Abstract
Wnt signaling plays an important role in development and disease. In this review we focus on the role of the canonical Wnt signaling pathway in somatic stem cell biology and its critical role in tissue homeostasis. We present current knowledge how Wnt/β-catenin signaling affects tissue stem cell behavior in various organ systems, including the gut, mammary gland, the hematopoietic and nervous system. We discuss evidence that canonical Wnt signaling can both maintain potency and an undifferentiated state as well as cause differentiation in somatic stem cells, depending on the cellular and environmental context. Based on studies by our lab and others, we will attempt to explain the dichotomous behavior of this signaling pathway in determining cell fate decisions and put special emphasis on the interaction of β-catenin with two highly homologous co-activator proteins, CBP and p300, to shed light on the their differential role in the outcome of Wnt/β-catenin signaling. Furthermore, we review current knowledge regarding the aberrant regulation of Wnt/β-catenin signaling in cancer biology, particularly its pivotal role in the context of cancer stem cells. Finally, we discuss data demonstrating that small molecule modulators of the β-catenin/co-activator interaction can be used to shift the balance between undifferentiated proliferation and differentiation, which potentially presents a promising therapeutic approach to stem cell based disease mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction/Background
By definition, stem cells are primitive cells that have the capability to self-renew (i.e. give rise to at least one identical daughter cell) as well as differentiate into more mature, specialized cell types. It is these two hallmark characteristics, self-renewal and diverse differentiation potential, that have spawned widespread interest in stem cell biology and engendered formidable research efforts. Stem cells come in different flavors and are generally classified according to their origin, which also dictates their biological capabilities. They can be of embryonic origin, hence embryonic stem cells (ESC), or of adult tissue origin, termed somatic stem cells (SSC). ESC can differentiate into any tissue type found in the adult organism and are therefore termed pluripotent (1, 2). Somatic stem cells have undergone a partial differentiation process, restricting their differentiation potential, and are hence called multi-, oligo- or bipotent (3, 4). Despite some evidence regarding trans-differentiation (5), most SSC normally give rise only to cells of the tissue or organ in which they are found (e.g. hematopoietic stem cells, neuronal stem cells, intestinal stem cells, etc.) and are critical in both tissue homeostasis and regeneration after injury (6–8). Recently, SSC have also gained prominence in the field of cancer research. Following malignant transformation, so called cancer stem cells (CSCs) are believed to play a major part in tumor initiation, therapy resistance and ultimately relapse (9). Consequently, one key focus in cancer research over the past decade has been to prospectively identify CSCs and to find therapeutic strategies to safely eliminate this cell population. A major hurdle to this goal lies in the identification of the key mechanisms that control survival and proliferation of CSC, which distinguish them from normal endogenous tissue stem cells. Increasingly, the basic molecular signaling networks governing stem cell behavior are coming into focus, providing critical knowledge about basic mechanisms that regulate cellular potency and differentiation. Intriguingly, the same evolutionarily conserved signaling pathways, which govern embryonic development, appear to control the behavior of both normal stem cells as well as cancer stem cells. The Wnt/β-catenin (10, 11), Hedgehog(12), and Notch(13) pathways have all been implicated in stem cell and cancer stem cell biology (see also(14)). In this review, we will focus on various aspects of SSC biology and will discuss the importance of Wnt/β-catenin signaling, which has emerged as a key player in stem cell biology, in tissue homeostasis and regeneration (15–17) on one hand, as well as malignant transformation and cancer on the other. Mounting evidence suggests that aberrant activation of these signaling pathways play critical roles in malignant cell transformation and neoplastic proliferation (15, 17–19). Controlled pharmacologic manipulation of stem cell proliferation and differentiation offers tremendous potential for regenerative medicine as well as the treatment of malignancies.
The Wnt Pathway
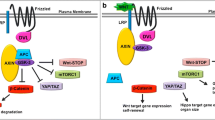
The Wnt-signaling pathway is an evolutionarily conserved and complex signaling cascade with important functions in both development and disease (17, 18, 20, 21). Its pivotal role in development and organismal homeostasis is strikingly illustrated by the diversity of fundamental cellular processes directed by the Wnt-pathway, such as cell fate determination during embryonic development, cellular polarity, cell proliferation, cell cycle arrest and differentiation as well as apoptosis and tissue homeostasis (22). Accordingly, aberrant regulation can cause severe developmental defects and has been linked to multiple disease processes, most notably cancer (20, 21). The pathway was first discovered in Drosophila (and termed wingless, because of it’s role as a morphogen in wing development), and later in the mouse (termed int1, where it was discovered due to its ability to promote tumor formation). Further research showed that both belong to the same evolutionary highly conserved signaling network, now commonly known as the Wnt-pathway (15, 23). Three ‘branches’ are often described: (1) the canonical Wnt pathway, which acts through the transcriptional activity of β-catenin, which will be discussed in more detail in this review article; plus two β-catenin- independent pathways: (2) the non-canonical planar cell polarity pathway, which effects cytoskeleton and cell shape (24, 25), and the (3) the non-canonical Wnt/calcium pathway (26, 27). Although these classifications are helpful in describing the distinct roles and functions of the Wnt pathway, it is important to note that these signaling events are not mutually exclusive — rather they are highly dynamic and coupled, with cross-talk occurring between all three ‘branches’ that is dependent on cell- or tissue-type and defined stages of development (28). Wnt-signaling involves a network of extra- and intracellular molecules. A family of 19 secreted glycoproteins constitutes the mammalian Wnt family, all of which are characterized by a series of conserved cysteine residues (29). Signal transduction is mediated by the binding of Wnt glycoproteins to a family of seven-pass transmembrane spanning receptors termed frizzled (Fz) receptors (16, 30). Several other membrane-bound proteins that function as co-receptors have been identified, such as members of the low-density lipoprotein (LDL) receptor-related protein (LPR5/6), as well as additional non-classical Wnt receptors (e.g. Ryk, Ror). When activated by Wnt binding, frizzled proteins recruit the scaffold protein Dishevelled, which is required for relaying the signal to all three signaling branches (18). In canonical Wnt-signaling, Dishevelled binding disrupts the β-catenin destruction complex (GSK-3β, APC and Axin), thereby preventing β-catenin phosphorylation and degradation and allowing β-catenin to accumulate in the cytoplasm. Subsequently β-catenin translocates to the nucleus where it forms a transcriptionally active complex and drives the expression of Wnt target genes (16, 31, 32). A key step in transcriptional activation is the formation of a complex between β-catenin and members of the T-cell factor (TCF)/lymphoid enhancer factor (LEF) family of transcription factors. The TCF family members alone have no transcriptional activation functions and are bound by inhibitors such as Groucho, CtBP or HBP1 (33–35). To generate a transcriptionally active complex, TCF/β-catenin recruits the transcriptional co-activator CREB-binding protein (CBP) or its closely related homolog p300, as well as other components of the basal transcription machinery, to initiate transcription (31, 32, 36) (Fig. 1). Beyond classical TCF driven gene-expression, it should be noted that the transcriptional role of β-catenin extends beyond the TCF/LEF family as β-catenin can partner with many other transcription factors (e.g. FOXOs, Nuclear Receptors, Sox, Smad, Oct4) that play important roles in stem cell biology (37–43).
Canonical Wnt signaling. a Without the binding of Wnt ligands to frizzled receptors, a multi-protein complex in the cytoplasm tightly regulates cellular β-catenin levels. This so-called destruction complex consists of Axin-1 and its interacting partners tumor suppressor adenomatous polyposis coli (APC), glycogen synthase kinase 3 beta (GSK3B) and casein kinase 1 (CK1). The complex degrades β-catenin by targeting it for ubiquitination and proteasomal degradation via phosphorylation on several amino acids, thereby maintaining low levels of β-catenin. Without nuclear β-catenin, transcription factors (TF) such as TCF/LEF form a repressive complex via binding to transcriptional co-repressors such as groucho, thereby inhibiting Wnt target gene expression. b Binding of Wnt proteins to the receptor/co-receptor complex of frizzled/Lrp5/6 leads to the recruitment of a negative regulator of the destruction complex called Dishevelled (Dsh). Dsh ultimately leads to the degradation of Axin and inactivation of GSK3B, thereby inhibiting their interaction with other components of the destruction complex. Disruption and sequestration of the destruction complex in turn allows β-catenin to accumulate in the cytoplasm and subsequently translocate to the nucleus. A transcriptionally active complex forms between β-catenin, transcription factors (e.g. of the TCF/Lef family) and co-activators (Co-TA) such as CBP and p300, driving the expression of Wnt target genes
CBP and p300
The transcriptional co-activators CBP and p300 are highly homologous Kat3 protein acetyltransferases that possess several conserved domains that bind a variety of transcriptional regulators and other proteins (44, 45). The ability of these multidomain proteins to acetylate histones and other proteins and serve as master organizers of many transcriptional events is critical for a wide array of biological processes (45, 46). Genetic alterations in both genes leading to functional inactivation have been linked to the rare human disease Rubinstein-Taybi syndrome (47). Gene dosage of both CBP and p300 are critical in mammalian development, as various knockout and mutagenesis studies in mice have demonstrated. Homozygous knock out embryos for p300 (−/−) die at or before E11.5 with severe central nervous system (CNS) and heart abnormalities, while p300 heterozygotes (+/−) exhibit neural tube closure defects and considerable lethality in utero (48). Curiously, compound CBP+/−, p300+/−mouse embryos display a phenotype similar to p300−/− or CBP−/−mouse embryos (49). Despite their high degree of homology, our group and others have demonstrated distinct, unique and non-redundant functions of CBP or p300 in the regulation of gene transcription, stem cell growth, differentiation as well as development (48, 50–52). For example, utilizing a hematopoietic stem cell (HSC) model, Rebel et al. concluded that CBP is essential for HSC self-renewal, whereas p300 is critical for proper hematopoietic differentiation (50). Ugai et al. found that p300, but not CBP, is absolutely required for RA-induced F9 differentiation (52). Our group has shown that Wnt/TCF/β-catenin mediated Survivin transcription provides evidence for non-compensatory roles for CBP and p300 (53).
Somatic Stem Cells (SSC)
The first concrete evidence for the existence of somatic stem cells (alternatively termed adult stem cells or tissue stem cells) came from the pioneering work of McCulloch and Till on mouse bone marrow stem cells (54). Subsequent research has identified SSC in many organs and tissues, including liver (55), gut (56), lung (57), heart (58), and CNS (59). Tissue stem cells have the ability to self-renew and proliferate as well as differentiate in a restricted manner (60, 61). They are understood to be the source of naturally occurring tissue regeneration and repair in adult tissues (60). The dichotomy between self-renewal and proliferation on the one hand and differentiation on the other is bridged by the ability of stem cells to switch between different modes of cell division: symmetric and asymmetric. Symmetric cell division, which is not unique to stem cells, can be further subdivided into differentiative or non-differentiative symmetric division (for detailed review, see (62)). The first produces two identical daughter cells with reduced differentiation potential and a higher degree of specification, while the later results in two daughter cells without changes in differentiation potential, thereby increasing the pool of stem cells. (Fig. 2B) Asymmetric division on the other hand results in the production of two distinct daughter cells: one retaining the characteristics of the parental (stem-) cell, the other entering differentiation and exiting the stem cell niche (Fig. 2A). Considerable efforts have been devoted to deciphering the molecular mechanisms that regulate SSC plasticity and to exploit their potential for therapeutic purposes. In particular evolutionary conserved developmental pathways have been implicated in the self-renewal and organ specific differentiation of somatic “stem/progenitor” cells (for review, see (63, 64)).
Mode of division. a and b. Stem cells (blue) have the unique capacity to either divide asymmetrically (panel A) or symmetrically (B). Asymmetric cell division will yield to different progenitors, one that maintains the same state of potency as the parental cell (blue), the other which will reduce its level of potency and initiate differentiation (green), first through a state of high proliferative capacity termed transiently amplifying state, and subsequently towards a terminally differentiated cell type (which many times will exit the cell cycle and remain in G0). Symmetric cell division on the other hand dictates that both progeny are identical, either maintaining the same differentiation status as the parental cell (blue) or reducing their level of potency through the initiation of differentiation (green) c After malignant transformation (red), stem cells basically maintain the same modes of division, albeit with a misregulation in terms of the divisions required for tissue homeostasis. Mutated stem cells may acquire increased potential to undergo non-differentiative symmetric cell divisions or will undergo differentiative symmetric or asymmetric cell divisions, but loose their ability to properly differentiate. The resulting undifferentiated or poorly differentiated progenitor cells also lack the capacity to terminally differentiate and maintain high proliferation capacity. Furthermore, partially differentiated cells might undergo and EMT-like process, leading to a reverse in differentiation and the acquisition of more stem-like properties. These mis-regulated cellular division events ultimately lead to the expansion of a tumor mass and the development of cancer
Wnt-Signaling in Somatic Stem Cells
The Wnt-signaling pathway has emerged as a pivotal player in the specification and maintenance of stem cell lineages and has been shown to have an important role in multiple stem cell compartments in a wide array of tissues and organs (65, 66) (67–71). For example, in 1998 the Clevers’ laboratory reported that elimination of the β-catenin interaction partner Tcf4 in mice resulted in the complete absence of the stem cell compartment in the small intestine (54, 72). Further work has shown that canonical Wnt signaling cooperates with BMP and Notch signaling in the intestinal stem cell niche to control stem cell self-renewal (69). The small intestine is organized into villi (apical) and crypts (basal) that are composed of various cell types. In particular, intestinal stem cells (ISC) and paneth cells require canonical Wnt-signaling for establishment and maintenance. ISC reside in intestinal crypts (73) and their proliferation is Wnt dependent (74). The loss of positive Wnt regulators, such as TCF4 or β-catenin, as well as the overexpression of negative Wnt regulators, such as Dickkopf1 (Dkk1), dramatically decreases the proliferation capacity of this cell compartment (72, 75). Two distinct stem cell populations have been described: (1) +4 label retaining cells (LRC) (76), which mainly remain in G0 and are apparently activated only during injury (76). These cells are characterized by the stem cell marker Bmi1 (77). (2) Crypt basis columnar cells (CBC), which can be identified by the expression of Lrg5 (an orphan G-protein coupled receptor) (77, 78). CBC continuously cycle and are responsible for sustained tissue homeostasis. Lgr5 is transmembrane receptor Wnt/β-catenin target gene that can associate with Wnt/β-catenin/Fzd/Lrp, thereby amplifying Wnt-signaling in CBC via a positive feedback loop (79). Paneth cells, through their secretion of various Wnt factors (Wnt3, 6, 9b, Fzd 4, 6, 7, Lrp5, Sfrp5) (80), are apparently an important source for Wnt ligands, which are crucial for the maintenance of ISCs (81). Supporting this notion is the fact that depletion of PC’s leads to a decrease in the number of ISCs (81). Wnt-signaling is also critical for expression of the gene Sox9, which is important for PC lineage commitment (82, 83).
In the hematopoietic system, Wnt3a has been implicated in self-renewal and proliferation (68, 84). Regulation of hematopoietic stem/progenitors, as well as lineage commitment of progenitors during hematopoiesis is highly Wnt dependent (85, 86). Survivin expression, which we have demonstrated is a Wnt/CBP/β-catenin regulated gene (53), is important during hematopoiesis and is prominently up-regulated in CD34+ hematopoietic stem/progenitor cells upon growth factor treatment (87). Survivin-deficient hematopoietic progenitors show defects in erythroid and megakaryocytic formation (88).
Canonical Wnt signaling is crucial for heart development (67, 89), but is usually shut-off in the adult heart (90, 91). Interestingly, several studies have shown that inhibition of Wnt signaling may yield a more favorable outcome in cardiac repair after ischemic injury (92). Transgenic mouse models for β-catenin (93) or disheveled knockdown demonstrated less scarring and better remodeling following injury. A study by Saraswati et al. provided evidence that a small molecule CK1α activator, pyrvinium, inhibited “canonical Wnt signaling” and thereby reduced adverse remodeling and improved cardiac function (94). Our group has recently demonstrated that Wnt signaling is important for physiological heart repair and that use of the co-activator CBP or p300 fundamentally and differentially influence the outcome (95). Sasaki et al. demonstrated that the disruption of CBP/β-catenin interaction via the small molecule inhibitor ICG-001 led to increased p300/β-catenin interaction in epicardial progenitor cells and resulted in a significant improvement of cardiac function in female rats post-infarction compared to vehicle control treated animals.
Wnt signaling has been implicated in mammary gland development and cell transformation (17, 65, 96, 97). Ectopic expression of ΔNβ-catenin (98) or Wnt1 (99) leads to ductal hyperplasia, while loss of function in β-catenin (using a dominant negative variant) has been shown to exert a negative effect on breast tissue development during pregnancy, particularly lobuloalveolar proliferation (100). Overexpression of inhibitors (such as Axin (101)) or loss of Lef1 function inhibits mammary differentiation of precursor cells (102). The bilayered mammary epithelium consists of luminal cells (Ck8+, Muc1+) and basal cells (Ck5+, p63+). Of these two cell types, the basal cells have been shown to express both Lrp5 and 6 (103), obligate canonical Wnt signaling receptors (70). Ductal mammary stem cells comprise a sub-population of basal epithelial cells and are capable of regenerating cleared mammary fat pads (104). Knockout studies for Lrp5 (105) and loss of function mutation for Lrp6 (106) receptor species showed significantly reduced activity in this cell compartment and impaired gland branching, suggesting impaired stem cell function.
Finally, Wnt-activity has been implicated in neuronal stem cell biology (107). The small molecule inhibitor XAV939, which stabilizes Axin2 and amplifies negative feedback signals in Wnt/β-catenin signaling, leads to accelerated differentiation of spinal cord stem cells and improved myelination after hypoxic and demyelinating injury (108). Our group had also previously shown that inhibition of CBP/β-catenin interaction can rescue neuronal differentiation defects in an Alzheimer’s Disease model (109).
Cancer Stem Cells (CSC)
Increasing evidence suggests the existence of a small subgroup of cells in cancer, termed cancer stem cells (CSC) or alternatively tumor initiating cells (TIC). The presence of CSC has forced a paradigm shift from the earlier model of tumor homogeneity towards one of “hierarchal clustering” in tumors, where CSCs play the central role in carcinogenesis (110, 111). The cancer stem cell concept postulates that the bulk tumor consists of rapidly proliferating and differentiated (albeit aberrantly or only partially differentiated) cells, with a small population of CSCs that provides for the long-term maintenance of the tumor. These cells are able to self-renew (112, 113), actively express telomerase (114) and activate anti-apoptotic and multidrug resistance pathways. They may remain relatively quiescent, but can give rise to rapidly dividing progeny, which form the bulk of tumor cells (Fig. 2C). Due to these characteristics, CSC are thought to be responsible for tumor initiation, progression and relapse, as well as metastasis and drug resistance (9, 115–118) . Supporting evidence exists that a stem-like signature contributes to cancer aggressiveness and is related to poor outcome (119). Although CSC resemble tissue stem cells in several characteristics, such as self-renewal and differentiation potential, Wicha et al. (120) pointed out that the term ‘cancer stem cell’ does not necessarily refer to the cell of origin, but rather refers to cells that have stem like properties. CSC could originate from tissue stem cells, transiently amplifying cells or potentially even differentiated cells. Vassiliou et al.(121) and others (122) suggest that stem cells, due to their longevity and self-renewing properties, may be more susceptible to minor genetic changes and have a far greater propensity to accumulate carcinogenic mutations (genetic mutations, epigenetic changes), which could markedly influence the behavior of those cells (e.g. accelerate self-renewal through a switch from asymmetric to symmetric division (112)). As stated above, it is possible that initial mutations occur in tissue stem cells, but final mutations that confer CSC properties might occur during neoplastic transformation in downstream progeny (123). Mutations that block differentiation have been identified (e.g. in breast cancer (124, 125)), which lead to an increase in stem/progenitor cells at the expense of differentiated cells (124). (Fig. 2C) It has also been speculated that cellular de-differentiation towards a cancer stem cell phenotype is involved in poorly differentiated cancer types. Observations that cancer cells can undergo a process called epithelial-mesenchymal-transition (EMT), which plays a critical role in development and other physiological processes such as wound healing or gastrulation, has led to the hypothesis that EMT plays a critical part in the formation of CSC (126, 127). Experimental evidence demonstrated that EMT can cause de-differentiation of cancer cells, involving a loss of epithelial polarity, and resulting in increased migratory and tumor initiating potential (128–130) although these may not be required for “stemness” per se. Several markers of EMT (e.g. ZEB1/2, Twist, Snail and Slug) have been described to be misregulated in various malignancies (131).
Despite still existing controversy regarding the CSC hypothesis (132), it is clear that distinct cancer cell populations have enhanced tumorigenic capacity compared to bulk tumor cells. Findings of cancer cells with enhanced tumor initiating properties were first reported in leukemia. Bruce et al. demonstrated that only a small subgroup of cells showed extensive proliferation in vivo and in vitro (133). In 1997 John Dick and colleagues isolated CSCs (known as leukemic stem cells, or LSCs) from bulk acute myeloid leukemia cells (134). Such LSC maintained or reacquired the ability to proliferate indefinitely while losing the ability to properly differentiate (113). Over the past decade, a large number of studies have identified CSC in several solid tumors, including brain tumors (135), melanoma (136), breast (137), liver (138), pancreatic (137) and colon cancer (139).
Wnt-Signaling and Cancer Stem Cells
Aberrant Wnt signaling has been shown to be involved in many malignancies (17). Considering the importance of the Wnt-pathway in stem cell biology, it is not surprising that aberrant Wnt signaling has been implicated in the tumorigenic potential of stem cells. A study by Blum et al. elegantly demonstrated the involvement of Wnt-signaling in tumor formation through stem cells by showing that continued expression of Survivin (a Wnt target gene) upon differentiation of hES cells in vivo is associated with enhanced teratoma formation (140). Telomerase activity endows cells with unlimited self-renewal capacity and is overexpressed in many cancers. Recently, it has been shown that Wnt/β-catenin signaling plays a role in telomerase expression (141). The process of EMT has been directly connected to activated β-catenin signaling (131, 142). Conacci-Sorrell et al. showed that slug, a strong inducer of EMT in tumors, is associated with nuclear accumulation of transcriptionally active β-catenin. Over-expression of EMT inducing factors twist and snail (both putative Wnt target genes) increases the expression of CSC markers (126). The functional connection between enhanced nuclear β-catenin signaling and EMT is further strengthened by the increasing number of β-catenin target genes (e.g. S100A4, fibronectin, L1CAM, CD44, MMP7, uPAR, etc.) whose expression is associated with invasion, migration and metastases (143–145). Moreover, Cdx-1 (146) and Id2 (147), two transcription factors associated with prevention of epithelial differentiation and maintenance of a more “stem like” state, have been identified as β-catenin-mediated genes. A typical approach to identify putative cancer stem cells is the use of cell surface markers (148), many of which have been found on normal tissue stem cells. Interestingly, many of these markers are direct Wnt targets (including LGR5/GPR49 (78), CD44 (149), CD24 (150), CD133 (151), ABC cassette genes (152, 153) and EpCAM (154, 155)). In fact, the first evidence for the existence of CSCs in solid tumors emerged from studies in breast cancer by Al hajj et al. (156), who showed that cells that are CD44highCD24low possess tumor-initiating capacity. Many subsequent studies confirmed these findings, showing that especially more aggressive breast cancer subtypes are characterized by a less differentiated phenotype and a higher percentage of CSC (157–159) . It has long been known that mis-expression of Wnt-ligands induces mammary adenocarcinomas. Pioneering work by Nusse and Varmus in 1982 showed that transgenic MMTV-Wnt1 mice, in which Wnt-signaling is constitutively activated, develop spontaneous mammary tumors (160). Further studies showed that canonical Wnt signaling is frequently up-regulated in breast tumors, particularly in aggressive, less differentiated basal type tumors (18, 161–163). Interestingly, these tumors show a stem cell-like transcriptional signature (164). Another hallmark of aggressive breast cancers is their enrichment of an EMT-like signatures (142, 165). Wnt1 has been shown to up-regulate twist (166), thereby favoring an EMT like process in breast cancer cells (167). In the case of Wnt/β-catenin driven transcription, β-catenin is relocated from the cytoplasm to the nucleus. This “loss” of cytoplasmic β-catenin leads to a disruption in cell polarity, resulting in EMT and a CSC-like phenotype with a significant increase in the CD44high, CD24low population (168)
The cell surface protein CD133, is expressed by normal primitive cells of the neural, hematopoietic, epithelial and endothelial cell lineages (139, 169–173). Recently, enrichment of CD133+ cells in colorectal cancer (CRC) samples has been shown to enrich for a population of CSC/TICs (139). Additionally, it has been shown that high levels of nuclear β-catenin characterize these stem-like cancer cells (174). Van de Wettering et al. showed that Wnt signaling imposes a progenitor cell phenotype on CRC cells and that the TCF4/β-catenin complex functions as a master regulator of proliferation and differentiation (175). It has been shown that Lrg5/GPR49 is overexpressed in the majority of colon tumors examined, compared to normal control tissue (173). Several studies have revealed that membrane transporters, including MDR-1, ABCG2, ABCA3 and BRCP1, are intrinsically expressed in stem/progenitor cells from multiple adult tissues and that they contribute to the side population (SP) phenotype of stem cells (176). The expression of these so-called multi-drug resistance genes has been shown to be related to therapy response and also identifies putative cancer stem cells (117, 177, 178). Wnt/β-catenin signaling appears to play an important role in ABCB1/MDR-1 transcription and putative TCF binding elements were identified in the ABCB1 promoter (−1,813 to −275 bp) (153). The side population assay has been applied to identify rare, drug resistant hematopoietic cancer stem cell/tumor initiating cell (CSC/TIC) populations (179). Such hematopoietic CSC/TIC populations, have been shown to be Wnt/β-catenin dependent (180). Furthermore, many Wnt signaling related genes are up-regulated in hematopoietic malignancies (181) and epigenetic silencing of negative regulators of the Wnt signaling cascade is frequently associated with leukemias, including CML (182). In a recent study, we demonstrated that drug resistant clones in ALL, which exhibit stem cell characteristics, are maintained by CBP/catenin driven gene transcription. Disruption of the CBP/catenin interaction leads to sensitization to chemotherapy and elimination of therapy resistant ALL clones (183).
Orchestrating Wnt/β-Catenin Signaling
Proliferation versus differentiation: a question of co-activator usage
It is quite clear that Wnt-signaling is important in stem cell biology. Coordination of the Wnt pathway is critical in regulating cell fate determination and migration during gastrulation and other developmental processes (184). Nevertheless there is no consensus as to whether Wnt is important for maintenance of the stem cell state (unlimited proliferation, pluripotency, multipotency), or the differentiation of stem/progenitor cells, or whether it is important for both by orchestrating cellular development in a context dependent manner (for ref. see (32, 185)). Wnt/β-catenin signaling has been demonstrated to expand undifferentiated stem cell populations (71, 186, 187). Yet it has been demonstrated to be essential for cellular differentiation of ES cells and plays a critical role in fate decision and lineage commitment (71, 89, 188–190). The dichotomous behavior of Wnt/β-catenin signaling in controlling both proliferation and differentiation has provoked substantial controversy. Throughout this review, we have provided several examples that illustrate the importance of the co-activator proteins CBP and p300 in controlling the outcome of Wnt/β-catenin signaling (also (191)). Using a chemical genomic approach, our group first identified the selective antagonist of the Wnt/CBP/β-catenin interaction, ICG-001, which led to the development of a model to explain the divergent activities of Wnt/β-catenin signaling (192, 193). Blocking the CBP/β-catenin interaction disrupts a subset of TCF/β-catenin responsive genes (i.e. Survivin, cyclin D1, axin2, hNkd) whereas it does not interfere or actually increases the expression of other TCF/β-catenin responsive genes (i.e. c-jun, fra-1, EphB2, Brachury T) (53, 109, 192–194). We proposed a distinct role for the co-activators CBP and p300 in the Wnt/β-catenin signaling cascade. CBP/β-catenin-mediated transcription is essential for stem and/or progenitor cell maintenance and proliferation, whereas a switch to p300/β-catenin-mediated transcription is the first critical step to initiate differentiation and a decrease in cellular potency. Accordingly, a change in co-activators interacting with β-catenin (or catenin-like molecules in the absence of β-catenin) (195), and more generally the basal transcriptional apparatus (196), is critical for a stem/progenitor cell deciding to either maintain its level of potency or to go on to differentiate. Several groups, including our own have shown that CBP/β-catenin mediated transcription maintains stem-cell gene expression (e.g. expression of Oct4, Survivin (197, 198)) and undifferentiated cell proliferation (199, 200) . Interestingly, our work showed that blocking the CBP/β-catenin interaction causes an increase in the p300/β-catenin interaction, leading to differentiation and a decrease in cellular potency (e.g., increasing the expression of c-jun, fra-1, etc.) (109). Endogenously, co-activator usage could be controlled via posttranslational modifications, as a substantial number of phosphorylation sites exist in the amino terminal regions of both CBP and p300, the region that interacts with beta-catenin (201).
Our model proposes that activation of the CBP/β-catenin arm is the default active pathway in stem cells which maintains a non-differentiative proliferative state. In order for regular development to proceed, cells must exit the cell cycle and initiate the process of differentiation (109). It is quite intriguing to speculate that an aberrant increase of the CBP/catenin interaction at the expense of the p300/catenin interaction increases the number of symmetric (non-differentiative) divisions at the expense of asymmetric divisions, leading to an inability to properly initiate and complete differentiation of somatic stem and/or progenitor as the underlying malfunction in essentially all cancers. Evidence supporting this proposition comes from a study by Cicalese et al., which suggests that asymmetric cell division may function as a tumor suppressor mechanism (202). We speculate that a wide range of mutations (some of which are cell type or tissue specific; e.g., bcr/abl, K-Ras, Her2, etc.) can lead to aberrant regulation of the underlying equilibrium between catenin/CBP and catenin/p300; i.e., between proliferation and maintenance of potency and the initiation of differentiation (Fig. 3). In support of our model, we have found that ICG-001, which selectively blocks CBP/β-catenin interaction, has differentiating effects on a wide array of stem/progenitor cells, including cancer stem cell populations (109, 183, 195).
p300/CBP differential co-activator usage model. Upon nuclear localization, the effect of β-catenin driven gene expression and cell fate decision is dependent on its association with either co-activator CBP or p300, which are critical for full activation of the transcriptional complex composed of β- catenin and TCF/Lef transcription factors. Binding with CBP maintains cell proliferation and inhibits differentiation while binding to p300 triggers the cell to exit the cell cycle and initiate differentiation. Via this differential co-activator usage, Wnt/β-catenin signaling can shift the balance between two fundamental cellular decisions, either to proliferate in an undifferentiated state (CBP/β-catenin signaling) or differentiation and cell fate decision (p300/β-catenin signaling). Many different internal and external factors may ultimately be combined and “funneled” into a relatively simple bimodal switch controlling cell fate decision
Concluding Remarks
Stem cells, particularly adult stem cells, play an important role in tissue regeneration and disease. Their fate is controlled in large part by developmentally preserved signaling pathways. One of these, the canonical Wnt pathway, has a prominent role in stem cell proliferation and differentiation. Aberrant regulation has been shown to effect stem cell behavior and can lead to the development of cancer. The effects of Wnt/β-catenin signaling are not merely dependent on a simple ‘on/off’ principle, but rather on the orchestration of different ‘arms’ in a temporally and spatially controlled fashion. A delicate equilibrium between the co-activator proteins CBP and p300 seems to be critically important for fundamental decision making in stem cells: namely whether to maintain potency and to proliferate or to initiate differentiation. This critical decision-making has important downstream effects on tissue homeostasis, repair and cancer development, which are essentially all stem cell related. Organized regulation of the equilibrium between CBP/β-catenin and p300/β-catenin signaling is important for normal development, while aberrant regulation leads to disease, including cancer. Our group has demonstrated that specific small molecule inhibitors of these interactions can be used to influence stem cell behavior, causing a switch between stem cell maintenance and differentiation. With the development of the second generation CBP/catenin antagonist PRI-724, by Prism Pharmaceuticals, and the initiation in 2011 of human clinical trials, this discovery moved from the bench to the bedside. We believe that the ability to pharmacologically regulate critical decision points in stem cell biology will have important implications for both regenerative medicine and cancer therapy.
Abbreviations
- CSC:
-
Cancer stem cell
- LSC:
-
Leukemic stem cell
- TIC:
-
Tumor initiating cell
- EMT:
-
Epithelial-mesenchymal transition
- SSC:
-
Somatic stem cell
- CBP:
-
CREB-binding protein
- p300:
-
E1A binding protein p300
- TCF:
-
T-cell factor
- LEF:
-
Lymphoid enhancer factor
- Fzd:
-
Frizzled
- APC:
-
Adenomatous polyposis coli
- GSK-3β:
-
Glycogen synthase kinase 3
- CNS:
-
Central nervous system
- Lrp:
-
Low-density lipoprotein receptor-related protein
References
Miki, T., Ring, A., & Gerlach, J. (2011). Hepatic differentiation of human embryonic stem cells is promoted by three-dimensional dynamic perfusion culture conditions. Tissue Engineering. Part C, Methods, 17(5), 557–68.
Jaenisch, R., & Young, R. (2008). Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell, 132(4), 567–82.
Trentin, A., Glavieux-Pardanaud, C., Le Douarin, N. M., & Dupin, E. (2004). Self-renewal capacity is a widespread property of various types of neural crest precursor cells. Proceedings of the National Academy of Sciences of the United States of America, 101(13), 4495–500.
Shyh-Chang, N., Daley, G. Q., & Cantley, L. C. (2013). Stem cell metabolism in tissue development and aging. Development, 140(12), 2535–47.
Ortiz-Gonzalez, X. R., Keene, C. D., Verfaillie, C. M., & Low, W. C. (2004). Neural induction of adult bone marrow and umbilical cord stem cells. Current Neurovascular Research, 1(3), 207–13.
Gage, F. H., & Temple, S. (2013). Neural stem cells: generating and regenerating the brain. Neuron, 80(3), 588–601.
Cullen, S. M., Mayle, A., Rossi, L., & Goodell, M. A. (2014). Hematopoietic stem cell development: an epigenetic journey. Current Topics in Developmental Biology, 107, 39–75.
Clevers, H. (2013). The intestinal crypt, a prototype stem cell compartment. Cell, 154(2), 274–84.
Reya, T., Morrison, S. J., Clarke, M. F., & Weissman, I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature, 414(6859), 105–11.
Miki, T., Yasuda, S. Y., & Kahn, M. (2011). Wnt/beta-catenin signaling in embryonic stem cell self-renewal and somatic cell reprogramming. Stem Cell Reviews, 7(4), 836–46.
Takahashi-Yanaga, F., & Kahn, M. (2010). Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clinical Cancer Research, 16(12), 3153–62.
Merchant, A. A., & Matsui, W. (2010). Targeting hedgehog–a cancer stem cell pathway. Clinical Cancer Research, 16(12), 3130–40.
Liu, J., Sato, C., Cerletti, M., & Wagers, A. (2010). Notch signaling in the regulation of stem cell self-renewal and differentiation. Current Topics in Developmental Biology, 92, 367–409.
Takebe, N., Harris, P. J., Warren, R. Q., & Ivy, S. P. (2011). Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nature Reviews. Clinical Oncology, 8(2), 97–106.
Nusse, R. (2005). Wnt signaling in disease and in development. Cell Research, 15(1), 28–32.
MacDonald, B. T., Tamai, K., & He, X. (2009). Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental Cell, 17(1), 9–26.
Klaus, A., & Birchmeier, W. (2008). Wnt signalling and its impact on development and cancer. Nature Reviews Cancer, 8(5), 387–98.
Gough NR. Focus issue: Wnt and beta-catenin signaling in development and disease. (2012) Sci Signal Jan 10;5 (206):eg2.
Fodde, R., & Brabletz, T. (2007). Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Current Opinion in Cell Biology, 19(2), 150–8.
Moon, R. T., Kohn, A. D., De Ferrari, G. V., & Kaykas, A. (2004). WNT and beta-catenin signalling: diseases and therapies. Nature Reviews Genetics, 5(9), 691–701.
Reya, T., & Clevers, H. (2005). Wnt signalling in stem cells and cancer. Nature, 434(7035), 843–50.
Dickinson, M. E., & McMahon, A. P. (1992). The role of Wnt genes in vertebrate development. Current Opinion in Genetics and Development, 2(4), 562–6.
Nusse, R., & Varmus, H. (2012). Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO Journal, 31(12), 2670–84.
Mlodzik, M. (2002). Planar cell polarization: do the same mechanisms regulate drosophila tissue polarity and vertebrate gastrulation? Trends in Genetics, 18(11), 564–71.
Wang, Y., & Nathans, J. (2007). Tissue/planar cell polarity in vertebrates: new insights and new questions. Development, 134(4), 647–58.
Veeman, M. T., Axelrod, J. D., & Moon, R. T. (2003). A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Developmental Cell, 5(3), 367–77.
Kohn, A. D., & Moon, R. T. (2005). Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium, 38(3–4), 439–46.
Florian, M. C., Nattamai, K. J., Dorr, K., Marka, G., Uberle, B., Vas, V., et al. (2013). A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature, 503(7476), 392–6.
Nusse, R. (2008). Wnt signaling and stem cell control. Cell Research, 18(5), 523–7.
Nusse, R., & Varmus, H. E. (1992). Wnt genes. Cell, 69(7), 1073–87.
Moon RT. Wnt/beta-catenin pathway. (2005) Sci STKE 2005 Feb 15 (271):cm1.
Teo, J. L., & Kahn, M. (2010). The Wnt signaling pathway in cellular proliferation and differentiation: a tale of two coactivators. Advanced Drug Delivery Reviews, 62(12), 1149–55.
Daniels, D. L., & Weis, W. I. (2005). Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nature Structural and Molecular Biology, 12(4), 364–71.
Fang, M., Li, J., Blauwkamp, T., Bhambhani, C., Campbell, N., & Cadigan, K. M. (2006). C-terminal-binding protein directly activates and represses Wnt transcriptional targets in Drosophila. EMBO Journal, 25(12), 2735–45.
Sampson, E. M., Haque, Z. K., Ku, M. C., Tevosian, S. G., Albanese, C., Pestell, R. G., et al. (2001). Negative regulation of the Wnt-beta-catenin pathway by the transcriptional repressor HBP1. EMBO Journal, 20(16), 4500–11.
Angers, S., & Moon, R. T. (2009). Proximal events in Wnt signal transduction. Nature Reviews Molecular Cell Biology, 10(7), 468–77.
Valenta, T., Hausmann, G., & Basler, K. (2012). The many faces and functions of beta-catenin. EMBO Journal, 31(12), 2714–36.
Zhang, N., Wei, P., Gong, A., Chiu, W. T., Lee, H. T., Colman, H., et al. (2011). FoxM1 promotes beta-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell, 20(4), 427–42.
Tang, Y., Liu, Z., Zhao, L., Clemens, T. L., & Cao, X. (2008). Smad7 stabilizes beta-catenin binding to E-cadherin complex and promotes cell-cell adhesion. Journal of Biological Chemistry, 283(35), 23956–63.
Liu, Z., Tang, Y., Qiu, T., Cao, X., & Clemens, T. L. (2006). A dishevelled-1/Smad1 interaction couples WNT and bone morphogenetic protein signaling pathways in uncommitted bone marrow stromal cells. Journal of Biological Chemistry, 281(25), 17156–63.
Saegusa, M., Hashimura, M., & Kuwata, T. (2012). Sox4 functions as a positive regulator of beta-catenin signaling through upregulation of TCF4 during morular differentiation of endometrial carcinomas. Laboratory Investigation, 92(4), 511–21.
Faunes, F., Hayward, P., Descalzo, S. M., Chatterjee, S. S., Balayo, T., Trott, J., et al. (2013). A membrane-associated beta-catenin/Oct4 complex correlates with ground-state pluripotency in mouse embryonic stem cells. Development, 140(6), 1171–83.
Scholtysek, C., Katzenbeisser, J., Fu, H., Uderhardt, S., Ipseiz, N., Stoll, C., et al. (2013). PPARbeta/delta governs Wnt signaling and bone turnover. Nature Medicine, 19(5), 608–13.
Dyson, H. J., & Wright, P. E. (2005). Intrinsically unstructured proteins and their functions. Nature Reviews Molecular Cell Biology, 6(3), 197–208.
Goodman, R. H., & Smolik, S. (2000). CBP/p300 in cell growth, transformation, and development. Genes and Development, 14(13), 1553–77.
Iyer, N. G., Ozdag, H., & Caldas, C. (2004). p300/CBP and cancer. Oncogene, 23(24), 4225–31.
Foley, P., Bunyan, D., Stratton, J., Dillon, M., & Lynch, S. A. (2009). Further case of Rubinstein-Taybi syndrome due to a deletion in EP300. American Journal of Medical Genetics Part A, 149A(5), 997–1000.
Yao, T. P., Oh, S. P., Fuchs, M., Zhou, N. D., Ch’ng, L. E., Newsome, D., et al. (1998). Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell, 93(3), 361–72.
Bedford, D. C., Kasper, L. H., Fukuyama, T., & Brindle, P. K. (2010). Target gene context influences the transcriptional requirement for the KAT3 family of CBP and p300 histone acetyltransferases. Epigenetics, 5(1), 9–15.
Rebel, V. I., Kung, A. L., Tanner, E. A., Yang, H., Bronson, R. T., & Livingston, D. M. (2002). Distinct roles for CREB-binding protein and p300 in hematopoietic stem cell self-renewal. Proceedings of the National Academy of Sciences of the United States of America, 99(23), 14789–94.
Roth, J. F., Shikama, N., Henzen, C., Desbaillets, I., Lutz, W., Marino, S., et al. (2003). Differential role of p300 and CBP acetyltransferase during myogenesis: p300 acts upstream of MyoD and Myf5. EMBO Journal, 22(19), 5186–96.
Kawasaki, H., Eckner, R., Yao, T. P., Taira, K., Chiu, R., Livingston, D. M., et al. (1998). Distinct roles of the co-activators p300 and CBP in retinoic-acid-induced F9-cell differentiation. Nature, 393(6682), 284–9.
Ma, H., Nguyen, C., Lee, K. S., & Kahn, M. (2005). Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene, 24(22), 3619–31.
Siminovitch, L., McCulloch, E. A., & Till, J. E. (1963). The distribution of colony-forming cells among spleen colonies. Journal Cell Physiology, 62, 327–36.
Stachelscheid, H., Urbaniak, T., Ring, A., Spengler, B., Gerlach, J. C., & Zeilinger, K. (2009). Isolation and characterization of adult human liver progenitors from ischemic liver tissue derived from therapeutic hepatectomies. Tissue Engineering Part A, 15(7), 1633–43.
Lin, S. A., & Barker, N. (2011). Gastrointestinal stem cells in self-renewal and cancer. Journal of Gastroenterology, 46(9), 1039–55.
Leeman, K. T., Fillmore, C. M., & Kim, C. F. (2014). Lung stem and progenitor cells in tissue homeostasis and disease. Current Topics in Developmental Biology, 107C, 207–33.
Anversa, P., & Leri, A. (2013). Innate regeneration in the aging heart: healing from within. Mayo Clinic Proceedings, 88(8), 871–83.
Williams A. (2014) Central nervous system regeneration--where are we? QJM Jan 13.
Blanpain, C., & Simons, B. D. (2013). Unravelling stem cell dynamics by lineage tracing. Nature Reviews Molecular Cell Biology, 14(8), 489–502.
Ring, A., Gerlach, J., Peters, G., Pazin, B. J., Minervini, C. F., Turner, M. E., et al. (2010). Hepatic maturation of human fetal hepatocytes in four-compartment three-dimensional perfusion culture. Tissue Engineering. Part C, Methods, 16(5), 835–45.
Morrison, S. J., & Kimble, J. (2006). Asymmetric and symmetric stem-cell divisions in development and cancer. Nature, 441(7097), 1068–74.
Lowry, W. E., & Richter, L. (2007). Signaling in adult stem cells. Frontiers in Bioscience, 12, 3911–27.
Blank, U., Karlsson, G., & Karlsson, S. (2008). Signaling pathways governing stem-cell fate. Blood, 111(2), 492–503.
Boras-Granic, K., & Wysolmerski, J. J. (2008). Wnt signaling in breast organogenesis. Organogenesis, 4(2), 116–22.
Inestrosa, N. C., & Arenas, E. (2010). Emerging roles of Wnts in the adult nervous system. Nature Review Neuroscience, 11(2), 77–86.
Naito, A. T., Shiojima, I., Akazawa, H., Hidaka, K., Morisaki, T., Kikuchi, A., et al. (2006). Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proceedings of the National Academy of Sciences of the United States of America, 103(52), 19812–7.
Reya, T., Duncan, A. W., Ailles, L., Domen, J., Scherer, D. C., Willert, K., et al. (2003). A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature, 423(6938), 409–14.
Clarke, A. R. (2006). Wnt signalling in the mouse intestine. Oncogene, 25(57), 7512–21.
Brennan, K. R., & Brown, A. M. (2004). Wnt proteins in mammary development and cancer. Journal of Mammary Gland Biology and Neoplasia, 9(2), 119–31.
Zechner, D., Fujita, Y., Hulsken, J., Muller, T., Walther, I., Taketo, M. M., et al. (2003). beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Developmental Biology, 258(2), 406–18.
Korinek, V., Barker, N., & Moerer, P. (1998). van DE, Huls G, Peters PJ, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nature Genetics, 19(4), 379–83.
Cheng, H., & Leblond, C. P. (1974). Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. unitarian theory of the origin of the four epithelial cell types. The American Journal of Anatomy, 141(4), 537–61.
Farin, H. F., van Es, J. H., & Clevers, H. (2012). Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology, 143(6), 1518–29.
Pinto, D., Gregorieff, A., Begthel, H., & Clevers, H. (2003). Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes and Development, 17(14), 1709–13.
Tian, H., Biehs, B., Warming, S., Leong, K. G., Rangell, L., Klein, O. D., et al. (2011). A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature, 478(7368), 255–9.
Yan, K. S., Chia, L. A., Li, X., Ootani, A., Su, J., Lee, J. Y., et al. (2012). The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proceedings of the National Academy of Sciences of the United States of America, 109(2), 466–71.
Barker, N., van Es, J. H., Kuipers, J., & Kujala, P. (2007). van den BM, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature, 449(7165), 1003–7.
De, L. W., Barker, N., Low, T. Y., Koo, B. K., Li, V. S., Teunissen, H., et al. (2011). Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature, 476(7360), 293–7.
Gregorieff, A., Pinto, D., Begthel, H., Destree, O., Kielman, M., & Clevers, H. (2005). Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology, 129(2), 626–38.
Sato, T., van Es, J. H., Snippert, H. J., Stange, D. E., Vries, R. G., van den, B. M., et al. (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature, 469(7330), 415–8.
Bastide, P., Darido, C., Pannequin, J., Kist, R., Robine, S., Marty-Double, C., et al. (2007). Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. Journal of Cell Biology, 178(4), 635–48.
Mori-Akiyama, Y., van den, B. M., van Es, J. H., Hamilton, S. R., Adams, H. P., Zhang, J., et al. (2007). SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology, 133(2), 539–46.
Willert, K., Brown, J. D., Danenberg, E., Duncan, A. W., Weissman, I. L., Reya, T., et al. (2003). Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature, 423(6938), 448–52.
Nemeth, M. J., Mak, K. K., Yang, Y., & Bodine, D. M. (2009). Beta-Catenin expression in the bone marrow microenvironment is required for long-term maintenance of primitive hematopoietic cells. Stem Cells, 27(5), 1109–19.
Malhotra, S., & Kincade, P. W. (2009). Wnt-related molecules and signaling pathway equilibrium in hematopoiesis. Cell Stem Cell, 4(1), 27–36.
Fukuda, S., & Pelus, L. M. (2001). Regulation of the inhibitor-of-apoptosis family member survivin in normal cord blood and bone marrow CD34 (+) cells by hematopoietic growth factors: implication of survivin expression in normal hematopoiesis. Blood, 98(7), 2091–100.
Fukuda, S., Foster, R. G., Porter, S. B., & Pelus, L. M. (2002). The antiapoptosis protein survivin is associated with cell cycle entry of normal cord blood CD34 (+) cells and modulates cell cycle and proliferation of mouse hematopoietic progenitor cells. Blood, 100(7), 2463–71.
Dravid, G., Ye, Z., Hammond, H., Chen, G., Pyle, A., Donovan, P., et al. (2005). Defining the role of Wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells, 23(10), 1489–501.
Gessert, S., & Kuhl, M. (2010). The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circulation Research, 107(2), 186–99.
Brade, T., Manner, J., & Kuhl, M. (2006). The role of Wnt signalling in cardiac development and tissue remodelling in the mature heart. Cardiovascular Research, 72(2), 198–209.
Baurand, A., Zelarayan, L., Betney, R., Gehrke, C., Dunger, S., Noack, C., et al. (2007). Beta-catenin downregulation is required for adaptive cardiac remodeling. Circulation Research, 100(9), 1353–62.
mini-Nik, S., Glancy, D., Boimer, C., Whetstone, H., Keller, C., & Alman, B. A. (2011). Pax7 expressing cells contribute to dermal wound repair, regulating scar size through a beta-catenin mediated process. Stem Cells, 29(9), 1371–9.
Saraswati, S., Alfaro, M. P., Thorne, C. A., Atkinson, J., Lee, E., & Young, P. P. (2010). Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS One, 5(11), e15521.
Sasaki, T., Hwang, H., Nguyen, C., Kloner, R. A., & Kahn, M. (2013). The small molecule Wnt signaling modulator ICG-001 improves contractile function in chronically infarcted rat myocardium. PLoS One, 8(9), e75010.
Zeng, Y. A., & Nusse, R. (2010). Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell, 6(6), 568–77.
Turashvili, G., Bouchal, J., Burkadze, G., & Kolar, Z. (2006). Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology, 73(5), 213–23.
Imbert, A., Eelkema, R., Jordan, S., Feiner, H., & Cowin, P. (2001). Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland. Journal of Cell Biology, 153(3), 555–68.
Liu, B. Y., McDermott, S. P., Khwaja, S. S., & Alexander, C. M. (2004). The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proceedings of the National Academy of Sciences of the United States of America, 101(12), 4158–63.
Tepera, S. B., McCrea, P. D., & Rosen, J. M. (2003). A beta-catenin survival signal is required for normal lobular development in the mammary gland. Journal of Cell Science, 116(Pt 6), 1137–49.
Hsu, W., Shakya, R., & Costantini, F. (2001). Impaired mammary gland and lymphoid development caused by inducible expression of Axin in transgenic mice. Journal of Cell Biology, 155(6), 1055–64.
Boras-Granic, K., Chang, H., Grosschedl, R., & Hamel, P. A. (2006). Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Developmental Biology, 295(1), 219–31.
Alexander CM, Goel S, Fakhraldeen SA, Kim S. (2012) Wnt signaling in mammary glands: plastic cell fates and combinatorial signaling. Cold Spring Harb Perspect Biol;4 (10).
Kordon, E. C., & Smith, G. H. (1998). An entire functional mammary gland may comprise the progeny from a single cell. Development, 125(10), 1921–30.
Badders, N. M., Goel, S., Clark, R. J., Klos, K. S., Kim, S., Bafico, A., et al. (2009). The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS One, 4(8), e6594.
Lindvall, C., Zylstra, C. R., Evans, N., West, R. A., Dykema, K., Furge, K. A., et al. (2009). The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PLoS One, 4(6), e5813.
Wexler, E. M., Paucer, A., Kornblum, H. I., Palmer, T. D., & Geschwind, D. H. (2009). Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells, 27(5), 1130–41.
Fancy, S. P., Harrington, E. P., Yuen, T. J., Silbereis, J. C., Zhao, C., Baranzini, S. E., et al. (2011). Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nature Neuroscience, 14(8), 1009–16.
Teo, J. L., Ma, H., Nguyen, C., Lam, C., & Kahn, M. (2005). Specific inhibition of CBP/beta-catenin interaction rescues defects in neuronal differentiation caused by a presenilin-1 mutation. Proceedings of the National Academy of Sciences of the United States of America, 102(34), 12171–6.
Shipitsin, M., Campbell, L. L., Argani, P., Weremowicz, S., Bloushtain-Qimron, N., Yao, J., et al. (2007). Molecular definition of breast tumor heterogeneity. Cancer Cell, 11(3), 259–73.
Visvader, J. E., & Lindeman, G. J. (2008). Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature Reviews Cancer, 8(10), 755–68.
Al-Hajj, M., & Clarke, M. F. (2004). Self-renewal and solid tumor stem cells. Oncogene, 23(43), 7274–82.
Jamieson, C. H., Weissman, I. L., & Passegue, E. (2004). Chronic versus acute myelogenous leukemia: a question of self-renewal. Cancer Cell, 6(6), 531–3.
Armanios, M., & Greider, C. W. (2005). Telomerase and cancer stem cells. Cold Spring Harbor Symposia on Quantitative Biology, 70, 205–8.
Clevers, H. (2011). The cancer stem cell: premises, promises and challenges. Nature Medicine, 17(3), 313–9.
Visvader, J. E., & Lindeman, G. J. (2010). Stem cells and cancer - the promise and puzzles. Molecular Oncology, 4(5), 369–72.
Hirschmann-Jax, C., Foster, A. E., Wulf, G. G., Goodell, M. A., & Brenner, M. K. (2005). A distinct “side population” of cells in human tumor cells: implications for tumor biology and therapy. Cell Cycle, 4(2), 203–5.
Hirschmann-Jax, C., Foster, A. E., Wulf, G. G., Nuchtern, J. G., Jax, T. W., Gobel, U., et al. (2004). A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proceedings of the National Academy of Sciences of the United States of America, 101(39), 14228–33.
Hussenet, T., Dembele, D., Martinet, N., Vignaud, J. M., & du, M. S. (2010). An adult tissue-specific stem cell molecular phenotype is activated in epithelial cancer stem cells and correlated to patient outcome. Cell Cycle, 9(2), 321–7.
Liu, S., & Wicha, M. S. (2010). Targeting breast cancer stem cells. Journal of Clinical Oncology, 28(25), 4006–12.
Vassiliou, G. S., Cooper, J. L., Rad, R., Li, J., Rice, S., Uren, A., et al. (2011). Mutant nucleophosmin and cooperating pathways drive leukemia initiation and progression in mice. Nature Genetics, 43(5), 470–5.
Gudjonsson, T., & Magnusson, M. K. (2005). Stem cell biology and the cellular pathways of carcinogenesis. APMIS, 113(11–12), 922–9.
Chaffer, C. L., Brueckmann, I., Scheel, C., Kaestli, A. J., Wiggins, P. A., Rodrigues, L. O., et al. (2011). Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proceedings of the National Academy of Sciences of the United States of America, 108(19), 7950–5.
Liu, S., Ginestier, C., Charafe-Jauffret, E., Foco, H., Kleer, C. G., Merajver, S. D., et al. (2008). BRCA1 regulates human mammary stem/progenitor cell fate. Proceedings of the National Academy of Sciences of the United States of America, 105(5), 1680–5.
Foulkes, W. D. (2004). BRCA1 functions as a breast stem cell regulator. Journal of Medical Genetics, 41(1), 1–5.
Mani, S. A., Guo, W., Liao, M. J., Eaton, E. N., Ayyanan, A., Zhou, A. Y., et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell, 133(4), 704–15.
Morel, A. P., Lievre, M., Thomas, C., Hinkal, G., Ansieau, S., & Puisieux, A. (2008). Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One, 3(8), e2888.
der PG, v. (2011). Epithelial plasticity, cancer stem cells and bone metastasis formation. Bone, 48(1), 37–43.
Godde, N. J., Galea, R. C., Elsum, I. A., & Humbert, P. O. (2010). Cell polarity in motion: redefining mammary tissue organization through EMT and cell polarity transitions. Journal of Mammary Gland Biology and Neoplasia, 15(2), 149–68.
Chaffer, C. L., & Weinberg, R. A. (2011). A perspective on cancer cell metastasis. Science, 331(6024), 1559–64.
Moreno-Bueno, G., Portillo, F., & Cano, A. (2008). Transcriptional regulation of cell polarity in EMT and cancer. Oncogene, 27(55), 6958–69.
Kelly, P. N., Dakic, A., Adams, J. M., Nutt, S. L., & Strasser, A. (2007). Tumor growth need not be driven by rare cancer stem cells. Science, 317(5836), 337.
Bruce, W., & Van Der, G. (1963). A quantitative assay for the Number of murine lymphoma cells capable of proliferation in vivo. Nature, 199, 79–80.
Bonnet, D., & Dick, J. E. (1997). Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine, 3(7), 730–7.
Singh, S. K., Hawkins, C., Clarke, I. D., Squire, J. A., Bayani, J., Hide, T., et al. (2004). Identification of human brain tumour initiating cells. Nature, 432(7015), 396–401.
Fang, D., Nguyen, T. K., Leishear, K., Finko, R., Kulp, A. N., Hotz, S., et al. (2005). A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Research, 65(20), 9328–37.
Li, C., Heidt, D. G., Dalerba, P., Burant, C. F., Zhang, L., Adsay, V., et al. (2007). Identification of pancreatic cancer stem cells. Cancer Research, 67(3), 1030–7.
Ma, S., Chan, K. W., Hu, L., Lee, T. K., Wo, J. Y., Ng, I. O., et al. (2007). Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology, 132(7), 2542–56.
O’Brien, C. A., Pollett, A., Gallinger, S., & Dick, J. E. (2007). A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature, 445(7123), 106–10.
Blum, B., Bar-Nur, O., Golan-Lev, T., & Benvenisty, N. (2009). The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nature Biotechnology, 27(3), 281–7.
Hoffmeyer, K., Raggioli, A., Rudloff, S., Anton, R., Hierholzer, A., Del, V. I., et al. (2012). Wnt/beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science, 336(6088), 1549–54.
DiMeo, T. A., Anderson, K., Phadke, P., Fan, C., Perou, C. M., Naber, S., et al. (2009). A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Research, 69(13), 5364–73.
Sack, U., Walther, W., Scudiero, D., Selby, M., Aumann, J., Lemos, C., et al. (2011). S100A4-induced cell motility and metastasis is restricted by the Wnt/beta-catenin pathway inhibitor calcimycin in colon cancer cells. Molecular Biology of the Cell, 22(18), 3344–54.
Uchino, M., Kojima, H., Wada, K., Imada, M., Onoda, F., Satofuka, H., et al. (2010). Nuclear beta-catenin and CD44 upregulation characterize invasive cell populations in non-aggressive MCF-7 breast cancer cells. BMC Cancer, 10, 414.
Yu, M., Ting, D. T., Stott, S. L., Wittner, B. S., Ozsolak, F., Paul, S., et al. (2012). RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature, 487(7408), 510–3.
Lickert, H., Domon, C., Huls, G., Wehrle, C., Duluc, I., Clevers, H., et al. (2000). Wnt/(beta)-catenin signaling regulates the expression of the homeobox gene Cdx1 in embryonic intestine. Development, 127(17), 3805–13.
Russell, R. G., Lasorella, A., Dettin, L. E., & Iavarone, A. (2004). Id2 drives differentiation and suppresses tumor formation in the intestinal epithelium. Cancer Research, 64(20), 7220–5.
Klonisch, T., Wiechec, E., Hombach-Klonisch, S., Ande, S. R., Wesselborg, S., Schulze-Osthoff, K., et al. (2008). Cancer stem cell markers in common cancers - therapeutic implications. Trends in Molecular Medicine, 14(10), 450–60.
Wielenga, V. J., Smits, R., Korinek, V., Smit, L., Kielman, M., Fodde, R., et al. (1999). Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. American Journal of Pathology, 154(2), 515–23.
Shulewitz, M., Soloviev, I., Wu, T., Koeppen, H., Polakis, P., & Sakanaka, C. (2006). Repressor roles for TCF-4 and Sfrp1 in Wnt signaling in breast cancer. Oncogene, 25(31), 4361–9.
Katoh, Y., & Katoh, M. (2007). Comparative genomics on PROM1 gene encoding stem cell marker CD133. International Journal of Molecular Medicine, 19(6), 967–70.
Correa, S., Binato, R., Du, R. B., Castelo-Branco, M. T., Pizzatti, L., & Abdelhay, E. (2012). Wnt/beta-catenin pathway regulates ABCB1 transcription in chronic myeloid leukemia. BMC Cancer, 12, 303.
Yamada, T., Takaoka, A. S., Naishiro, Y., Hayashi, R., Maruyama, K., Maesawa, C., et al. (2000). Transactivation of the multidrug resistance 1 gene by T-cell factor 4/beta-catenin complex in early colorectal carcinogenesis. Cancer Research, 60(17), 4761–6.
Munz, M., Baeuerle, P. A., & Gires, O. (2009). The emerging role of EpCAM in cancer and stem cell signaling. Cancer Research, 69(14), 5627–9.
Yamashita, T., Budhu, A., Forgues, M., & Wang, X. W. (2007). Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Research, 67(22), 10831–9.
Al-Hajj, M., Wicha, M. S., ito-Hernandez, A., Morrison, S. J., & Clarke, M. F. (2003). Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 100(7), 3983–8.
Sheridan, C., Kishimoto, H., Fuchs, R. K., Mehrotra, S., Bhat-Nakshatri, P., Turner, C. H., et al. (2006). CD44+/. Breast Cancer Research, 8(5), R59.
Fillmore, C. M., & Kuperwasser, C. (2008). Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Research, 10(2), R25.
Hennessy, B. T., Gonzalez-Angulo, A. M., Stemke-Hale, K., Gilcrease, M. Z., Krishnamurthy, S., Lee, J. S., et al. (2009). Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Research, 69(10), 4116–24.
Nusse, R., & Varmus, H. E. (1982). Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell, 31(1), 99–109.
Howe, L. R., & Brown, A. M. (2004). Wnt signaling and breast cancer. Cancer Biology and Therapy, 3(1), 36–41.
Mukherjee, N., Bhattacharya, N., Alam, N., Roy, A., Roychoudhury, S., & Panda, C. K. (2012). Subtype-specific alterations of the Wnt signaling pathway in breast cancer: clinical and prognostic significance. Cancer Science, 103(2), 210–20.
Khramtsov, A. I., Khramtsova, G. F., Tretiakova, M., Huo, D., Olopade, O. I., & Goss, K. H. (2010). Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. American Journal of Pathology, 176(6), 2911–20.
Lim, E., Wu, D., Pal, B., & Bouras, T. (2010). sselin-Labat ML, Vaillant F, et al. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Research, 12(2), R21.
Zhang, Y., Toy, K. A., & Kleer, C. G. (2012). Metaplastic breast carcinomas are enriched in markers of tumor-initiating cells and epithelial to mesenchymal transition. Modern Pathology, 25(2), 178–84.
Howe, L. R., Watanabe, O., Leonard, J., & Brown, A. M. (2003). Twist is up-regulated in response to Wnt1 and inhibits mouse mammary cell differentiation. Cancer Research, 63(8), 1906–13.
Takebe, N., Warren, R. Q., & Ivy, S. P. (2011). Breast cancer growth and metastasis: interplay between cancer stem cells, embryonic signaling pathways and epithelial-to-mesenchymal transition. Breast Cancer Research, 13(3), 211.
Bhat-Nakshatri, P., Appaiah, H., Ballas, C., Pick-Franke, P., Goulet, R., Jr., Badve, S., et al. (2010). SLUG/SNAI2 and tumor necrosis factor generate breast cells with CD44+/CD24- phenotype. BMC Cancer, 10, 411.
Wang, J., O’Bara, M. A., Pol, S. U., & Sim, F. J. (2013). CD133/CD140a-based isolation of distinct human multipotent neural progenitor cells and oligodendrocyte progenitor cells. Stem Cells and Development, 22(15), 2121–31.
Shmelkov, S. V., St, C. R., Lyden, D., & Rafii, S. (2005). AC133/CD133/Prominin-1. International Journal of Biochemistry and Cell Biology, 37(4), 715–9.
Corbeil, D., Marzesco, A. M., Wilsch-Brauninger, M., & Huttner, W. B. (2010). The intriguing links between prominin-1 (CD133), cholesterol-based membrane microdomains, remodeling of apical plasma membrane protrusions, extracellular membrane particles, and (neuro) epithelial cell differentiation. FEBS Letters, 584(9), 1659–64.
Yin, A. H., Miraglia, S., & Zanjani, E. D. (1997). meida-Porada G, Ogawa M, Leary AG, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood, 90(12), 5002–12.
Uchida, H., Yamazaki, K., Fukuma, M., Yamada, T., Hayashida, T., Hasegawa, H., et al. (2010). Overexpression of leucine-rich repeat-containing G protein-coupled receptor 5 in colorectal cancer. Cancer Science, 101(7), 1731–7.
Vermeulen, L., Todaro, M., de Sousa, M. F., Sprick, M. R., Kemper, K., Perez, A. M., et al. (2008). Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proceedings of the National Academy of Sciences of the United States of America, 105(36), 13427–32.
van de, W. M., Sancho, E., Verweij, C., de, L. W., Oving, I., Hurlstone, A., et al. (2002). The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell, 111(2), 241–50.
Haraguchi, N., Utsunomiya, T., Inoue, H., Tanaka, F., Mimori, K., Barnard, G. F., et al. (2006). Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells, 24(3), 506–13.
Hirschmann-Jax, C., Foster, A. E., Wulf, G. G., Nuchtern, J. G., Jax, T. W., Gobel, U., et al. (2004). A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proceedings of the National Academy of Sciences of the United States of America, 101(39), 14228–33.
O’Brien, C. A., Kreso, A., & Jamieson, C. H. (2010). Cancer stem cells and self-renewal. Clinical Cancer Research, 16(12), 3113–20.
Moshaver, B. (2008). van RA, Kelder A, van der PM, Terwijn M, Bachas C, et al. Identification of a small subpopulation of candidate leukemia-initiating cells in the side population of patients with acute myeloid leukemia. Stem Cells, 26(12), 3059–67.
Wang, Y., Krivtsov, A. V., Sinha, A. U., North, T. E., Goessling, W., Feng, Z., et al. (2010). The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science, 327(5973), 1650–3.
Radich, J. P., Dai, H., Mao, M., Oehler, V., Schelter, J., Druker, B., et al. (2006). Gene expression changes associated with progression and response in chronic myeloid leukemia. Proceedings of the National Academy of Sciences of the United States of America, 103(8), 2794–9.
Pehlivan, M., Sercan, Z., & Sercan, H. O. (2009). sFRP1 promoter methylation is associated with persistent Philadelphia chromosome in chronic myeloid leukemia. Leukemia Research, 33(8), 1062–7.
Gang EJ, Hsieh YT, Pham J, Zhao Y, Nguyen C, Huantes S, et al. (2013) Small-molecule inhibition of CBP/catenin interactions eliminates drug-resistant clones in acute lymphoblastic leukemia. Oncogene Jun 3.
Cavodeassi, F., Carreira-Barbosa, F., Young, R. M., Concha, M. L., Allende, M. L., Houart, C., et al. (2005). Early stages of zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/beta-catenin pathway. Neuron, 47(1), 43–56.
Rudloff, S., & Kemler, R. (2012). Differential requirements for beta-catenin during mouse development. Development, 139(20), 3711–21.
Otto, A., Schmidt, C., Luke, G., Allen, S., Valasek, P., Muntoni, F., et al. (2008). Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. Journal of Cell Science, 121(Pt 17), 2939–50.
Le, G. F., Jones, A. E., Seale, V., Scime, A., & Rudnicki, M. A. (2009). Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell, 4(6), 535–47.
Otero, J. J., Fu, W., Kan, L., Cuadra, A. E., & Kessler, J. A. (2004). Beta-catenin signaling is required for neural differentiation of embryonic stem cells. Development, 131(15), 3545–57.
Wang, X., Kopinke, D., Lin, J., McPherson, A. D., Duncan, R. N., Otsuna, H., et al. (2012). Wnt signaling regulates postembryonic hypothalamic progenitor differentiation. Developmental Cell, 23(3), 624–36.
Chenn, A., & Walsh, C. A. (2002). Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science, 297(5580), 365–9.
Li, J., Sutter, C., Parker, D. S., Blauwkamp, T., Fang, M., & Cadigan, K. M. (2007). CBP/p300 are bimodal regulators of Wnt signaling. EMBO Journal, 26(9), 2284–94.
Emami, K. H., Nguyen, C., Ma, H., Kim, D. H., Jeong, K. W., Eguchi, M., et al. (2004). A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proceedings of the National Academy of Sciences of the United States of America, 101(34), 12682–7.
McMillan, M., & Kahn, M. (2005). Investigating Wnt signaling: a chemogenomic safari. Drug Discovery Today, 10(21), 1467–74.
Kumar, S. R., Scehnet, J. S., Ley, E. J., Singh, J., Krasnoperov, V., Liu, R., et al. (2009). Preferential induction of EphB4 over EphB2 and its implication in colorectal cancer progression. Cancer Research, 69(9), 3736–45.
Kim, Y. M., Ma, H., Oehler, V. G., Gang, E. J., Nguyen, C., Masiello, D., et al. (2011). The gamma catenin/CBP complex maintains survivin transcription in beta-catenin deficient/depleted cancer cells. Current Cancer Drug Targets, 11(2), 213–25.
Deato, M. D., & Tjian, R. (2008). An unexpected role of TAFs and TRFs in skeletal muscle differentiation: switching core promoter complexes. Cold Spring Harbor Symposia on Quantitative Biology, 73, 217–25.
Creyghton, M. P., Roel, G., Eichhorn, P. J., Hijmans, E. M., Maurer, I., Destree, O., et al. (2005). PR72, a novel regulator of Wnt signaling required for naked cuticle function. Genes and Development, 19(3), 376–86.
Zeng, W., Wharton, K. A., Jr., Mack, J. A., Wang, K., Gadbaw, M., Suyama, K., et al. (2000). naked cuticle encodes an inducible antagonist of Wnt signalling. Nature, 403(6771), 789–95.
Miyabayashi, T., Teo, J. L., Yamamoto, M., McMillan, M., Nguyen, C., & Kahn, M. (2007). Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proceedings of the National Academy of Sciences of the United States of America, 104(13), 5668–73.
Hasegawa, K., Yasuda, S. Y., Teo, J. L., Nguyen, C., McMillan, M., Hsieh, C. L., et al. (2012). Wnt signaling orchestration with a small molecule DYRK inhibitor provides long-term xeno-free human pluripotent cell expansion. Stem Cells Translational Medecine, 1(1), 18–28.
Corcoran, E. E., Joseph, J. D., MacDonald, J. A., Kane, C. D., Haystead, T. A., & Means, A. R. (2003). Proteomic analysis of calcium/calmodulin-dependent protein kinase I and IV in vitro substrates reveals distinct catalytic preferences. Journal of Biological Chemistry, 278(12), 10516–22.
Cicalese, A., Bonizzi, G., Pasi, C. E., Faretta, M., Ronzoni, S., Giulini, B., et al. (2009). The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell, 138(6), 1083–95.
Cohnheim, J. (1867). Ueber entzuendung und eiterung. PathologyAnatomyPhysiologyKlinicheskaia Meditsina, 40, 1–79.
Acknowledgments
MK gratefully acknowledges support from USC Norris Comprehensive Cancer Center Support Grant P30 CA014089, NIH 1R01CA166161-01A1 and NIH 1R01 HL112638-01.
YMK gratefully acknowledges support from NIH Grant R01CA172896.
AR gratefully acknowledges support from the California Institute for Regenerative Medicine (CIRM) clinical fellowship TG2-01161.
Disclosure
Michael Kahn is a consultant and equity holder in Prism Pharmaceuticals.
The remaining authors indicate no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ring, A., Kim, YM. & Kahn, M. Wnt/Catenin Signaling in Adult Stem Cell Physiology and Disease. Stem Cell Rev and Rep 10, 512–525 (2014). https://doi.org/10.1007/s12015-014-9515-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-014-9515-2