis-1,3,5-naphthalenetrisulfonic acid hexa-sodium salt (NF279) were ordered from Tocris Bioscienza (Abingdon, UK).
Cell Culture
Human subcutaneous adipose-derived stromal cells (S-ASCs) were purchased from the Zen-Bio Company (Research Triangle Park, NC, USA) and cultured using a growth medium consisting of MEM Alpha Medium, 10% heat-inactivated Fetal Bovine Serum (FBS, Gibco, Thermo Fisher Scientific, MA, USA), 1% penicillin/streptomycin, 1% Amphotericin B. The mean age of 6 human subjects (females) was 28 ± 3 years. Cultures were incubated at 37 °C and 5% CO2, and the medium was changed twice a week. Experiments were performed only in the first six-eight cell passages.
The osteogenic differentiation was induced by culturing the human S-ASCs with MEM alpha medium supplemented with 5% heat-inactivated FBS, 0.05 mM ascorbic acid, 10 mM β-glycerophosphate and 10 nM dexamethasone up to 28 days, with changes of medium every 3 days. Cultures were stained at different time points with Alizarin Red S (ARS, Sigma-Aldrich) to identify calcium depots, that were quantified by spectrophotometric measurement. Briefly, 800 μl 10% (v/v) acetic acid were added to each well; cells were incubated for 30 min with shaking, then removed by scraping, transferred into a 1.5-ml vial and vortexed for 30 s. The obtained suspension was overlaid with 500 μl mineral oil (Sigma–Aldrich), heated to 85 °C for 10 min, then transferred to ice for 5 min, carefully avoiding the opening of the tubes until fully cooled, and centrifuged at 20,000 g for 15 min. The samples were acidified (pH between 4.1 and 4.5) with 200 μl of 10% (v/v) ammonium hydroxide. Aliquots (150 μl) were read in triplicate at 405 nm by a spectrophotometer (Spectramax SM190, Molecular Devices, Sunnyvale, CA, USA).
Cell Proliferation
To assess cell number and therefore the proliferation trend, the trypan blue exclusion method was used. Briefly, cells were harvested after different culture periods, incubated with trypan blue, and counted with an hemocytometer (three different fields for each sample evaluated in triplicate). Results are expressed as number of live cells/ml.
Quantification of Apoptosis by Caspase 3/7 Activity
Quantification of apoptosis in S-ASCs, treated or not with appropriate concentration of P2X7R agonist/antagonist, was performed using Caspase-Glo Assay Tecnology by providing a luminogenic caspase 3/7 substrate, which contains the tetrapeptide sequence DEVD, in a reagent optimized for caspase acitivty, the luciferase activity. Luciferase activity is proportional to the amount of caspase activity present. The assay was carried out according to the instructions of the supplier company (Promega Italia, Milan, Italy).
Lactate Dehydrogenase Assay
Lactate dehydrogenase (LDH) levels are widely used to estimate necrotic cell death since LDH is a cytoplasm enzyme that can be released following cell membrane damage. Cells, seeded (2 × 103 cells/well) in 96-well plates, were incubated with drugs following the usual protocol. At different time points (7, 14, 21 and 28 days) a part of these cells were incubated at 37 °C and 5% CO2 for 45 min with specific lysis buffer and then, the plate was centrifuged at 250 g for 4 min. Subsequently, 50 μl of supernatant from each well, transferred to a new 96-well plate, were added to 50 μl of substrate buffer consisting of 0.7 mM p-iodonitrotetrazolium Violet, 50 mM L-lactic acid, 0.3 mM phenazine methoxysulfate, 0.4 mM NAD and 0.2 M Tris-HCl pH 8.0. The plate suitably blanket was incubated in the dark at room temperature for 30 min, and finally the reaction was stopped by addition of 50 μl/well of stop solution. The absorbance was measured spectrophotometrically at 490 nm and the results were expressed as a percentage of total LDH released from the positive control calculated as follows:
All reagents were from Promega Italia (Milan, Italy).
Calcium Measurements on Single Cells
Intracellular Ca2+ levels were monitored in the cells using the Fluo4-acetoxymethylesterdye (Fluo4/AM, Thermo Fisher Scientific, Monza, Italy) and an upright microscope (Zeiss Axio Examiner, Jena, Germany), equipped with a 20 × 0.75NA water-immersion objectives. The microscope was connected by an optical fiber to a 75 W Xenon lamp and a monochromator (OptoScan; Cairn Instrument, Faversham, UK). Cells, seeded on 35 mm plates at a density of 20 × 103 cells/cm2, were incubated in a normal external solution (NES: 140 mM NaCl, 2.8 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM glucose and 10 mM HEPES, pH 7.3) supplemented with 10 mg/ml bovine serum albumin and 5 μM of Fluo4-AM, for 40 min at 37 °C. After a washing, the fluo4-loaded cells were bathed in NES and excited at 488 nm. The fluorescence images were acquired using a 505–530 nm band-pass filter (to select the proper emission wavelength), at 2 frames/s with a 16 bit digital EM-CCD camera (PhotoEvolve 512; Photometrics; Tucson, AZ, USA). After 2 min-recordings, the cells were stimulated with 100 μM ATP, after 6 min the cells were washed with NES for 5 min followed by 100 μM BzATP addition. The same recording protocol was performed after a 30 min pre-incubation with and in the presence of 10 μM A438079, an inhibitor of P2X7R. The temporal analysis of intracellular Ca2+ measurements was calculated as the mean fluorescence intensity signal in a selected cell and reported as f/f 0, where f is the fluorescence intensity of a single loaded cell that was acquired during the time lapse, and f0 is the mean fluorescence intensity of the same cell calculated from images acquired before the first stimulus addition. The maximum peak (peakmax) amplitude was calculated as the ratio between the maximum peak and the baseline [27]. Temporal analyses were shown as representative traces from at least 160 tested cells from three independent experiments.
Pore Formation
In order to analyze the effect of BzATP on pore formation, 5 × 105 cells were incubated with BzATP (100 μM) and ethidium bromide (20 μM) for 5 min. The plasma membrane permeability to ethidium bromide was analyzed by flow cytometry using a flow cytometer (BD FACSCalibur, Becton Dickinson Bioscience, San Jose, CA, USA.). Ethidium bromide emission fluorescence was recorded using a blue laser (488 nm) and an emission BP filter 574/26 nm (BL2 channel). The results were analyzed using FlowJo v10.1r5 software (Ashland, OR, USA). Cells not treated with BzATP were used as control.
Scratch Assay
The effects of BzATP on S-ASCs migration were evaluated in a scratch assay. Briefly, S-ASCs were seeded in 6-well plates at 3 × 105 cells/well and cultured normally. When the cell confluence reached 80%, the S-ASCs were pretreated with 5 μg/ml mitomycin-C (Sigma-Aldrich, cat. M-0503) at 37 °C with 5% CO2 for 3 h to prevent cell proliferation; then, the medium was replaced with fresh FBS-free medium after two washes with PBS, the confluent cell monolayer was scratched using a sterile 200-μl pipette tip, the cells were washed, and the edge of the scratch was smoothed with PBS. Different concentrations of BzATP (50 and 100 μM) were added to the wells whereas the pretreatment with the P2X7R antagonist A438079 (10 μM), when present, started 1 h prior to BzATP addition. Images were recorded prior to (0 h) and at 6 and 24 h after the monolayers were scratched. The migration area was quantified by densitometric analysis using ImageJ software (U.S. National Institutes of Health, Bethesda, MD) and assessed as follows: percentage (%) of open wound = (A0 – An)/A0 × 100, where A0 represents the initial wound area (t = 0 h) and An represents the residual area of the wound at the time of measurement (t = n h).
Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
This technique was used for the evaluation of the mRNA expression both for some osteogenic markers and P2X7R A and B splice variants. Total RNA was extracted from S-ASCs with Trizol reagent (Invitrogen, Thermo Fisher Scientific). RNA content was determined using a Nanodrop 2000 spectrophotometer (Thermo Scientific, Milano, Italia) and RNA integrity was checked by electrophoresis on 1.5% agarose gel in Tris Borate EDTA (TBE) (89 mM Tris, 89 mM boric acid, 20 mM EDTA, pH 8.0). Gels were analyzed by a RED analyzer (Cell Biosciences, Santa Clara, CA, USA). All samples were further treated with amplification grade with Turbo DNA-free kit (Invitrogen). Reverse transcription was performed starting from 1 μg of total RNA/sample, with high Capacity cDNA Reverse Transcription kit (Applied Biosystems) as described by the manufacturer. The reaction mixture was loaded to the Gene Amp PCR system 9700 (Applied Biosystem, Foster City, CA, USA) undergoing the cycle at 37 °C for 120 min.
Real-Time PCR was carried out with the ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Expression of Alkaline Phosphatase (ALP), Runt-related transcription factor 2 (RUNX2) and osteopontin (OPN) was evaluated at 0, 3, 7 and 14 days in cells cultured in osteogenic medium whereas that of P2X7R A and B splice variants was evaluated in undifferentiated S-ASCs and in S-ASCs submitted to osteogenic differentiation at different time points up to 28 days. Commercially available TaqMan Gene Expression Assays were used for osteogenic markers (RUNX2, Hs00231692_m1, ALP, Hs01029144_m1, OPN, Hs00960942_m1) and for pan-P2X7R (Hs00175721_m1, recognizing both the A and B isoforms of the P2X7R), whereas TaqMan Gene Expression custom assays were purchased to identify P2X7AR and P2X7BR, as previously described [27]. Moreover, the TaqMan Universal PCR Master Mix (Applied Biosystems) was used according to standard protocols. Gene expression levels were normalized (ΔCt) by using the house keeping β2-microglobulin as endogenous control (B2M, Hs99999907_m1, Applied Biosystems, Foster City, CA, USA). The results were analyzed for relative quantitation among groups using the comparative 2 ΔΔCt method [28].
Western Blot Analysis
Cells were harvested at 4 °C in a RIPA lysis Buffer with Protease Inhibitor cocktail (Sigma-Aldrich) and centrifuged (14, 000 rpm, 8 min, 4 °C). Protein concentration was determined by BioRad protein assay (Bio-Rad Laboratories, Milan, Italy). Samples (60 μg), diluted in sodium dodecyl sulphate (SDS)-bromophenol blue buffer, were boiled (5 min) and separated on 10% (as for P2X7R and P2X7AR) or 12.5% (as for P2X7BR) SDS polyacrylamide gels. Proteins were transferred on a polyvinylidene fluoride membrane, blocked with PBS/0.1% Tween20/5% nonfat milk (Bio-Rad Laboratories) for 2 h at 4 °C, incubated overnight at 4 °C with specific primary antibodies [polyclonal rabbit anti-P2X7R (extracellular), dilution 1:200 (#APR-008, Alomone Labs, Jerusalem, Israel)]; polyclonal rabbit anti-P2X7R (intracellular, C-terminus) dilution 1:300 (#P8232, Sigma-Aldrich); polyclonal rabbit anti-P2X1 receptor (P2X1R), dilution 1:200 (# APR-001, Alomone Labs); polyclonal rabbit anti-P2X3 receptor (P2X3R), dilution 1:200 (#APR-016, Alomone Labs)] and then exposed for 1 h at room temperature to goat anti-rabbit HPR-conjugated secondary antibody at final dilution 1:5000 (Bethyl Laboratories Inc., Montgomery, TX, USA). Subsequently, the blots were stripped and reprobed with an antibody against the protein ß-actin assumed as loading control (dilution 1:1000, incubation overnight at 4 °C; Santa Cruz Biotechnologies, Heidelberg, Germany). Immunocomplexes were visualized using the enhancing chemiluminescence (ECL) detection system (GE Healthcare Life Sciences, Milan, Italy) and quantified by densitometric analysis using ImageJ software (U.S. National Institutes of Health, Bethesda, MD).
Statistical Analysis
All results are represented as means ± standard error of mean (SEM). Comparisons among experimental groups were performed by Student t-test followed by Sidak’s multiple comparisons test or by one-way ANOVA followed by Dunnett’s post hoc test, using GraphPad Prism 6.01 (San Diego, CA, USA), as indicated. Difference was considered to be statistically significant at a value of P < 0.05.
Results
Presence and Influence of P2X7R Activation on Different Functions in Undifferentiated S-ASCs
In undifferentiated S-ASCs, P2X7R expression increased along the culture period of 28 days, as evaluated by qRT-PCR (Fig. 1a). To assess receptor activity in the same cells, the intracellular Ca2+ levels were measured using fluorescence video-imaging technique and the fluorescent Fluo-4, a specific Ca2+ indicator (see Fig. 1b illustrating the scheme of the time course of the cellular fluorescence recordings). The activation of purinergic P2 receptors by the nonselective agonist ATP (100 μM) induced intracellular Ca2+ variations in 75.0 ± 7.0% of tested cells. These ATP-induced variations were represented by isolated single Ca2+ spike in 53.3 ± 2.7% cells (Fig. 1b, black trace) and by a spike followed by ionic waves in 47.8 ± 3.0% cells (Fig. 1b, grey trace). In contrast, the rather selective P2X7R agonist BzATP (100 μM) triggered a single intracellular Ca2+ increase in 31 ± 7.0% cells that were also responsive to ATP (Fig. 1b, black and grey traces after washing). The pre-incubation and the presence of the specific P2X7R antagonist A438079 during the assay did not significantly modify the number of ATP-responsive cells, but reduced the amplitude of ATP-evoked Ca2+ increase while abolishing the BzATP effect (Fig. 1c, d). However, neither BzATP nor A438079 modified cell viability evaluated by the count of live cells in culture up to day 14 (Fig. 1e). Finally, BzATP at 100 μM did not induce membrane pore formation, as demonstrated by the lack of fluorescence due to ethidium bromide entry inside the cells (data not shown). In contrast, the exposure of undifferentiated S-ASCs to BzATP up to 24 h enhanced cell migration, as shown by the scratch assay (Fig. 2). Also in this case, the effect was counteracted by the P2X7R antagonist A438079.
Expression of P2X7R and effect of its stimulation in S-ASCs grown in basal medium. a Gene expression of the total P2X7R evaluated by qRT-PCR at different times of cultured cell growth under non differentiating condition. mRNA levels were normalized (ΔCt) by using the house keeping β2-microglobulin as endogenous control and the results were analyzed for relative quantitation among groups using the comparative 2 ΔΔCt method. The obtained data were compared with mRNA levels of S-ASCs at the beginning of the experiment (time 0). The values are the mean ± SEM of 5 independent samples. b-d Intracellular Ca2+ variation evoked by ATP and BzATP. In B the scheme of the time course of the cellular fluorescence recordings, during stimuli addition, is reported. In the panels C representative temporal analyses of intracellular Ca2+ levels are shown expressed as fluorescence ratios (f/f0), during stimuli addition (100 μM ATP followed by 100 μM BzATP after washing) in the absence (left) or presence (right) of 10 μM A438079. The traces are related to cells responsive to ATP with an isolated single Ca2+ spike (black trace) and with a spike followed by ionic waves (grey trace). In D there is the quantification of intracellular Ca2+ response parameters including: the percentage of cells responsive to each stimulus calculated on the total tested cells and the amplitude of Ca2+ increase calculated as the ratio of the f/f0 at the maximum peak to the basal f/f0. Values are the mean ± SEM of at least three separate experiments. Statistical significance of values was estimated by Student’s t test. In the panel A: *p < 0.05; **p < 0.01; *** p < 0.001 calculated in undifferentiated cells at different times of growth in culture versus those at the time 0. In the Table D: *** p < 0.001 vs ATP stimulus
P2X7R mediates wound healing in undifferentiated S-ASCs. Nearly confluent S-ASCs (80%) human S-ASCs were incubated in the presence or absence of BzATP and when present, the P2X7R antagonist A438079 was added 1 h prior to the agonist. Cell were then scratch wounded and observed at the indicated times after injury using a Nikon Eclipse TS100 phase contrast microscope, acquiring images with the Zoom Browser EX software. a Images of control and treated cells prior to (0 h) and 6 and 24 h after injury are representative of four independent experiments (scale bar = 100 μm for all panels). b Quantification of the wound size in the presence or absence of pharmacological treatments was performed using ImageJ and graphed as percentage of open wound. Data are given as means±SEM and analyzed by one way ANOVA and Dunnett’s post hoc test (B) *P < 0.05 vs control
Influence of P2X7R Activation in S-ASCs Induced to Osteogenic Differentiation
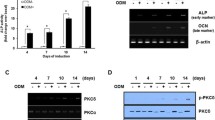
We previously demonstrated that S-ASCs are able to undergo an efficient osteogenic differentiation when grown in appropriate conditions along a 28 day period [29]. Here, we showed that BzATP, added at concentrations ranging from 25 up to 100 μM to S-ASCs at each culture medium change during cell commitment towards osteogenesis, caused a dose-dependent decrease of extracellular matrix mineralization that was particularly evident at 21 and 28 days (Fig. 3a–d). This effect was coupled to a decrease in the expression of early transcription factors such as RUNX2, ALP and OPN associated to osteoblast differentiation, as evaluated at 4, 7 and 14 days (Fig. 4a–c). Cell exposure to the P2X7R antagonist A438079 alone inhibited S-ASC osteogenic differentiation, whereas it significantly enhanced the negative effect produced by BzATP on cell differentiation at 7 and 14 days (Table 1). In contrast, in the subsequent 21 and 28 days, A438079 alone enhanced the differentiation process while counteracting the BzATP inhibitory effect on extracellular matrix mineralization. This effect was similar to that exhibited by apyrase (2 U/ml), the enzyme converting ATP and ADP into AMP (Table 1). Indeed, apyrase, when administered alone, restrained the osteogenic differentiation of S-ASCs at 7 and 14 days and enhanced this process in the following 21 and 28 days. Moreover, when given in combination with BzATP, apyrase did not modify the inhibitory effect of the P2X7R agonist on cell differentiation in the first 14 days, whereas in the next 21 and 28 days the presence of apyrase, like that of the P2X7R antagonist, reversed the inhibitory BzATP effect on S-ASC osteogenesis, (Fig. 3a–d). Altogether, the findings related to the activity of A438079 or apyrase would suggest that endogenous ATP exerts an activity of support along the osteogenic differentiation of S-ASCs, especially during the first 14 days, mainly interacting with P2X7R.
Effect of the stimulation of P2X7R on S-ASC osteogenic differentiation. The cell treatment with different concentrations of BzATP was performed in S-ASCs for different periods from cell induction till the osteogenic differentiation. The effect on the extracellular matrix mineralization was evaluated by ARS staining and its spectrophotometrical analysis (a-d). The values are the mean ± SEM of six independent experiments, in which different cell samples were used. §§§P < 0.001 significantly different from undifferentiated cells (UD); *P < 0.05, **P < 0.01, and ***P < 0.001: significantly different from differentiated S-ASCs (one way ANOVA followed by Dunnett’s post hoc test)
Effect of the stimulation of P2X7R on the expression of some early osteogenic markers during S-ASC osteogenic differentiation. Cell exposure to the P2X7R agonist BzATP was carried out for different periods in S-ASCs induced to differentiate towards an osteogenic differentiation. The expression of osteogenic markers (RUNX2, ALP and OPN) was evaluated by real-time PCR. mRNA levels of the osteogenic markers were normalized by using the house keeping β2-microglobulin as endogenous control. The values are the mean ± SEM of six independent experiments, in which different cell samples were used. §P < 0.05; §§§P < 0.001 significantly different from undifferentiated cells (UD); *P < 0.05, **P < 0.01, and ***P < 0.001: significantly different from differentiated S-ASCs (one way ANOVA followed by Dunnett’s post hoc test)
We also verified the possible activity of BzATP on other P2 receptors, namely P2X1R and P2X3R, for which it also showed affinity [30]. Since it was recently shown that these receptors are not expressed in human ASCs [31], we first checked if this occurred in our cells. Western blot analysis demonstrated that both receptors are expressed by undifferentiated cells even though their levels, which were similar at the days 0 and 7, substantially decreased in the following period. Conversely, in cells committed towards osteogenesis, the protein levels of both receptors were uniformly low, except an increase of P2X1R at the day 28. However, P2X3R seemed to be more expressed than P2X1R at 7, 14 and 21 days. Finally, protein levels of P2X1R and P2X3R were substantially unaffected by cell exposure to BzATP except for the day 28 (Fig. 1 Suppl.). Accordingly, the exposure of S-ASCs to only NF279, an antagonist of P2X1R and P2X3R at the dose used (10 μM), did not modify the trend of their osteogenic differentiation, likely because endogenous ATP interacts with much more expressed P2 receptors, including P2X7R. In contrast, NF279, when given in combination with BzATP (50 μM), partially reversed the inhibitory effect of the latter at 7 and 14 days, but not at longer times (Table 1). These data would indicate that the BzATP interacted also with other receptors, likely the P2X3R, during a limited period of S-ASC differentiation.
We wondered whether the inhibitory effect caused by P2X7R stimulation was due to an influence on cell viability. However, P2X7R stimulation did not influence the number of cultured cells, as assayed by cell counting at different times over a period of 28 days (data not shown). Also the extracellular levels of LDH, assumed as an index of necrotic death and measured in the medium of S-ASCs induced to osteogenic differentiation, were not modified by cell treatment with BzATP as compared to the control, showing even a reduction at the end of this process (28 days) (Fig. 5a). Additionally, the caspase 3/7 activity was decreased by the exposure of the same cells to BzATP (Fig. 5b), confirming that the decrease in the osteogenic differentiation induced by P2X7R stimulation was not due to cell death.
Evaluation of cell necrotic or apoptotic death induced by modulation of P2X7R. S-ASCs induced towards an osteogenic differentiation were treated with BzATP, in the absence or presence of the P2X7R antagonist A438079, administered 1 h prior to the agonist. After different times from the beginning of the pharmacological treatments the activity of LDH or caspase 3/7 was assayed in the medium of all cells. a LDH release from cells, assumed as an index of necrotic death, was measured as reported in the Methods section. Values are expressed as the percentage of the total amount of the enzyme released in the medium from the cells after their lysis. b Apoptic death was assessed by the evaluation of the release of caspase 3 and 7, the most involved in this process, by luminescence using a commercial kit and following the manufacturer’s instruction. The values in A and B are the mean ± SEM of four independent experiments in which each sample was tested in triplicate. *P < 0.05**P < 0.01, ***P < 0.001: statistical significance vs. untreated cells; ##p < 0.01: statistical significance vs. cells treated with BzATP (one-way ANOVA plus Dunnett’s test)
Expression of the Splice Variants P2X7AR and P2X7BR in S-ASCs when Undifferentiated and along their Commitment towards Osteogenesis
We investigated if the effect caused by BzATP could be due to a modification in the expression of P2X7R. In comparison to undifferentiated S-ASCs, the expression of this receptor in cells under differentiation was significantly increased during the observation period of 28 days (Fig. 6a). Since in literature it has been reported that the two functional splice variants of P2X7R, namely P2X7AR and P2X7BR, are expressed in human cells, differently influencing cell growth and differentiation [26, 32], we investigated this issue in our cells. In undifferentiated S-ASCs the expression of the full length P2X7AR monomer was increasing whereas that of the shorter form of P2X7BR remained almost invariant during the period of 28 days, although it was decreased in comparison to the day 0. In contrast, in S-ASCs induced to osteogenic differentiation, the expression of both splice variants was significantly increased and that of P2X7BR monomer was greater than that of P2X7AR at the end of the differentiation period (Fig. 6b, c). Western blot analysis was also performed using two different antibodies, one directed against an extracellular loop common to both P2X7R splice variants A and B, and the another one, directed towards the C-terminal tail, present only in the A variant. In this way, we identified immune-bands with different molecular weights. In particular, both antibodies recognized bands at about 70 KDa, which corresponds to the monomer constituting the P2X7AR (Fig. 7b, c), whereas the antibody against the extracellular loop recognized also a protein at about 50 KDa, compatible with the shorter splice variant present in P2X7BR (Fig. 7c). Western blot analysis substantially confirmed an increased presence of the proteins related to the two splice variants A and B along the differentiation process, with a prevalence of the variant B on the A one (Fig. 7b). When the cells were exposed to BzATP, mRNA expression of both isoforms decreased, in the period up to 21 days and mainly at the highest drug concentration (100 μM) (Fig. 7a). Accordingly, western blot analysis showed that BzATP, even at 50 μM, caused a potent decrease in the protein content of both P2X7AR and P2X7BR monomers along the S-ASC differentiation period (28 days) (Fig. 7b, c).
Expression of P2X7R and the two main human P2X7R splice variants A and B in S-ASCs when undifferentiated and along the process of their osteogenic differentiation. By qRT-PCR the evaluation of the mRNA levels was performed both in relation to the total P2X7R (panel a) and to its principal splice variants in humans that are the full length form P2X7AR and the truncated form (lacking the carboxy-terminal tail) P2X7BR (panel b). mRNA levels were normalized by using the house keeping β2-microglobulin as endogenous control. Values, calculated as fold of increase vs undifferentiated cells, are the mean ± SEM of four independent experiments in which each sample was tested in duplicate. *P < 0.05, **P < 0.01, ***P < 0.001: statistical significance vs. undifferentiated cells, as for the data reported in the panel A and in the graph on the right in the panel B, or vs. undifferentiated S-ASCs at the beginning of the experiment (day 0) as for the data of the graph on the left of the panel B; ###p < 0.01 statistical significance vs. cells grown in OS (one-way ANOVA plus Dunnett’s test)
Modulation of the expression of the P2X7R splice variants A and B in S-ASCs exposed to BzATP along their osteogenic differentiation. S-ASCs during their commitment towards osteoblast-like cells (control) were in part exposed to BzATP. Undifferentiated cells grown in basal medium (BM) were also assayed, besides cells grown in osteogenic medium (OS). a At the indicated time periods cells were collected to obtain their mRNA to analyze for the gene expression of the two splice variants A and B of P2X7R. mRNA levels were normalized by using the house keeping β2-microglobulin as endogenous control. Values, calculated as fold of increase vs undifferentiated cells, are the mean ± SEM. of three independent experiments in which each sample was tested in duplicate. B-C) Protein levels of P2X7AR and P2X7BR monomers were determined by Western blot analysis (60 μg of proteins were loaded per lane). Immunoblots were obtained by exposing membranes to two antibodies, recognizing proteins at about 70 KDa (b) and 50 KDa (c), respectively. Subsequently, immunoblots were reprobed with antibody against β actin, to verify equal sample loading and quantified by densitometric analysis, the values of which, normalized to β actin, are reported in the histograms. Densitometric values are the mean ± SEM of three independent experiments with very similar results. *P < 0.05, **P < 0.01, ***P < 0.001: statistical significance vs. undifferentiated S-ASCs, grown in basal medium (BM); #P < 0.05, ##P < 0.01, ###P < 0.001: statistical significance vs untreated cells grown in osteogenic medium (OS) (one-way ANOVA plus Dunnett’s test)
Discussion
In this paper, we first demonstrated that S-ASCs either when undifferentiated or committed towards osteogenic differentiation express the P2X7R evaluated in its total form. While this receptor seems to be absent in rat adipose-derived stromal cells [33], transcripts for purine receptors, including the P2X7R subtype, have recently been detected in human ASCs [31, 34]. Here, we showed that P2X7R is functionally active in undifferentiated ASCs as demonstrated by the increase in the intracellular Ca2+ levels induced by ATP or BzATP that was reduced or abolished, respectively, by cell pretreatment with the P2X7R antagonist A438079. Two aspects should be underlined: 1) a certain number of cells responding to ATP showed Ca2+ peaks at different times. According to literature, the first peak should correspond to activation of different P2YR [31, 34], whereas the second ones, which were delayed, should be caused also by P2X7R activation, which normally shows a longer latency [35]; 2) only a smaller amount of the total cell number was responsive to the stimulation by BzATP as compared to ATP. This finding is consistent with data previously reported on the existence of P2X7R in a subset of cell population [36, 37]. As demonstrated for other cells types [38], this cell subset should be the only one both sensitive to the pro-osteogenic activity of ATP, released from cells in “physiological” culture condition, via P2X7R, and responsive to the inhibitory effects on P2X7R expression and osteogenic differentiation caused by prolonged S-ASC exposure to BzATP. However, as discussed below, it is also to consider that the expression (and thereby the function) of P2X7R was increased during S-ASC commitment towards osteogenesis. Whether this finding is related to an increase in receptors on the same cell or involves a larger number of cells, it needs to be better investigated.
As expected, 100 μM BzATP was unable to open pores in the plasma membranes and to alter cell duplication. This likely indicated that BzATP, at the maximal concentration used in all our experiments, did not induce cell damage, as confirmed by the absence of modifications in the number of S-ASCs committed towards osteogenic differentiation and exposed to BzATP as well as in LDH release and caspase 3/7 activity assayed in the osteogenic medium of the same cells. These findings, especially those related to caspase 3, are consistent with recent data from another laboratory [39]. Interestingly, BzATP enhanced S-ASC motility, as demonstrated by the reduced time in scratch closure. This last finding fits well with the increase in the intracellular Ca2+ levels, since this signaling is very important for MSC migration [14, 40] and for the reported P2X7R activity in favouring scratch closure of dendritic cells [41]. In our opinion, the properties shown by the activation of P2X7R could be of some importance for stimulating the first phases of tissue repair. Indeed, high levels of ATP are found in the extracellular space as a consequence of tissue damage and they may contribute to mobilize endogenous MSCs. On the other hand, the P2X7R stimulation in undifferentiated MSCs, in our case S-ASCs, could be exploited to accelerate cell homing towards the site of tissue alteration, in case of employment of these cells for regenerative medicine.
Looking at S-ASC osteogenic differentiation, P2X7R expression increased along this process, as reported also in osteoblasts [42, 43]. However, differently from other findings [44, 45], a prolonged P2X7R stimulation in S-ASCs under osteogenic conditions impairs the differentiation process, as demonstrated by the reduction in the accumulation of calcium depots in the extracellular matrix (Alizarin Red S staining) and by the decrease in the expression of early osteogenic genes (Runx-2, ALP and OPN).
Intriguingly, the P2X7R antagonist A438079, when given alone, inhibited cell commitment towards osteogenesis during the first 14 days, while enhancing it later on. Moreover, A438079 did not reverse the inhibitory effects caused by BzATP up to 14 days, but only when it was administered for prolonged periods (up to 28 days). These findings would suggest that A438079 alone, by blocking the P2X7R activity, could counteract a physiological pro-osteogenic effect induced by endogenously released ATP via P2X7R. This also accounts for the lack of reversion of the BzATP effect by the P2X7R antagonist during the first 14 days. In contrast, a prolonged P2X7R blockade, either in the presence or in the absence of BzATP, could favor a delayed pro-osteogenic activity of adenosine, formed by extracellular ATP metabolism, acting in particular on A2B receptor, as previously reported [15,16,17,18]. This last speculation seems to be supported by the effect induced by S-ASC exposure to apyrase, which causes the rapid disappearance of ATP (and also of ADP) as well as an increase in the extracellular amount of adenosine. In this way, also apyrase counteracted the negative BzATP effect on S-ASC differentiation.
Finally, our findings on P2X1R and P2X3R expression are consistent with data reported by Kotova et al. [31], who found no expression for these receptors in human undifferentiated ASCs, likely because they evaluated this aspect in cells cultured for a relatively long period. Anyway, P2X1R and P2X3R do not seem to be involved in the osteogenic differentiation of S-ASCs, as suggested by their low expression during this process and by the lack of effect by the antagonist NF279, when administered alone. However, as reported for bone mineralization [42], these receptors, likely P2X3R more than P2X1R, might play an inhibitory role in the first phase of S-ASC differentiation, when pharmacologically stimulated by BzATP. Indeed, even if they both receptors showed a low expression, BzATP could interact also with them, given its good affinity for these receptors, as previously reported [30], and as demonstrated by the effect of the P2X1/3R antagonist, which partially reversed the inhibitory BzATP effect. It is conceivable that, in the second period (14–28 days) of S-ASC osteogenic differentiation, during which NF279 (but not A438079) was no longer able to prevent the BzATP effect, BzATP mostly interacted with P2X7R, favored by the persistent low expression mainly of P2X3R (see the Scheme 1 herein enclosed).
Effect of the stimulation of purinergic receptors on cultured S-ASCs during their osteogenic differentiation. In the upper panel, S-ASCs are represented during their osteogenic differentiation in the absence of BzATP stimulation. Cells are known to release ATP, which is in turn transformed into adenosine (ADO) by ecto-enzymes present on cell membranes. These endogenous molecules can favor, even though with a different time trend, S-ASC differentiation towards osteoblasts (OB) by stimulating some of their own receptors, including P2X7R, the expression of which is increased along cell differentiation, and likely A2B receptors responsive to ADO, as reported in literature [15,16,17,18]. Indeed, the P2X7R blockade by A438079 counteracts the “physiological” pro-osteogenic effect of endogenous ATP during the first 14 days, while probably favoring ADO effect, mainly in the second period of S-ASC differentiation. Also apyrase, which causes a rapid ATP disappearance as well as an increase in the extracellular ADO amount, shows effects similar to P2X7R blockade. P2X1R and P2X3R, present at low levels in differentiating cells, do not seem to be involved in this process, as demonstrated by the lack of effect by the antagonist NF279 when administered alone. Different findings were observed following S-ASC exposure to a prolonged P2X7R stimulation by BzATP during cell commitment towards osteogenesis (lower panel). This pharmacological treatment decreased P2X7R expression (as evaluated in relation to the two main P2X7R splice variants) and/or possibly activated different P2X7R-related molecular mechanisms leading to a decreased cell differentiation. Cell pretreatment with NF279 (antagonist of P2X1R and P2X3R) prior to cell exposure to the “partial” agonist BzATP reduced the inhibitory effect of this agent during the first differentiation period, whereas in the subsequent period NF279 was ineffective, possibly because the expression of both receptors was persistently low, favoring a major interaction of BzATP with P2X7R. In contrast, the P2X7R antagonist A438079 reversed the BzATP inhibitory effect in the second differentiation period, while potentiating it in the first one. These findings are compatible with an antagonism by A438079 not only against BzATP effect, but also against the pro-osteogenic activity of endogenous ATP, allowing a late positive effect by endogenous ADO on cell differentiation. Also apyrase, reasonably favoring the activity of endogenous ADO, decreased the BzATP inhibitory effect in the second period of cell differentiation, without modifying the BzATP effect during the first 14 days, likely because apyrase did not block P2X7R
Since our data showed that cell exposure to BzATP up to 100 μM neither diminished the number of active cells nor induced cell death by necrosis or apoptosis, we investigated whether there was a possible BzATP effect on the expression of P2X7R, including also that of the two functional splice variants forming P2X7AR and P2X7BR. In undifferentiated cells, both isoforms are expressed to a similar extent, whereas the S-ASC osteogenic differentiation could be mostly due to an increase in the P2X7BR monomer expression. Importantly, a prolonged cell stimulation with BzATP induced a remarkable reduction in the expression of both P2X7R splice variants. As previously reported, such a reduction could be ascribed to P2X7R endocytosis by caveolin-1, a lipid chaperone usually present in the plasma membrane that exerts a regulatory activity against a prolonged signaling [46]. Noteworthy, P2X7R protein loss could result in an attenuation of the receptor-ligand interaction and mostly accounts for the reduction in the osteogenic differentiation induced by BzATP in S-ASCs.
In conclusion, our results would suggest that, using undifferentiated cells, a brief pharmacological stimulation of P2X7R may enhance their migration and likely their homing to the site of interest. In contrast, if there is need to induce an osteogenic differentiation, the “physiological” stimulation of P2X7R, that is at low extracellular ATP levels (low nanomolar range), may contribute to cell differentiation, considering that the number/expression of these receptors tends to increase during osteogenesis, as if the cells needed a greater support by their activity. In this regard, it is noteworthy that the enhanced co-expression of the variants P2X7AR and P2X7BR increases the receptor affinity for ATP as well as the ability of endogenous ATP to support cell energy and metabolism through the interaction with P2X7R [47]. In contrast, to avoid an overstimulation of this greater P2X7R number by a pharmacological and/or prolonged stimulation, that could compromise cell viability [48], cells defend themselves reducing receptor expression/number/function and this results in a decrease of their osteogenic commitment. If this process should occur in vivo, it could retard bone repair. Clearly, bone remodeling associated to bone repair following a damage is not limited to the involvement of MSCs/progenitor cells/osteoblasts, but includes other cells like osteoclasts, which are also provided with P2X7R. Interestingly, activation of this receptor in osteoclasts by high levels of extracellular ATP has a clear inhibitory effect on osteoclastic bone resorption, whereas the pharmacological blockade or the induced deficiency of P2X7R increased the osteoclastic bone resorption with a tendency toward increased survival of these cells [49]. Thus, in a wider scenario, our findings confirm the fundamental role played by the local extracellular concentration of purines in bone healing/remodeling that should be carefully monitored to favor/increase the usefulness of MSCs in bone regenerative medicine.
Abbreviations
- A438079:
-
3-[[5-(2,3-Dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine hydrochloride
- ADO:
-
Adnosine
- ALP:
-
Alkaline phosphatase
- ARS:
-
Alizarin Red S
- BzATP:
-
2′(3′)-O-(4-benzoylbenzoyl)-ATP
- FBS:
-
fetal bovine serum
- MSCs:
-
mesenchymal stromal/stem cells
- NF279:
-
8,8′-[carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino)]bis-1,3,5-naphthalenetrisulfonic acid hexasodium salt
- OPN:
-
osteopontin
- P2X7R:
-
P2X7 receptors
- P2X1R:
-
P2X1 receptors
- P2X3R:
-
P2X3 receptors
- P2X7AR:
-
P2X7 receptor splice variant A
- P2X7BR:
-
P2X7 receptor splice variant B
- qRT-PCR:
-
quantitative real time polymerase chain reaction
- RUNX2:
-
Runt-related transcription factor 2
- S-ASCs:
-
subcutaneous adipose tissue-derived stromal stem cells.
References
Salem, H. K., & Thiemermann, C. (2010). Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells, 28, 585–596. https://doi.org/10.1002/stem.269.
Egusa, H., Sonoyama, W., Nishimura, M., Atsuta, I., & Akiyama, K. (2012). Stem cells in dentistry–part I: Clinical applications. Journal of Prosthodontic Research, 56, 229–248. https://doi.org/10.1016/j.jpor.2012.10.001.
Wagner, W., Horn, P., Castoldi, M., Diehlmann, A., Bork, S., Saffrich, R., Benes, V., Blake, J., Pfister, S., Eckstein, V., & Ho, A. D. (2008). Replicative senescence of mesenchymal stem cells: A continuous and organized process. PLoS One, 3, e2213.
Li, S., Wang, Y., Guan, L., & Ji, M. (2015). Characteristics of human umbilical cord mesenchymal stem cells during ex vivo expansion. Molecular Medicine Reports, 12, 4320–43255. https://doi.org/10.3892/mmr.2015.3999.
Dufrane, D. (2017). Impact of age on human adipose stem cells for bone tissue engineering. Cell Transplantation, 26, 1496–1504. https://doi.org/10.1177/0963689717721203.
Lavoie, J. R., & Rosu-Myles, M. (2013). Uncovering the secretes of mesenchymal stem cells. Biochimie, 95, 2212–2221. https://doi.org/10.1016/j.biochi.2013.06.017.
Lazarowski, E. R. (2012). Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal, 8, 359–373. https://doi.org/10.1007/s11302-012-9304-9.
Noronha-Matos, J. B., & Correia-de-Sá, P. (2016). Mesenchymal stem cells ageing: Targeting the "Purinome" to promote osteogenic differentiation and bone repair. Journal of Cellular Physiology, 231, 1852–1861. https://doi.org/10.1002/jcp.25303.
Fredholm, B. B., IJzerman, A. P., Jacobson, K. A., Linden, J., & Muller, C. E. (2011). International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors–an update. Pharmacological Reviews, 63, 1–34. https://doi.org/10.1124/pr.110.003285.
Burnstock, G. (2014). Purinergic signalling: From discovery to current developments. Experimental Physiology, 99, 16–34. https://doi.org/10.1113/expphysiol.2013.071951.
Gartland, A., Orriss, I. R., Rumney, R. M., Bond, A. P., Arnett, T., & Gallagher, J. A. (2012). Purinergic signalling in osteoblasts. Frontiers in Bioscience, 17, 16–29.
Jorgensen, N. R., Syberg, S., & Ellegaard, M. (2015). The role of P2X receptors in bone biology. Current Medicinal Chemistry, 22, 902–914.
Orriss, I. R., Guneri, D., Hajjawi, M.O.R., Shaw, K., Patel, J. J., &. Arnett, T. R. (2017) Activation of the P2Y(2) receptor regulates bone cell function by enhancing ATP release. The Journal of Endocrinology, 233, 341–356. doi: https://doi.org/10.1530/JOE-17-0042.
Jiang, L.-H., Hao, Y., Mousawi, F., Peng, H., & Yang, X. (2017). Expression of P2 purinergic receptors in mesenchymal stem cells and their roles in extracellular nucleotide regulation of cell functions. Journal of Cellular Physiology, 232, 287–297. https://doi.org/10.1002/jcp.25484.
Gharibi, B., Abraham, A. A., Ham, J., & Evans, B. A. (2011). Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. Journal of Bone and Mineral Research, 26, 2112–2124. https://doi.org/10.1002/jbmr.424.
Carrol, S. H., Wigner, N. A., Kulkarni, N., Johnston-Cox, H., Gerstenfeld, L. C., & Ravid, K. (2012). A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. The Journal of Biological Chemistry, 287, 15718–15727. https://doi.org/10.1074/jbc.M112.344994.
He, W., Mazumder, A., Wilder, T., & Cronstein, B. N. (2013). Adenosine regulates bone metabolism via A1, A2A, and A2B receptors in bone marrow cells from normal humans and patients with multiple myeloma. The FASEB Journal, 27, 3446–3454. https://doi.org/10.1096/fj.13-231233.
Ciciarello, M., Zini, R., Rossi, L., Salvestrini, V., Ferrari, D., Manfredini, R., & Lemoli, R. M. (2013). Extracellular purines promote the differentiation of human bone marrow-derived mesenchymal stem cells to the osteogenic and adipogenic lineages. Stem Cells and Development, 22, 1097–1111. https://doi.org/10.1089/scd.2012.0432.
Agrawal, A., & Gartland, A. (2015). P2X7 receptors: Role in bone cell formation and function. Journal of Molecular Endocrinology, 54, R75–R88. https://doi.org/10.1530/JME-14-0226.
Lenertz, L. Y., Baughman, C. J., Waldschmidt, N. V., Thaler, R., & van Wijnen, A. J. (2015). Control of bone development by P2X and P2Y receptors expressed in mesenchymal and hematopoietic cells. Gene, 570, 1–7. https://doi.org/10.1016/j.gene.2015.06.031.
Jiang, L. H., Baldwin, J. M., Roger, S., & Baldwin, S. A. (2013). Insights into the molecular mechanisms underlying mammalian P2X7 receptor functions and contributions in diseases, revealed by structural modeling and single nucleotide polymorphisms. Frontiers in Pharmacology, 4, 55. https://doi.org/10.3389/fphar.2013.00055.
Sluyter, R. (2017). The P2X7 receptor. Advances in Experimental Medicine and Biology, 1051, 17–53. https://doi.org/10.1007/5584_2017_59.
Surprenant, A., Rassendren, F., Kawashima, E., North, R. A., & Buell, G. (1996). The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science, 272, 735–738. https://doi.org/10.1126/science.272.522.735.
Cheewatrakoolpong, B., Gilchrest, H., Anthes, J. C., & Greenfeder, S. (2005). Identification and characterization of splice variants of the human P2X7 ATP channel. Biochemical and Biophysical Research Communications, 332, 17–27.
Feng, Y. H., Li, X., Wang, L., Zhou, L., & Gorodeski, G. I. (2006). A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. The Journal of Biological Chemistry, 281, 17228–17237.
Adinolfi, E., Cirillo, M., Woltersdorf, R., Falzoni, S., Chiozzi, P., Pellegatti, P., Callegari, M. G., Sandonà, D., Markwardt, F., Schmalzing, G., & Di Virgilio, F. (2010). Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. The FASEB Journal, 24, 3393–3404. https://doi.org/10.1096/fj.09-153601.
Caprara, G. A., Morabito, C., Perni, S., Navarra, R., Guarnieri, S., & Mariggio, M. A. (2016). Evidence for altered Ca2+ handling in growth associated protein 43-knockout skeletal muscle. Frontiers in Physiology, 7, 493 eCollection 2016.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−11CT method. Methods, 25, 402–408. https://doi.org/10.1006/meth.2001.1262.
D’Alimonte, I., Mastrangelo, F., Giuliani, P., Pierdomenico, L., Marchisio, M., Zuccarini, M., Di Iorio, P., Quaresima, R., Caciagli, F., & Ciccarelli, R. (2017). Osteogenic differentiation of mesenchymal stromal cells: A comparative analysis between human subcutaneous adipose tissue and dental pulp. Stem Cells and Development, 26, 843–855. https://doi.org/10.1089/scd.2016.0190.
Bianchi, B. R., Lynch, K. J., Touma, E., Niforatos, W., Burgard, E. C., Alexander, K. M. H., Park, S., Yu, H., Metzger, R., Kowaluk, E., Jarvis, M. F., & van Biesen, T. (1999). Pharmacological characterization of recombinant human and rat P2X receptor subtypes. European Journal of Pharmacology, 376, 127–138.
Kotova, P. D., Bystrova, M. F., Rogachevskaja, O. A., Khokhlov, A. A., Sysoeva, V. Y., Tkachuk, V. A., & Kolesnikov, S. S. (2018). Coupling of P2Y receptors to ca(2+) mobilization in mesenchymal stromal cells from the human adipose tissue. Cell Calcium, 71, 1–14. https://doi.org/10.1016/j.ceca.2017.11.001.
Giuliani, A. L., Colognesi, D., Ricco, T., Roncato, C., Capece, M., Amoroso, F., Wang, Q. G., De Marchi, E., Gartland, A., Di Virgilio, F., & Adinolfi, E. (2014). Trophic activity of human P2X7 receptor isoforms a and B in osteosarcoma. PLoS One, 9, e107224. https://doi.org/10.1371/journal.pone.0107224.
Forostyak, O., Butenko, O., Anderova, M., Forostyak, S., Sykova, E., Verkhratsky, A., & Dayanithi, G. (2016). Specific profiles of ion channels and ionotropic receptors define adipose- and bone marrow derived stromal cells. Stem Cell Research, 16, 622–634. https://doi.org/10.1016/j.scr.2016.03.010.
Ali, S., Turner, J., & Fountain, S. J. (2018) P2Y(2) and P2Y(6) receptor activation elicits intracellular calcium responses in human adipose-derived mesenchymal stromal cells. Purinergic Signal. Aug. 7 [Epub ahead of print] https://doi.org/10.1007/s11302-018-9618-3.
Xing, S., Grol, M. W., Grutter, P. H., Dixon, S. J., & Komarova, S. V. (2016). Modeling interactions among individual P2 receptors to explain complex response patterns over a wide range of ATP concentrations. Frontiers in Physiology, 7, 294. https://doi.org/10.3389/fphys.2016.00294.
Gartland, A., Hipskind, R. A., Gallagher, J. A., & Bowler, W. B. (2001). Expression of a P2X7 receptor by a subpopulation of human osteoblasts. Journal of Bone and Mineral Research, 16, 846–856.
Nakamura, E., Uezono, Y., Narusawa, K., Shibuya, I., Oishi, Y., Tanaka, M., Yanagihara, N., Nakamura, T., & Izumi, F. (2000). ATP activates DNA synthesis by acting on P2X receptors in human osteoblast-like MG-63 cells. American Journal of Physiology. Cell Physiology, 279, C510–C519.
Tamajusuku, A. S., Villodre, E. S., Paulus, R., Coutinho-Silva, R., Battasstini, A. M., Wink, M. R., & Lenz, G. (2010). Characterization of ATP-induced cell death in the GL261 mouse glioma. Journal of Cellular Biochemistry, 109, 983–991. https://doi.org/10.1002/jcb.22478.
Agrawal, A., Henriksen, Z., Syberg, S., Petersen, S., Aslan, D., Solgaard, M., Nissen, N., Larsen, T. K., Schwarz, P., Steinberg, T. H., & Jørgensen, N. R. (2017). P2X7Rs are involved in cell death, growth and cellular signaling in primary human osteoblasts. Bone., 95, 91–101. https://doi.org/10.1016/j.bone.2016.11.011.
Jiang, L. H., Mousawi, F., Yang, X., & Roger, S. (2017). ATP-induced ca(2+)-signalling mechanisms in the regulation of mesenchymal stem cell migration. Cellular and Molecular Life Sciences, 74, 3697–3710. https://doi.org/10.1007/s00018-017-2545-6.
Adinolfi, E., Capece, M., Franceschini, A., Falzoni, S., Giuliani, A. L., Rotondo, A., Sarti, A. C., Bonora, M., Syberg, S., Corigliano, D., Pinton, P., Jorgensen, N. R., Abelli, L., Emionite, L., Raffaghello, L., Pistoia, V., & Di Virgilio, F. (2015). Accelerated tumor progression in mice lacking the ATP receptor P2X7. Cancer Research, 75, 635–644. https://doi.org/10.1158/0008-5472.CAN-14-1259.
Orriss, I. R., Knight, G. E., Ranasinghe, S., Burnstock, G., & Arnett, T. R. (2006). Osteoblast responses to nucleotides increase during differentiation. Bone, 39, 300–309.
Orriss, I. R., Key, M. L., Brandao-Burch, A., Patel, J. J., Burnstock, G., & Arnett, T. R. (2012). The regulation of osteoblast function and bone mineralisation by extracellular nucleotides: The role of P2X receptors. Bone, 51, 389–400. https://doi.org/10.1016/j.bone.2012.06.013.
Panupinthu, N., Rogers, R. J., Zhao, L., Solano-Flores, L. P., Possmayer, F., Sims, S. M., & Dixon, S. J. (2008). P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: A signaling axis promoting osteogenesis. The Journal of Cell Biology, 181, 859–871. https://doi.org/10.1083/jcb.200708037.
Noronha-Matos, J. B., Coimbra, J., Sa-e-Sousa, R. R., Marinhas, J., Freitas, R., Guerra-Gomes, S., Ferreirinha, F., Costa, M. A., & Correia-de-Sá, P. (2014). P2X7-induced zeiosis promotes osteogenic differentiation andmineralization of postmenopausal bone marrow-derived mesenchymal stem cells. The FASEB Journal, 28, 5208–5222. https://doi.org/10.1096/fj.14-257923.
Gangadharan, V., Nohe, A., Caplan, J., Czymmek, K., & Duncan, R. L. (2015). Caveolin-1 regulates P2X7 receptor signaling in osteoblasts. American Journal of Physiology. Cell Physiology, 308, C41–C50. https://doi.org/10.1152/ajpcell.00037.2014.
Di Virgilio, F., Schmalzing, G., & Markwardt, F. (2018). The elusive P2X7 macropore. Trends in Cell Biology, 28, 392–404. https://doi.org/10.1016/j.tcb.2018.01.005.
Mackenzie, A. B., Young, M. T., Adinolfi, E., & Surprenant, A. (2005). Pseudoapoptosis induced by brief activation of ATP-gated P2X7 receptors. The Journal of Biological Chemistry, 280, 33968–33976.
Miyazaki, T., Iwasawa, M., Nakashima, T., Mori, S., Shigemoto, K., Nakamura, H., Katagiri, H., Takayanagi, H., & Tanaka, S. (2012). Intracellular and extracellular ATP coordinately regulate the inverse correlation between osteoclast survival and bone resorption. The Journal of Biological Chemistry, 287, 37808–37823. https://doi.org/10.1074/jbc.M112.385369.
Funding
This work was supported by funds for research to RC and PDI from the University of Chieti-Pescara.
Author information
Authors and Affiliations
Contributions
FC, PDI and RC conceived and designed the experimental work, assembled and supervised the overall project, analyzed and interpreted the data and finalized the manuscript. MC initiated the project and along with SZ, MZ, PG, CM, ML, MAM, EA and EO performed the experimental work, assembled and analyzed the data, drafted/revised the manuscript. All authors have read and approved the manuscript for publication.
Corresponding author
Ethics declarations
Consent for Publication
This manuscript has been approved by all authors and is solely the work of the authors named.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marzia Carluccio and Mariachiara Zuccarini equally co-authored
Electronic supplementary material
Expression of P2X1R and P2X3R in S-ASCs along their osteogenic differentiation in the presence or absence of BzATP.
Protein levels of P2X1R and P2X3R were determined by Western blot analysis (60 μg of proteins were loaded per lane). Immunoblots were obtained by exposing membranes to two antibodies, recognizing proteins (the monomeric subunit of each receptor) at 50 KDa (A) and about 60 KDa (B), respectively. Subsequently, immunoblots were reprobed with an antibody against β actin, to verify equal sample loading, and quantified by densitometric analysis, the values of which, normalized to β actin, are reported as such in the two lower histograms, whereas in the upper histograms they are reported assuming the values of cells at the day 0 equal to 1. All values are the mean ± SEM of three independent experiments with very similar results. *P < 0.05, **P < 0.01, ***P < 0.001: statistical significance vs. S-ASCs at the day 0 (in the upper histograms) or vs. cells grown in osteogenic medium for 7 days (in the lower histograms); #P < 0.05, ###P < 0.001: statistical significance of values measured in cells exposed to BzATP vs the corresponding untreated cells grown in osteogenic medium (one-way ANOVA plus Dunnett’s test). (PPTX 326 kb)
Rights and permissions
About this article
Cite this article
Carluccio, M., Zuccarini, M., Ziberi, S. et al. Involvement of P2X7 Receptors in the Osteogenic Differentiation of Mesenchymal Stromal/Stem Cells Derived from Human Subcutaneous Adipose Tissue. Stem Cell Rev and Rep 15, 574–589 (2019). https://doi.org/10.1007/s12015-019-09883-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-019-09883-6